Abstract
Background and Purpose
Fibroblast growth factor 23 (FGF23) is a hormone that regulates phosphorus and vitamin D metabolism. Elevated FGF23 concentrations are associated with excess risk of cardiovascular disease. Associations of FGF23 with stroke outcomes are less clear.
Methods
Using a case-cohort study design, we examined the association of baseline plasma FGF23 concentrations with incident stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a cohort of black and white adults ≥45 years of age. FGF23 was measured in 615 participants who developed incident stroke (cases) and in 936 participants randomly selected from the REGARDS cohort (comparison sub-cohort).
Results
In multivariable-adjusted models, higher calcium and phosphorus concentrations, lower estimated glomerular filtration rate and higher urine albumin excretion were independently associated with higher FGF23. There was no statistically significant association of FGF23 with risk of all-cause stroke in Cox models adjusted for demographic factors and established stroke risk factors (hazard ratio [HR] comparing fourth to first quartile 1.19, 95%CI 0.78,1.82). In pre-specified models stratified by stroke subtypes, there was a graded association of FGF23 with risk of cardioembolic stroke in fully adjusted models (quartile 1 ref; quartile 2 HR 1.48, 95%CI 0.63,3.47; quartile 3 HR 1.99, 95%CI 0.89,4.44; quartile 4 HR 2.52, 95%CI 1.08,5.91). There were no statistically significant associations of FGF23 with other ischemic stroke subtypes or with hemorrhagic strokes.
Conclusions
Higher FGF23 concentrations were associated with higher risk of cardioembolic but not other stroke subtypes in community-dwelling adults. Future studies should delineate reasons for these findings.
Keywords: cerebrovascular disease, stroke, fibroblast growth factor, embolic stroke
INTRODUCTION
Fibroblast growth factor 23 (FGF23) is a hormone secreted by bone cells that regulates vitamin D and phosphorus homeostasis.1 Higher FGF23 concentrations have been associated with greater prevalence of cardiovascular and kidney disease independently of traditional risk factors.2–5 Higher FGF23 concentrations have also been linked to greater risk of incident cardiovascular disease events and death,6–9 establishing increased circulating FGF23 as a novel cardiovascular risk factor. Experimental data showing that FGF23 directly induces cardiac hypertrophy in animal models10 provides biological plausibility for a direct association of excess FGF23 with cardiovascular disease.
Most of the studies that examined the association of FGF23 with incident cardiovascular disease risk used a composite of events as the primary outcome, but there is evidence that the strength of the association of FGF23 with cardiovascular disease events substantially varies by outcome. For example, higher FGF23 concentrations have been more strongly associated with cardiovascular events linked to heart failure than those linked to atherosclerotic disease.8, 11–13 Few studies examined the association of FGF23 with stroke as a distinct endpoint, and those that did were limited by a low number of events,8, 14, 15 or did not examine the association of FGF23 with different ischemic stroke subtypes.16 This is important in that a recent study showed that higher FGF23 concentrations were independently associated with higher risk of incident atrial fibrillation, a major risk factor for cardioembolic strokes.17 As such, it is possible that the association between FGF23 and stroke risk may differ by etiologic subtype. The primary focus of the current study was to examine the association of FGF23 with incident stroke events overall and by etiologic subtypes in participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.
METHODS
The REGARDS study is a population-based investigation of stroke incidence in black and white US adults ≥ 45 years of age. Details of the study design have been reviewed elsewhere.18 Briefly, the study was designed to provide approximately equal representation of men and women and oversampled individuals who were black as well as individuals living in the US stroke belt/buckle. Trained interviewers conducted computer-assisted telephone interviews to obtain information including participants’ socio-demographics, cardiovascular risk factors, and use of anti-hypertensive, anti-glycemic, and cholesterol-lowering medication. Following this call, health professionals conducted an in-home study visit that included an electrocardiograph (ECG) recording, blood pressure, height and weight measurements, inventory of medications and collection of blood and urine samples. Overall, 30,239 individuals were enrolled between January 2003 and October 2007 (42% black, 55% women). The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided informed consent.
Primary Exposure
The exposure of interest was FGF23 concentrations measured in baseline plasma samples using a second generation, C-terminal enzyme linked immunosorbent assay (Immutopics, Santa Clara, CA) with coefficients of variation <10%.
Outcome of Interest
The outcome of interest was incident stroke. Suspected strokes were reported via telephone follow-up with participants every 6 months. Medical records were requested for stroke events and reviewed by at least 2 physician members of a committee of stroke experts to validate and classify potential strokes. Stroke events were defined according to the World Health Organization (WHO) definition of stroke.19 Events not meeting this definition but characterized by symptoms lasting < 24 hours, with neuroimaging consistent with acute ischemia or hemorrhage were classified as “clinical strokes” and included as stroke events. Cases in which adjudicators agreed that the event was likely a stroke or stroke-related death but information was incomplete for WHO or clinical classification were classified as “probable strokes” and also included as stroke events. Strokes were classified as ischemic or hemorrhagic, and ischemic strokes were further sub-classified into etiologic subtypes of small vessel occlusion, large vessel atherosclerosis, cardioembolic or unclassified.20 Ischemic stroke subtype classifications were based upon the potential stroke etiology discovered during post-stroke evaluation as per other stroke epidemiology studies.21–23
Covariates of Interest
Age, race, sex, smoking history, annual family income, and educational attainment were determined by self-report. Systolic and diastolic blood pressure were defined as the average of two seated measures taken after a 5 minute rest. History of coronary heart disease (CHD) was defined as having any of the following: evidence of myocardial infarction on the baseline ECG, self-report of a prior history of a cardiac procedure (coronary artery bypass surgery or percutaneous coronary intervention), or self-reported history of myocardial infarction. Diabetes was defined as self-reported use of insulin or oral hypoglycemic agents, fasting blood glucose concentration of 6.9 mmol/L or higher, or a non-fasting blood glucose concentration of 11.1 mmol/L or higher. History of atrial fibrillation was ascertained from self-report or by detection in ECG recordings obtained during the baseline study visit. Left ventricular hypertrophy (LVH) was classified using ECG criteria.24 Phosphorus and calcium concentrations were measured in baseline plasma samples using standard assays. N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations were measured using an electrochemiluminescence immunoassay (Roche Elecsys 2010 analyzer; Roche Diagnostics Indianapolis, IN). High sensitivity C-reactive protein (hsCRP) was measured by particle-enhanced immunonephelometry (BNII nephelometer, Dade Behring). Estimated glomerular filtration rate (eGFR) was determined from serum creatinine measurements using the Chronic Kidney Disease (CKD) Epidemiology Collaboration equation.25 Urine albumin measured by the BNII ProSpec nephelometer (Siemens AG) and urine creatinine measured by the rate Jaffé method (Roche/Hitachi, Basel, Switzerland) were used to calculate urine albumin to creatinine ratio (ACR).
Derivation of Case Cohort
We used a case-cohort study design. This approach provides an unbiased estimate of the relative hazard of an outcome(s) without requiring measurement of biomarkers in all participants and is considered a gold standard for minimizing the cost of expensive assays without compromising the power advantage of large cohort studies.26 Cases included all participants who developed an incident stroke during follow-up through September, 2011. The cohort random sample (comparison group) was selected using stratified sampling to ensure sufficient representation of high-risk groups. All participants with at least one follow-up contact (n=29,653) were categorized into 20 strata based on age (45–54, 55–64, 65–74, 75–84, ≥85 years), race (black or white), and sex (male or female).27 In each stratum, participants were randomly selected to fulfill the desired distribution: 50% black, 50% white, 50% female, 50% male, 20% age 45–54, 20% age 55–64, 25% age 65–74, 25% age 75–84, and 10% age ≥85. Given a random cohort size of ~1,000 individuals and 615 stroke events, we estimated that we would be able to detect a minimal effect size (hazard ratio) of 1.6 when comparing the highest to the lowest quartile of FGF23.
Statistical Analysis
Descriptive statistics were used to compare participant characteristics across quartiles of FGF23 within the cohort random sample using appropriate weights to account for the stratified sampling design. Factors that were associated with FGF23 concentrations in unadjusted analyses were then entered into a multivariable linear regression model to identify independent predictors of FGF23 in the cohort random sample.
After confirming the proportionality of hazards, Cox regression models for case-cohort studies28 were used to estimate the hazard ratio of incident stroke as a function of baseline FGF23 in sequential models.26 Model 1 adjusted for age, sex, race, and an age x race interaction term because associations of race with stroke are greater at younger ages, as previously reported.29 Model 2 adjusted for variables in Model 1 plus systolic blood pressure and clinical factors (history of diabetes, history of heart disease, atrial fibrillation, current smoking, and left ventricular hypertrophy). Model 3 further adjusted for laboratory factors (eGFR, natural log-transformed urine ACR, phosphorus, and calcium concentrations). In all models, FGF23 was analyzed in quartiles, with the lowest quartile serving as the referent group. Since a prior study showed that the association of FGF23 with incident cardiovascular disease events differed by CKD status,8 we examined for effect modification by CKD (defined as an eGFR < 60 ml/min/1.73 m2 or an ACR ≥ 30 mg/g) by testing the statistical significance of a multiplicative interaction term in the model. In pre-specified analyses, we examined the association of FGF23 with stroke risk in models stratified by ischemic vs. hemorrhagic strokes and in models further stratified by ischemic stroke subtypes. For the latter analyses, when more than one etiologic subtype was considered present, that case was counted in each subtype group. A two-tailed P value < 0.05 was considered statistically significant. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics of Study Participants
A total of 619 participants who developed a stroke during follow-up and 1,104 participants randomly selected from the REGARDS Study cohort were included in the study. After excluding 93 participants who reported a history of stroke at the baseline visit and 79 participants who had missing FGF23 concentrations, a total of 1,551 participants constituted the final analyzed sample (615 cases and 936 participants in the cohort random sample). In general, as compared to individuals who were stroke-free during follow-up, individuals who developed a stroke were older, more likely to be male, have less than a high school education, make less than $20,000 a year, be a current smoker, and have diabetes, hypertension, coronary heart disease, atrial fibrillation, and chronic kidney disease.
Table 1 reports the baseline characteristics of participants in the cohort random sample by quartiles of FGF23. Higher concentrations of FGF23 were associated with older age, female sex, residence in US stroke belt/buckle states, lower diastolic blood pressure, lower educational achievement, lower annual income, current smoking, diabetes, CHD, LVH, atrial fibrillation, lower eGFR, higher urine ACR, and higher calcium, phosphorus, hsCRP and NT-proBNP concentrations. In a linear regression model adjusted for variables significantly associated with FGF23 in Table 1, only higher calcium and phosphorus concentrations, higher urine ACR and lower eGFR remained independently associated with higher FGF23 concentrations.
Table 1.
Baseline characteristics of study participants in the cohort random sample by quartiles of fibroblast growth factor 23.
| FGF23 Quartile 1 (< 53 RU/ml) | FGF23 Quartile 2 (53 – 70 RU/ml) | FGF23 Quartile 3 (70.5–100 RU/ml) | FGF23 Quartile 4 (> 100 RU/ml) | P | |
|---|---|---|---|---|---|
| Weighted N* | 6561 | 6565 | 6589 | 6577 | |
| Age | 62.1 (0.5) | 63.6 (0.5) | 65.9 (0.6) | 66.9 (0.6) | < 0.001 |
| Female (%) | 43 | 54 | 54 | 70 | < 0.001 |
| Black (%) | 45 | 36 | 45 | 35 | 0.11 |
| Region (%) | <0.001 | ||||
| Belt | 39 | 31 | 31 | 34 | |
| Buckle | 14 | 20 | 16 | 25 | |
| Non-belt | 47 | 49 | 53 | 40 | |
| Body mass index (kg/m2) | 28.2 (0.4) | 29.5 (0.5) | 29.7 (0.4) | 29.6 (0.5) | 0.06 |
| Waist circumference (cm) | 93.6 (1.0) | 96.1 (1.3) | 97.2 (0.9) | 95.8 (1.1) | 0.07 |
| Systolic blood pressure (mm Hg) | 128.0 (1.4) | 126.6 (1.1) | 127.5 (1.3) | 127.2 (1.0) | 0.87 |
| Diastolic blood pressure (mm Hg) | 77.6 (0.7) | 76.9 (0.7) | 76.4 (0.8) | 75.0 (0.7) | 0.04 |
| Less than high school education (%) | 11 | 9 | 12 | 15 | <0.001 |
| Annual income <$20,000/year (%) | 14 | 11 | 20 | 25 | <0.001 |
| Co-morbidities | |||||
| Current smoking (%) | 11 | 9 | 13 | 21 | <0.001 |
| Diabetes (%) | 17 | 15 | 22 | 29 | <0.001 |
| Coronary heart disease (%) | 13 | 11 | 15 | 25 | <0.001 |
| Left ventricular hypertrophy (%) | 5 | 5 | 11 | 9 | <0.001 |
| Atrial fibrillation (%) | 8 | 7 | 6 | 14 | <0.001 |
| eGFR (ml/min/1.73 m2) | 93.3 (1.2) | 89.3 (1.2) | 85.7 (1.3) | 77.2 (1.6) | < 0.001 |
| UACR (mg/g) | 6.5 [4.2, 11.5] | 6.6 [4.2, 11.5] | 6.9 [4.9,13.8] | 9.3 [5.1, 26.6] | <0.001 |
| Calcium (mg/dL) | 9.2 (0.04) | 9.1 (0.09) | 9.1 (0.05) | 9.4 (0.04) | <0.001 |
| Phosphorus (mg/dL) | 3.4 (0.04) | 3.4 (0.04) | 3.5 (0.04) | 3.7(0.05) | <0.001 |
| C-reactive protein (mg/L) | 1.6 [0.7, 3.8] | 2.0 [0.9, 4.3] | 2.4 [0.9, 5.1] | 2.7 [1.1, 5.9] | <0.001 |
| NT-proBNP (pg/ml) | 44.7 [27.2, 101.2] | 56.5 [29.9, 100.5] | 73.3 [36.9, 160.5] | 87.8 [41.1, 244.0] | <0.001 |
| Fibroblast growth factor 23 (RU/ml) | 44.1 [39.2, 48.4] | 60.8 [56.9, 64.7] | 78.9 [73.4, 86.4] | 140.5 [115.1, 219.0] | <0.001 |
Analysis weighted to the full cohort
Abbreviations: eGFR, estimated glomerular filtration rate; UACR, urine albumin to creatinine ratio; NT-proBNP, N-terminal pro B-type natriuretic peptide; FGF23, fibroblast growth factor 23.
Associations of FGF23 with incident stroke
Table 2 depicts the hazard ratios (HR) of incident stroke by quartiles of FGF23. Higher quartiles of FGF23 were associated with higher risk of incident stroke in models adjusted for age, race, age x race interaction, and sex (quartile 1 reference; quartile 2 HR 1.42, 95% confidence interval [CI] 1.01, 1.99; quartile 3 HR 1.35, 95%CI 0.97, 1.88; quartile 4 HR 1.84, 95%CI 1.31, 2.58, Ptrend= 0.001). The magnitude and strength of the associations were modestly attenuated after adjustment for systolic blood pressure, diabetes, cigarette smoking, CHD, atrial fibrillation, and LVH (HR comparing fourth to first quartile, 1.59, 95%CI 1.09, 2.35, Ptrend= 0.04). After further adjustment for phosphorus, calcium, eGFR, and natural log-transformed urine ACR, the association of higher FGF23 with higher incident stroke risk was completely attenuated (HR comparing the highest to lowest quartile of FGF23 1.19, 95%CI 0.78, 1.82, Ptrend= 0.77). Estimated GFR and urine ACR were the variables that were primarily responsible for this attenuation. The association of FGF23 with stroke risk was not modified by CKD status (P interaction=0.24)
Table 2.
Hazard ratios of stroke (95% confidence intervals) by quartiles of fibroblast growth factor 23 (FGF23). Models include both ischemic and hemorrhagic strokes.
| FGF23 Quartile 1 (< 53 RU/ml) | FGF23 Quartile 2 (53 – 70 RU/ml) | FGF23 Quartile 3 (70.5–100 RU/ml) | FGF23 Quartile 4 (> 100 RU/ml) | P trend | |
|---|---|---|---|---|---|
| N | 102 | 142 | 162 | 209 | |
| Model 1* | ref | 1.42 (1.01, 1.99) | 1.35 (0.97, 1.88) | 1.84 (1.31, 2.58) | 0.001 |
| Model 2† | ref | 1.42 (0.98, 2.06) | 1.23 (0.85, 1.79) | 1.59 (1.09, 2.35) | 0.04 |
| Model 3‡ | ref | 1.34 (0.91, 1.99) | 1.09 (0.74, 1.63) | 1.19 (0.78, 1.82) | 0.77 |
Abbreviations: FGF23, fibroblast growth factor 23.
Adjusted for age, race, age x race interaction, and sex;
Adjusted for variables in Model 1 plus systolic blood pressure, diabetes, cigarette smoking, coronary heart disease, atrial fibrillation, and left ventricular hypertrophy;
Adjusted for variables in Model 2 plus plasma phosphorus, plasma calcium, estimated glomerular filtration rate, and natural log-transformed albumin to creatinine ratio.
Associations of FGF23 with stroke subtypes
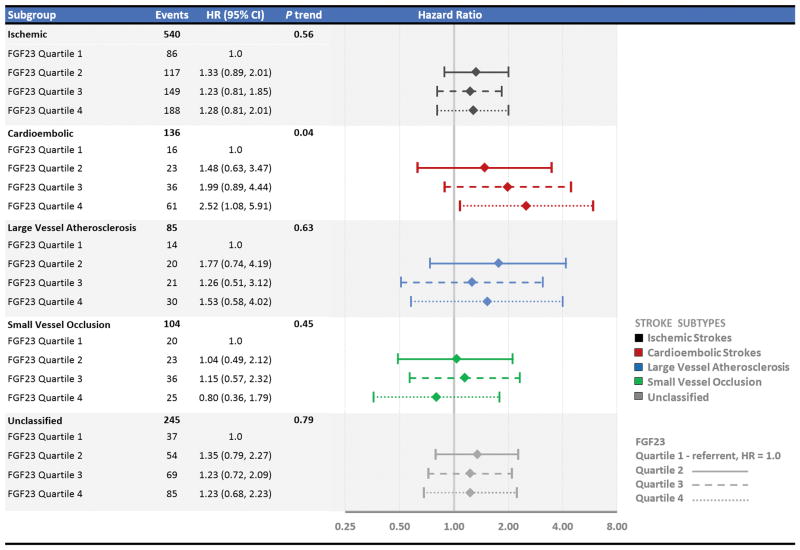
A total of 540 strokes were classified as ischemic in etiology and 75 as hemorrhagic. There were no statistically significant associations of FGF23 with risk of hemorrhagic stroke in unadjusted or adjusted analyses (data not shown). When the analysis was restricted to ischemic strokes, the results were similar to analyses using all-cause strokes—higher FGF23 quartiles were not associated with higher risk of incident stroke in fully adjusted models (HR comparing the fourth to first quartile 1.28, 95%CI 0.81, 2.01, Ptrend= 0.56) (Figure 1).
Figure 1.
Hazard ratios (95% confidence intervals) of stroke by quartiles of fibroblast growth factor 23 (FGF23) restricted to ischemic strokes and then further stratified by ischemic stroke subtypes (cardioembolic, large vessel, small vessel occlusion, and unclassified).
The model was adjusted for age, race, age x race interaction, sex, systolic blood pressure, diabetes, cigarette smoking, coronary heart disease, atrial fibrillation, left ventricular hypertrophy, plasma phosphorus, plasma calcium, estimated glomerular filtration rate, and natural log-transformed albumin to creatinine ratio.
A total of 136 ischemic strokes were further sub-classified as being cardioembolic in etiology, 85 as being due to large vessel atherosclerosis, 104 as being due to small vessel occlusion, and 245 were unclassified (the total was greater than 540 because more than one etiologic subtype was identified for some events). In pre-specified analyses restricted to cardioembolic stroke subtypes, higher quartiles of FGF23 were associated with higher risk of incident stroke after adjustment for demographic characteristics, clinical factors and laboratory measures including indices of kidney function (HR comparing the fourth to first quartile 2.52, 95%CI 1.08, 5.91, Ptrend= 0.04) (Figure 1). When the model was further adjusted for NT-proBNP concentrations as a surrogate measure of volume overload/left ventricular wall stress, the association between higher FGF23 and higher risk of cardioembolic stroke was no longer statistically significant but the magnitude of the association only minimally changed (HR comparing the fourth to first quartile 2.47, 95%CI 0.98, 6.22, Ptrend= 0.08). In contrast, in analyses restricted to large or small vessel disease subtypes, there were no statistically significant associations of FGF23 with stroke risk in fully adjusted models. Similarly, there was no statistically significant association of FGF23 with unclassified stroke subtypes in the fully adjusted model.
DISCUSSION
FGF23 is a hormone that regulates phosphorus and vitamin D metabolism.1 In states of phosphorus excess such as high dietary phosphorus intake or CKD, FGF23 secretion is stimulated to help maintain normal phosphorus balance by increasing urinary phosphorus excretion and reducing dietary phosphorus absorption via inhibition of vitamin D activation.30 While this helps to preserve phosphorus homeostasis in the short term, a growing body of literature suggests that chronic elevations in circulating FGF23 have long-term cardiovascular consequences.6–9 In the current study, we found no statistically significant association of FGF23 concentrations with risk of all-cause incident stroke when accounting for established stroke risk factors, particularly baseline CKD. However, in pre-specified analyses, higher FGF23 was associated with greater risk of incident cardioembolic stroke but not other ischemic stroke subtypes or hemorrhagic stroke in models adjusted for established stroke risk factors and other parameters of mineral metabolism.
Prior studies have examined the association of FGF23 with stroke risk but their results were inconsistent. The Heart and Soul Study reported that higher FGF23 was associated with higher risk of incident stroke or transient ischemic attack (total n=36) independently of other risk factors (HR per doubling of FGF23 1.50, 95%CI 1.11, 2.04),15 whereas Kendrick et al. found no statistically significant association of FGF23 with stroke (n=43) in individuals with advanced CKD.14 A Cardiovascular Health Study (CHS) report found that FGF23 was independently associated with higher risk of incident stroke among individuals with CKD (total events=168, HR per doubling of FGF23 1.29, 95%CI 1.08, 1.54) but not among individuals with preserved kidney function (total events=218, HR per doubling of FGF23 0.99 95%CI 0.81, 1.20).8 Consistent with the results of the current study, the Northern Manhattan Study (NOMAS) reported no association of FGF23 with ischemic strokes (n=212) after adjusting for traditional stroke risk factors.16 Unlike our study, however, they found an independent association of FGF23 with hemorrhagic strokes (n=26). Importantly, none of the aforementioned studies examined the association of FGF23 with ischemic stroke subtypes. Thus, the results of the current study help to extend these previous findings by demonstrating that the magnitude and strength of the association of FGF23 with ischemic stroke are greater for strokes due to cardioembolism than for those linked to large vessel atherosclerosis or small vessel ischemic disease, potentially explaining the inconsistency in the association of FGF23 with stroke in these prior studies.
The reason why FGF23 was associated with higher risk of cardioembolic but not other subtypes of ischemic stroke is unclear. Nonetheless, given the strong link between heart failure and cardioembolic stroke events, these results are broadly consistent with prior studies showing that higher FGF23 more strongly associates with cardiovascular disease events linked to heart failure than to large or small vessel atherosclerotic disease.8, 11–13 In line with this, increased FGF23 has been independently associated with increased left ventricular mass,2, 5, 31, 32 increased prevalence of left ventricular hypertrophy,2, 10, 31 and reduced ejection fraction,4, 32 but not vascular calcification or peripheral arterial disease.13, 33 Further, FGF23 was shown to directly induce cardiomyocyte hypertrophy in vitro 10, 34 and stimulate adverse left ventricular remodeling in vivo,10 providing biological plausibility for a direct toxic effect of FGF23 on the myocardium. Higher FGF23 concentrations were also associated with higher risk of incident atrial fibrillation independently of potential confounders in two large community-based cohorts.17 Collectively, these data bolster the body of evidence suggesting that elevated FGF23 concentrations have a multitude of adverse effects on heart structure and function, potentially promoting adverse downstream consequences such as increased cardioembolic stroke risk.
Given the tight association of FGF23 with kidney disease, it is also conceivable that the association of FGF23 with cardioembolic stroke may reflect known associations of kidney injury with LVH, heart failure and atrial fibrillation. Studies with more direct measures of kidney function or with longitudinal measures of eGFR and urinary ACR are needed to determine the role of kidney disease in explaining the results observed herein.
We did not have direct measurements of left ventricular size or function in REGARDS participants or clinical exam findings to delineate who did or did not have prevalent heart failure at the time of FGF23 measurement, and so we were not able to determine the association of FGF23 with heart failure at baseline or whether heart failure may have mediated an association of FGF23 with cardioembolic stroke risk. Similarly, since we had to rely on self-report or detection on baseline ECG to determine whether individual participants had atrial fibrillation, we could not reliably determine whether the association of FGF23 with cardioembolic stroke risk was explained in whole or in part by an association of FGF23 with atrial fibrillation, as has been reported previously.4, 17 Future studies will need to examine the association of FGF23 with the development of atrial fibrillation following the baseline visit to examine this possibility. Other limitations of our study included having only one baseline measure of FGF23 and inclusion of only black or white adults limiting our ability to extrapolate these findings to other races/ethnicities. Our study also had a number of strengths including standardized collection of baseline data as well as prospective data including physician-adjudicated stroke events and ischemic stroke subtypes.
Summary/Conclusions
In conclusion, higher FGF23 was an independent risk factor for cardioembolic strokes but not other stroke subtypes in this large national cohort of community-dwelling adults. Future studies are needed to delineate reasons for the differential association of FGF23 by stroke subtype in this study and potential implications of these findings for clinical practice.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
SOURCES OF FUNDING: This study was supported by a cooperative agreement U01 NS041588 and by R01NS080850 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation of the manuscript.
Footnotes
DISCLOSURES: No authors list disclosures relevant to this manuscript.
References
- 1.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: The heart and soul study. Nephrol Dial Transplant. 2010;25:993–997. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, et al. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–2696. doi: 10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovich A, Ix JH, Gottdiener J, McFann K, Katz R, Kestenbaum B, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231:114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. New England Journal of Medicine. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: Chs (cardiovascular health study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: The atherosclerosis risk in communities study. J Am Heart Assoc. 2014;3:e000936. doi: 10.1161/JAHA.114.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, et al. Fgf23 induces left ventricular hypertrophy. The Journal of clinical investigation. 2011;121:4393. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al. Fibroblast growth factor-23 and cardiovascular events in ckd. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. American heart journal. 2011;161:956–962. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garimella PS, Ix JH, Katz R, Chonchol MB, Kestenbaum BR, de Boer IH, et al. Fibroblast growth factor 23, the ankle-brachial index, and incident peripheral artery disease in the cardiovascular health study. Atherosclerosis. 2014;233:91–96. doi: 10.1016/j.atherosclerosis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, et al. Fgf-23 associates with death, cardiovascular events, and initiation of chronic dialysis. Journal of the American Society of Nephrology. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix gla protein with mortality in coronary artery disease: The heart and soul study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CB, Dong C, Stark M, Silverberg S, Rundek T, Elkind MS, et al. Plasma fgf23 and the risk of stroke: The northern manhattan study (nomas) Neurology. 2014;82:1700–1706. doi: 10.1212/WNL.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, et al. Fibroblast growth factor-23 and incident atrial fibrillation: The multi-ethnic study of atherosclerosis (mesa) and the cardiovascular health study (chs) Circulation. 2014;130:298–307. doi: 10.1161/CIRCULATIONAHA.113.005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 19.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, et al. Ischemic stroke subtypes: A population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 22.Wiklund PG, Brown WM, Brott TG, Stegmayr B, Brown RD, Jr, Nilsson-Ardnor S, et al. Lack of aggregation of ischemic stroke subtypes within affected sibling pairs. Neurology. 2007;68:427–431. doi: 10.1212/01.wnl.0000252955.17126.6a. [DOI] [PubMed] [Google Scholar]
- 23.Stead LG, Gilmore RM, Bellolio MF, Jain A, Rabinstein AA, Decker WW, et al. Cardioembolic but not other stroke subtypes predict mortality independent of stroke severity at presentation. Stroke Res Treat. 2011;2011:281496. doi: 10.4061/2011/281496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating cornell voltage from nonstandard chest electrode recording site in the reasons for geographic and racial differences in stroke study. Journal of electrocardiology. 2010;43:209–214. doi: 10.1016/j.jelectrocard.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 27.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, et al. N-terminal pro-b-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45:1646–1650. doi: 10.1161/STROKEAHA.114.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onland-Moret NC, van der AD, van der Schouw YT, Buschers W, Elias SG, van Gils CH, et al. Analysis of case-cohort data: A comparison of different methods. J Clin Epidemiol. 2007;60:350–355. doi: 10.1016/j.jclinepi.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutiérrez OM. Fibroblast growth factor 23 and disordered vitamin d metabolism in chronic kidney disease: Updating the “trade-off” hypothesis. Clinical Journal of the American Society of Nephrology. 2010;5:1710–1716. doi: 10.2215/CJN.02640310. [DOI] [PubMed] [Google Scholar]
- 31.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact fgf23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Shibata K, Fujita S, Morita H, Okamoto Y, Sohmiya K, Hoshiga M, et al. Association between circulating fibroblast growth factor 23, alpha-klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLoS One. 2013;8:e73184. doi: 10.1371/journal.pone.0073184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney international. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, et al. Fgf23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304:E863–873. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]