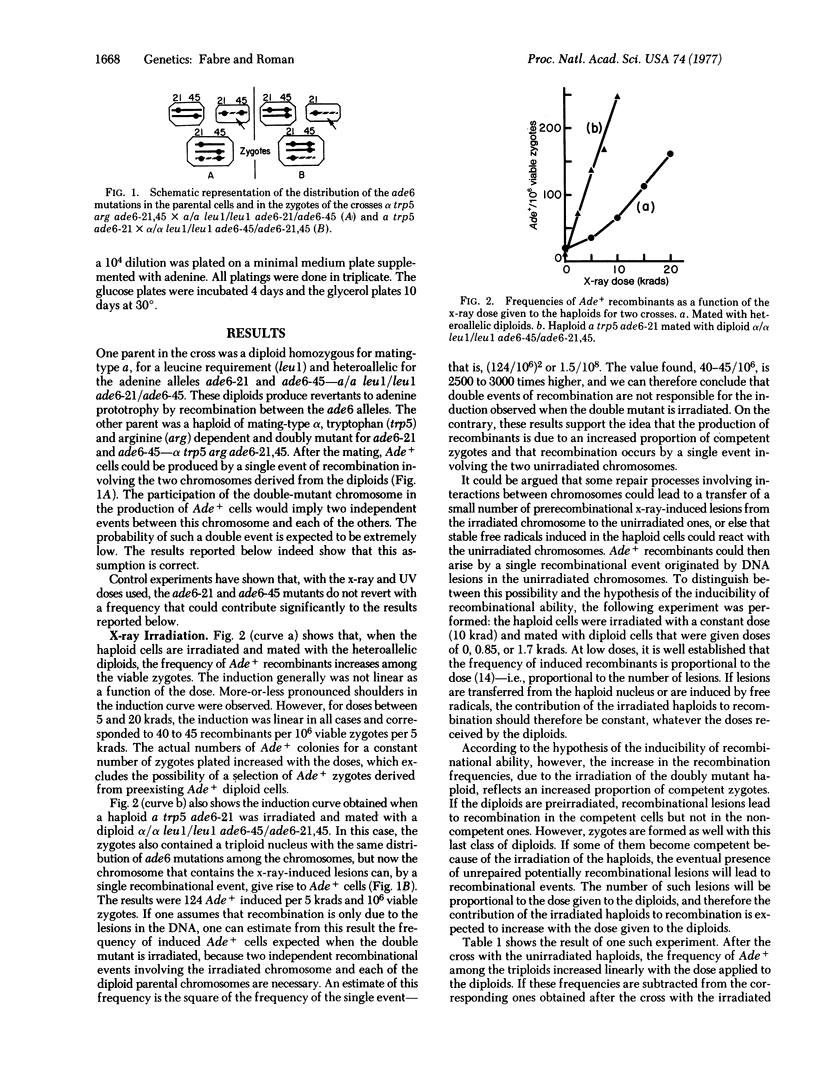
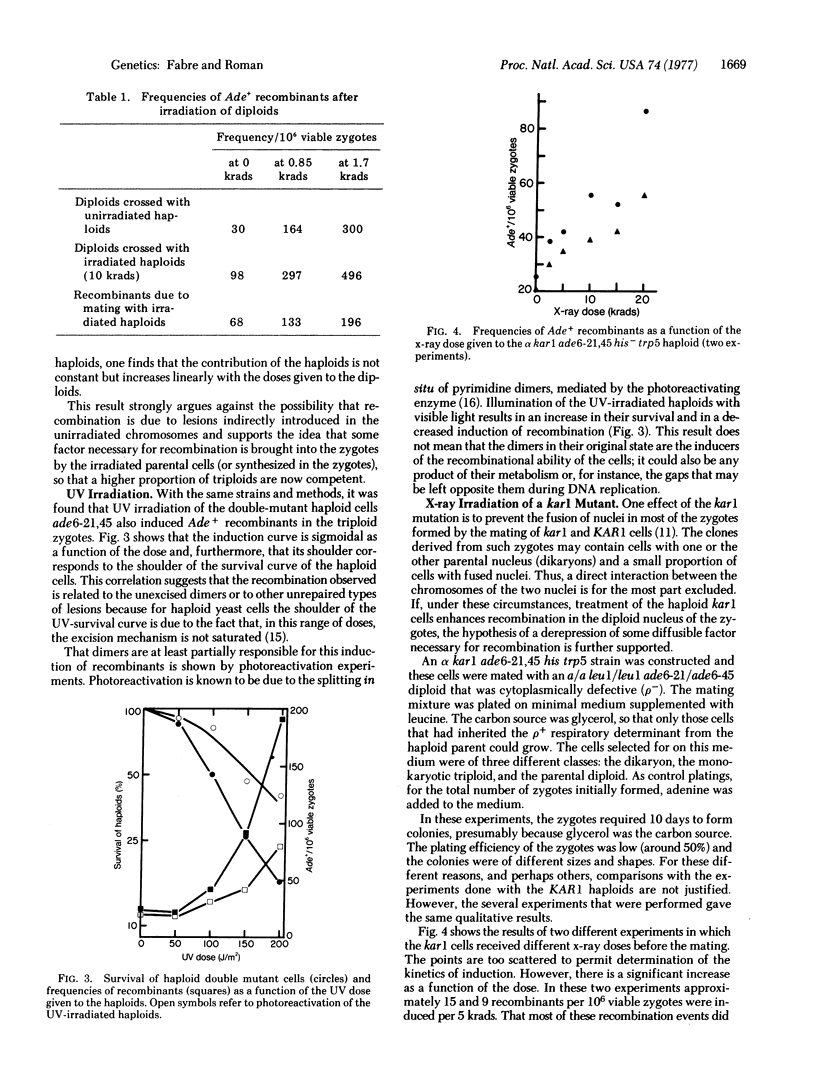
Abstract
Recombination between unirradiated chromosomes was induced by UV or x-ray irradiation of haploids followed by a mating with heteroallelic diploids of Saccharomyces cerevisiae. The selected event of intragenic recombination did not involve the participation of the irradiated chromosome and apparently was not caused by lesions introduced into the unirradiated chromosomes by some indirect process. The results favor the idea that recombination is repressed in the majority of vegetative cells and that one effect of radiation is the release of some factor(s) necessary for recombination. Consequently, the proportion of competent cells (i.e., cells able to recombine) in the population increases. This competent state seems necessary not only for the recombinational repair of radiation-induced lesions but also, since recombinants are produced in the absence of such lesions, for spontaneous recombination. Photoreactivation of the UV-irradiated haploids led to a decrease in the production of recombinants. Hence, lesions in the DNA appear to be responsible for the induction of the recombinational ability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boram W. R., Roman H. Recombination in Saccharomyces cerevisiae: a DNA repair mutation associated with elevated mitotic gene conversion. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2828–2832. doi: 10.1073/pnas.73.8.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. A. The Induction of Mitotic Gene Conversion by X-Irradiation of Haploid SACCHAROMYCES CEREVISIAE. Genetics. 1973 Jun;74(2):243–258. doi: 10.1093/genetics/74.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R. The control of dark repair mechanisms in meiotic cells. Mol Gen Genet. 1967;100(2):140–149. doi: 10.1007/BF00333600. [DOI] [PubMed] [Google Scholar]
- Esposito R. E., Esposito M. S. Genetic recombination and commitment to meiosis in Saccharomyces. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3172–3176. doi: 10.1073/pnas.71.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOGEL S., HURST D. D. Coincidence relations between gene conversion and mitotic recombination in Saccharomyces. Genetics. 1963 Mar;48:321–328. doi: 10.1093/genetics/48.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURST D. D., FOGEL S. MITOTIC RECOMBINATION AND HETEROALLELIC REPAIR IN SACCHAROMYCES CEREVISIAE. Genetics. 1964 Sep;50:435–458. doi: 10.1093/genetics/50.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Biochemical measure of the time and frequency of radiation-induced allelic recombination in Ustilago. Nat New Biol. 1971 Aug 25;232(34):233–236. doi: 10.1038/newbio232233a0. [DOI] [PubMed] [Google Scholar]
- Holliday R. Further evidence for an inducible recombination repair system in Ustilago maydis. Mutat Res. 1975 Jul;29(1):149–153. doi: 10.1016/0027-5107(75)90029-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Hénaut A., Luzzati M. Contrôle de l'aptitude à recombiner pendant la phase végétative chez Saccharomyces cerevisiae. Mol Gen Genet. 1972;116(1):26–34. doi: 10.1007/BF00334256. [DOI] [PubMed] [Google Scholar]
- MANNEY T. R., MORTIMER R. K. ALLELIC MAPPING IN YEAST BY X-RAY-INDUCED MITOTIC REVERSION. Science. 1964 Feb 7;143(3606):581–583. doi: 10.1126/science.143.3606.581. [DOI] [PubMed] [Google Scholar]
- MORTIMER R. K. Evidence of two types of x-ray-induced lethal damage in Saccharomyces cerevisiae. Radiat Res. 1955 Jun;2(4):361–368. [PubMed] [Google Scholar]
- Moore P. D. Radiation-sensitive pyrimidine auxotrophs of Ustilago maydis. II. A study of repair mechanisms and UV recovery in pyr I. Mutat Res. 1975 Jun;28(3):367–380. doi: 10.1016/0027-5107(75)90231-6. [DOI] [PubMed] [Google Scholar]
- Parry E. M., Parry J. M. Genetic analysis of UV inactivation, recovery and regulatory phenomena in a strain of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1973 Aug 10;124(2):117–133. doi: 10.1007/BF00265145. [DOI] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K. Photoreactivation. Radiat Res. 1966;(Suppl):141+–141+. [PubMed] [Google Scholar]
- Unrau P. The excision of pyrimidine dimers from the DNA of mutant and wild-type strains of Ustilago. Mutat Res. 1975 Jul;29(1):53–65. doi: 10.1016/0027-5107(75)90020-2. [DOI] [PubMed] [Google Scholar]
- WILKIE D., LEWIS D. THE EFFECT OF ULTRAVIOLET LIGHT ON RECOMBINATION IN YEAST. Genetics. 1963 Dec;48:1701–1716. doi: 10.1093/genetics/48.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. Dose dependence of the excision of ultraviolet-induced pyrimidine dimers from nuclear deoxyribonucleic acids of haploid and diploid Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):901–906. doi: 10.1128/jb.121.3.901-906.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


