Abstract
Background
Alcohol [ethanol (EtOH)] intoxication antagonizes stimulation of muscle protein synthesis and mTOR signaling. However, whether the anabolic response can be reversed when alcohol is consumed after the stimulus is unknown.
Methods
A single bout of electrically stimulated muscle contractions (10 sets of 6 contractions) were induced in fasted male C57BL/6 mice 2 h prior to alcohol intoxication. EtOH was injected IP (3g/kg) and the gastrocnemius/plantaris muscle complex was collected 2 h later from the stimulated and contralateral unstimulated control leg.
Results
Muscle contraction increased protein synthesis 28% in control mice while EtOH abolished this stimulation-induced increase. Further, EtOH suppressed the rate of synthesis ~75% compared to control muscle irrespective of stimulation. This decrease was associated with impaired protein elongation as EtOH increased the phosphorylation of eEF2 Thr56. In contrast, stimulation-induced increases in mTORC1 (S6K1 Thr421/Ser424, S6K1 Thr389, rpS6 Ser240/244, 4E-BP1 Thr37/46) and MAPK (JNK Thr183/Tyr185, p38 Thr180/Tyr182, and rpS6S235/236) signaling were not reversed by acute EtOH.
Conclusion
These data suggest that EtOH-induced decreases in protein synthesis in fasted mice may be independent of mTORC1 and MAPK signaling following muscle contraction and instead due to the antagonistic actions of EtOH on mRNA translation elongation. Therefore, EtOH suppresses the contraction-induced increase in protein synthesis and over time has the potential to prevent skeletal muscle hypertrophy induced by repeated muscle contraction.
Keywords: Ethanol, S6K1, skeletal muscle metabolism, anabolic signaling, electrical stimulation
Introduction
Skeletal muscle growth is regulated by the coordinated balance between rates of muscle protein synthesis and degradation in response to anabolic stimuli; however, several conditions including alcohol intoxication may impair this normal anabolic response (Kumar et al., 2002, Lang et al., 2003, Lang and Frost, 2006, Lang et al., 2012). The importance of the mammalian target of rapamycin (mTOR) protein complex-1 (mTORC1) in the stimulation of protein synthesis and muscle hypertrophy is widely accepted (Baar and Esser, 1999, Bodine et al., 2001, Goodman et al., 2011) and in contrast to alcohol intoxication, muscle contraction/resistance exercise stimulates mTORC1, increases protein synthesis and induces hypertrophy when repeated over time (Bodine et al., 2001, Drummond et al., 2009, Kubica et al., 2005). Upon activation, mTORC1 phosphorylates its primary substrates, S6K1 and 4E-BP1. S6K1 phosphorylates several downstream substrates including ribosomal protein S6 (rpS6) and elongation factor 2 kinase (eEF2K), ultimately leading to translation initiation, peptide chain elongation and presumably protein synthesis (Magnuson et al., 2012, Wang et al., 2001). On the other hand, phosphorylation of 4E-BP1 is hierarchical and its activation induces its release from eIF-4E, allowing the cap-binding eIF4F complex to be formed (with eIF-4E) and cap-dependent protein translation to proceed (Gingras et al., 2001, Mothe-Satney et al., 2000).
Conversely, both acute alcohol intoxication and chronic alcohol consumption decrease protein synthesis predominately in type II muscle fibers in part by impairing mTOR-dependent translation initiation (Lang et al., 2000, Lang et al., 2001, Kumar et al., 2002). More specifically, basal phosphorylation of rpS6 and 4E-BP1 is decreased and binding of 4E-BP1 with eIF-4E is increased in skeletal muscle of rats following acute alcohol intoxication (Lang et al., 2004, Lang et al., 2009, Lang et al., 2010a). Further, administration of a bolus dose of alcohol to mimic binge drinking behavior prevents the normal anabolic response to several stimuli including hormones [insulin and insulin-like growth factor-1 (IGF-1)] and nutrients (re-feeding, leucine) (Sneddon et al., 2003, Lang et al., 2004, Lang et al., 2010a, Lang et al., 2003, Kumar et al., 2002). These findings show that alcohol intoxication prior to stimulation produces a transient anabolic resistance within muscle which, in the case of chronic alcoholics, may contribute to skeletal muscle wasting and myopathy.
While current research primarily focuses on the central role of mTORC1 signaling in the stimulation of protein synthesis following muscle contraction, the activation of the mitogen activated protein kinase (MAPK) pathway may also contribute via its regulation of proteins both within and independent of the canonical mTORC1 pathway (Wang et al., 2001, Roux et al., 2007, Anjum and Blenis, 2008). For example, phosphorylation of protein kinase p90RSK by several MAPK proteins (Sutherland et al., 1993, Zaru et al., 2007) (Jensen et al., 1999) can inhibit tuberous sclerosis-2 (TSC2) (on Ser1796 and Ser939) (Roux et al., 2004), and activation of raptor (Ser719) (Carrière et al., 2008), eIF4B (Ser422) (Shahbazian et al., 2006), and rpS6 (Ser235/236) (Roux et al., 2007), to promote protein translation. Further, muscle contraction induces the phosphorylation of p38, ERK and p90RSK (Ryder et al., 2000, Widegren et al., 2001) while EtOH can suppress ERK signaling (Vary and Lang, 2008).
Initiation is documented as the rate-limiting step of protein translation, but the inhibition of elongation factors including eEF1A and eEF2 may also impair synthesis (Browne and Proud, 2002, Jørgensen et al., 2006). Whole-body knock out of eEF1A-2/S1 leads to profound muscle wasting due to the loss of eEF1A2 in neurons (Doig et al., 2013), while phosphorylation of eEF2 Thr56 decreases the rate of elongation by preventing the movement of peptidyl-tRNA to the P-site (from the A-site) of the ribosome. Accordingly, phosphorylation of eEF2 is typically decreased in the recovery period after maximal muscle contractions to facilitate peptide chain elongation (Atherton et al., 2005).
The majority of the previous work investigating the detrimental effects of acute alcohol intoxication on anabolic signaling has utilized an experimental paradigm in which alcohol precedes the anabolic stimulus. This work however fails to determine whether alcohol administered after a given anabolic stimulus reverses the increase in muscle protein synthesis which represents a more relevant model as it is less likely that large amounts of alcohol will be consumed prior to a training session or competition. Hence, the present study examined whether the contraction-induced increase in skeletal muscle protein synthesis and mTORC1 signaling is reversed by an acute dose of alcohol intended to mimic an episode of binge drinking.
Materials and Methods
Animals
Male viral antibody free C57BL/6 mice aged 11–13 weeks were purchased from Charles River Laboratories (Wilmington, MA) and acclimated for at least 1 week to the animal facility at the College of Medicine at Penn State Hershey prior to experimental use. Mice were housed (1–4/cage) in shoe-box cages with corn cob bedding under controlled environmental conditions (12:12 light:dark), and were provided Teklad Global 2019 (Harlan Teklad, Boston, MA) and water ad libitum until the start of the experiment. All experimental procedures were performed in accordance with the National Institutes of Health (NIH) guidelines for the use of experimental animals and were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine.
Experimental design and muscle contraction protocol
The stimulated muscle contraction protocol was performed in the morning between 8 am and 12 pm following an overnight fast intended to produce a uniform baseline and allow for measurement of a maximal anabolic response. Mice were randomly assigned to either alcohol (EtOH) (n=10) or control (Con) (n=9) treatment groups and underwent the muscle contraction protocol in an alternating manner (i.e., one mouse from the control group followed by one from the EtOH group), so as to minimize any variation potentially caused by differences in the duration of the fast. In preparation for muscle contraction induced via sciatic nerve stimulation, mice were anesthetized with isoflurane (2–3% in O2 with 1.5% maintenance) (Butler Schein Animal Health; Dublin, OH) and hair was shaved from the right leg/hip where a small incision was then made through the skin and muscle to expose the sciatic nerve. After placement of needle electrodes over the nerve, a current stimulator (Model A365, World Precision Instruments, Sarasota, Fl) interfaced with Powerlab 4/35 and LabChart software (ADI Instruments, Colorado Springs CO) was used to elicit maximal muscular contractions. The stimulation protocol was performed as previously described by Baar and Esser (1999). Briefly, 10 sets of 6 contractions (each lasting 3 s) with 10 s rest between contractions and 60 s rest after each set of 6 contractions was administered. Pulse height was set to 6 volts (V) and current to 1 milliamperes (mA) to ensure maximal contraction of the musculature. Each contraction produced a lengthening action of the tibialis anterior and extensor digitorum longus muscles, with a concomitant shortening of the triceps surae complex resulting in plantar flexion of the foot. The contralateral leg served as the non-contracted control muscle. After the ~20 min protocol, the incision was closed with metal wound clips and mice were subcutaneously injected with 500 µL of warm sterile 0.9% saline before being returned to their individual cage for recovery. Two hours after cessation of the muscle contraction protocol, mice in the EtOH group were administered alcohol at a dose of 3 g/kg (using 70% EtOH v/v solution) via intraperitoneal (IP) injection while Con mice were injected with an equal volume of 0.9% sterile saline. The route of administration of EtOH (IP injection vs. oral gavage) does not affect resulting blood alcohol concentrations (BAC) as absorption and clearance rates are similar with each method (Chen et al., 2013). Following treatment with either EtOH or saline, mice remained in the fasted state with free access to water for another 2 h until they were deeply anesthetized via isoflurane inhalation and the gastrocnemius muscle complex (i.e. gastrocnemius and plantaris muscle) (referred to as muscle from here on) was excised from the stimulated and non-stimulated leg. Muscles were then frozen between aluminum blocks precooled to the temperature of liquid nitrogen. Blood was collected from the vena cava in heparinized syringes and centrifuged (10,000×g for 10 min) for isolation of plasma. Both frozen tissue and plasma were stored at −80°C until analysis. This time point corresponds to 4 h after the cessation of the muscle contraction protocol and is a time at which preliminary investigations showed protein synthesis and mTORC1 signaling to be elevated by this electrical stimulation protocol.
Western blotting
Half of the frozen gastrocnemius complex (50–90 mg) was homogenized using a glass mortar and pestle in 10 volumes of ice cold buffer consisting of (in mmol/L): 50 HEPES, 0.1% Triton-X, 4 EGTA, 10 EDTA, 15 sodium pyrophosphate, 100 β-Glycerophosphate, 25 sodium fluoride, 5 sodium orthovanadate and 1 cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail Tablet (Roche Applied Science, Madison, WI) per 10 mL of buffer. Protein concentration was quantified using Bio-Rad Protein Assay Dye reagent concentrate (Hercules, CA) and SDS-PAGE was carried out using equal amounts of total protein per sample and Criterion 4–20% gradient gels (Bio-Rad, Hercules, CA). PVDF membranes were incubated overnight at 4°C with primary antibody (Cell Signaling, Beverly, MA, unless otherwise noted) including S6K1, S6K1 (Thr389 and Thr421/Ser424), rpS6, rpS6 (Ser240/244 and Ser235/236), 4E-BP1 (Bethyl Laboratories, Montgomery, TX), 4E-BP1 (Thr37/46 and Ser65), Regulated in Response to DNA damage-1 (REDD1) (ProteinTech, Chicago, IL), Akt, Akt (Ser473 and Thr308), eEF2, eEF2 (Thr56), eEF2 kinase (Bethyl Laboratories, Montgomery, TX), eEF2 kinase (Ser366) (Santa Cruz Biotechnology), eEF1A, eEF1A2 (Ser358) (Abgent, San Diego, CA), protein phosphatase 2 A (PP2A) (A Subunit, B Subunit, C Subunit), PP2A-Cα/β (Tyr307) (Santa Cruz, Dallas, TX), ERK1/2 (Thr202/Tyr204), p42/44 MAPK, p38 (Thr180/Tyr182), p38, JNK, JNK (Thr183/Tyr185), PDK1, PDK1 (Ser241), p90RSK, and p90RSK (Ser380 and Thr573). Protein loading was verified using Ponceau S stain (Aqua Solutions, Deer Park, TX) prior to blocking since the measurement of total protein for each target was performed on a separate membrane than the corresponding phosphorylated protein. The FluorChem M Multifluor System (ProteinSimple, San Jose, CA) was used for visualization following exposure to ECL reagent (Thermo Scientific, Waltham, MA). Images were analyzed within the linear range using AlphaView (ProteinSimple) and Image J software (NIH, Bethesda, MD). Although intervening lanes have been removed in some of the figures as demarcated by a solid line, each representative blot shown contains samples from the same animal (non-stimulated and stimulated muscle) run on the same gel.
Protein synthesis
A separate group of male C57BL/6 mice were used to determine the in vivo rate of muscle protein synthesis in response to muscle contraction in the presence or absence of EtOH. All experimental protocols were performed identically as described above except that at 4 h post-muscle contraction (2 h after EtOH treatment) mice were injected I.P. with a flooding dose of L-[2,3,4,5,6-3H]phenylalanine (Phe; 150 mM, 30 µCi/ml; 0.5mL) and muscles were collected 15 min later. Mice were anesthetized with isoflurane and blood was collected in heparinized syringes from the inferior vena cava for measurement of plasma Phe concentration and radioactivity. Muscles were excised and immediately clamped between aluminum blocks precooled to the temperature of liquid nitrogen. High performance liquid chromatography (HPLC) was used for the measurement of specific radioactivity of plasma Phe levels in the supernatant from trichloroacetic acid (TCA) treated plasma extracts. The global rate of [3H]Phe incorporation into protein within the muscle was assessed exactly as previously described by our laboratory (Lang et al., 2000).
Blood alcohol concentration
A rapid analyzer (Analox Instruments, Lunenburg, MA) was used to measure the plasma concentrations of alcohol in both the control and EtOH treated mice. Duplicate measurements were performed and the average ± SEM is reported.
Statistical analysis
Commercially available statistical software (SigmaPlot,Systat, San Jose, CA) was used to analyze all data using a repeated measures two-way ANOVA (contraction×alcohol) with Student-Neuman-Keuls post hoc tests performed when appropriate. Data are presented as mean ± SEM and considered significant when P<0.05.
Results
Blood alcohol concentration
Two hours after the injection of 3 g/kg of EtOH the blood alcohol concentration in the EtOH treated mice was 60 ± 4 mmol/L (~275 mg/dL). No alcohol was detected in the plasma of the saline treated control mice.
Muscle protein synthesis is impaired by EtOH
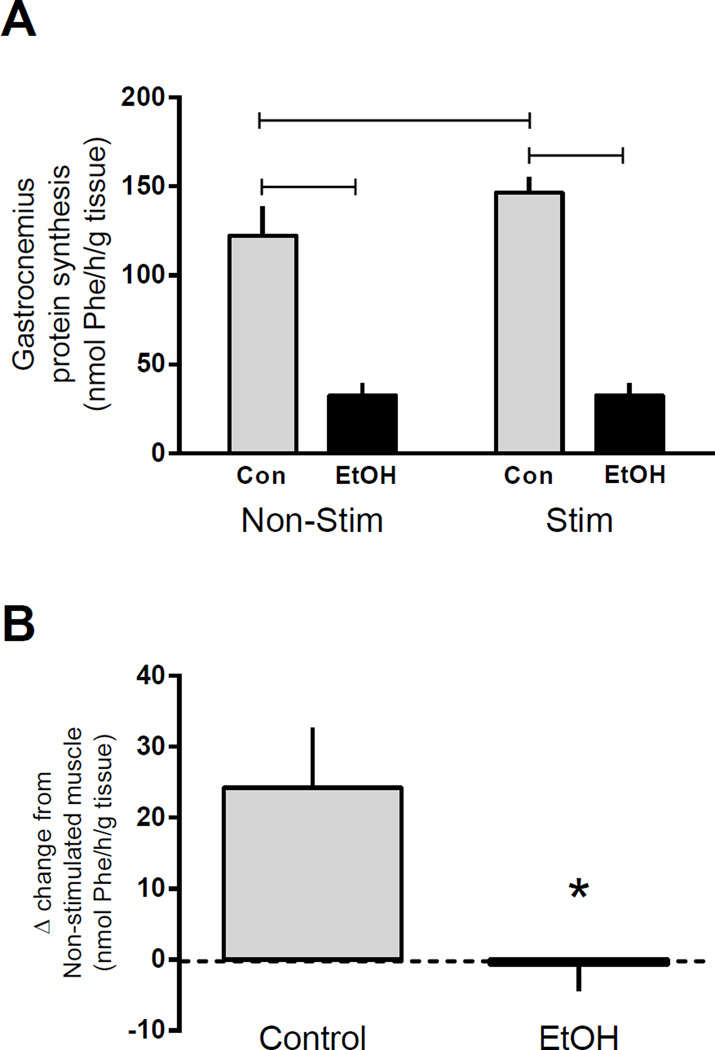
Stimulated muscle contraction increased protein synthesis 28% in control mice (Figure 1A). EtOH decreased the basal rate of synthesis in the non-stimulated control muscle by 74% and completely reversed the stimulation-induced increase in synthesis. When the delta change (stimulated minus non-stimulated muscle within the same animal) in protein synthesis was calculated for each animal (Figure 1B), it was evident that alcohol administered post-stimulation completely revered the stimulation-induced increase in protein synthesis as rates were not changed from basal levels.
Figure 1.
Protein synthesis following alcohol and/or muscle contraction. Rates of synthesis were assessed in the gastrocnemius/plantaris muscle complex at 4 h post muscle contraction and 2 h after EtOH (3 g/kg) intoxication (A) and the delta change induced by muscle contraction (B). Black bars correspond to alcohol (EtOH) treated mice (n=9) and light bars represent control (Con) mice (n=7). ‘Non-Stim’ indicates control condition, while ‘Stim’ indicates muscle which underwent electrically stimulated muscle contraction. Horizontal bars indicate statistical differences between groups (P<0.05). *p<0.05 compared to control value. Values are expressed as means + SE.
mTORC1 signaling induced by muscle contraction
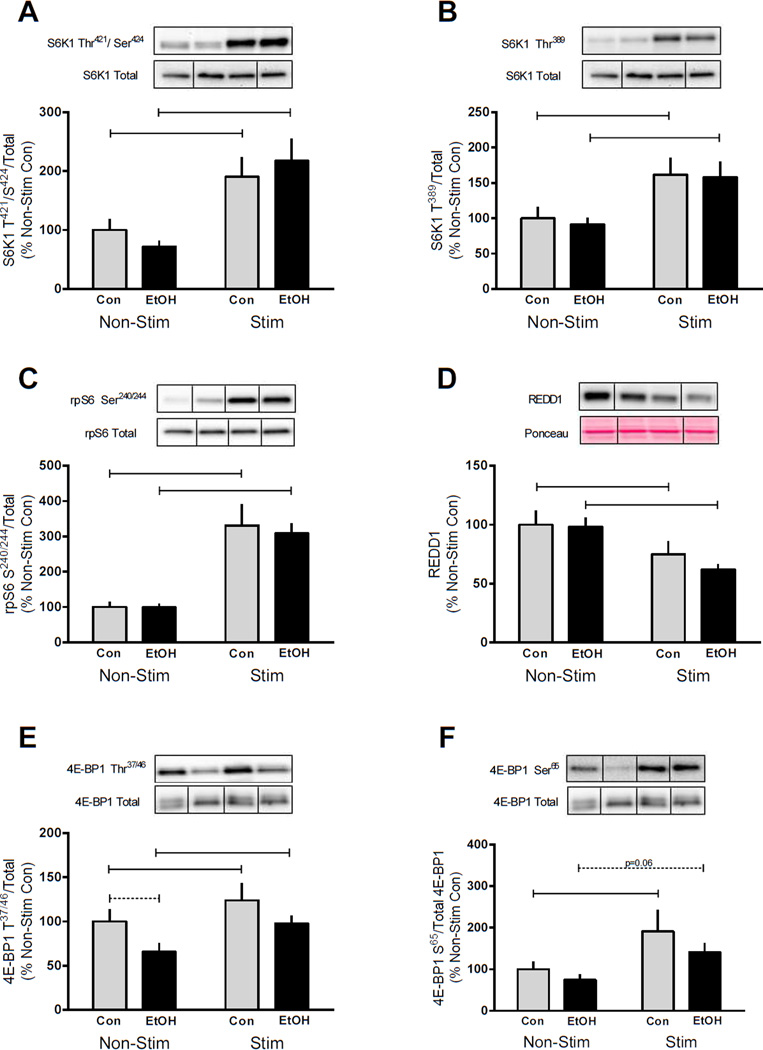
Electrically stimulated muscle contraction increased the phosphorylation of S6K1 Thr421/Ser424 (90%), S6K1 Thr389 (60%), and phosphorylation of its substrate rpS6 Ser240/244 (230%) (Figure 2A, B and C, respectively). EtOH intoxication did not alter the phosphorylation of these proteins in the control non-stimulated muscle or reverse stimulation-induced increases in phosphorylation. The phosphorylation of S6K1 Thr421/Ser424 (200%), S6K1 Thr389 (~60%) and rpS6 Ser240/244 (210%) was elevated after muscle contraction in the EtOH treated mice compared to their non-stimulated muscle, and phosphorylation did not differ from the stimulated muscle of control mice (Figure 2A, B and C). Signaling upstream of mTORC1 was also preserved in EtOH treated mice following muscle contraction as REDD1 protein was decreased similarly by electrically stimulated muscle contraction in both treatment groups (Figure 2D). Additionally, phosphorylation of Akt on either Ser473 or Thr308 was not affected by either alcohol or muscle contraction (data not shown) suggesting that the decrease in protein synthesis was independent of changes in PI3K/Akt signaling.
Figure 2.
Effects of alcohol and muscle contraction on mTORC1 signaling. S6K1 (A, B), rpS6Ser240/244 (C), REDD1 (D) and 4E-BP1 (E, F) were assessed 4 h post-muscle contraction and 2 h after EtOH (3 g/kg) intoxication in the gastrocnemius/plantaris muscle complex. Bar graphs represent quantification of Western blot images normalized to the total amount of the respective protein with the control non-contracted value set to 100%. Black bars correspond to alcohol (EtOH) treated mice (n=10) and light bars represent control (Con) mice (n=9). ‘Non-Stim’ indicates control condition, while ‘Stim’ indicates muscle which underwent electrically stimulated muscle contraction. Horizontal bars indicate statistical differences between groups (P<0.05). Values are expressed as means + SE.
4E-BP1 is also an mTORC1 substrate and the phosphorylation of this protein was increased on both Thr37/46 and Ser65 in the contracted muscle of control mice (Figure 2E and F). Muscle contraction also increased the phosphorylation of Thr37/46 and Ser65 (p=0.06) in EtOH treated mice (Figure 2E and F). Collectively, these data suggest the ability of EtOH to reverse contraction-induced changes in protein synthesis is largely independent of changes in mTORC1 signaling.
Differential effects of EtOH and muscle contraction on MAPK signaling
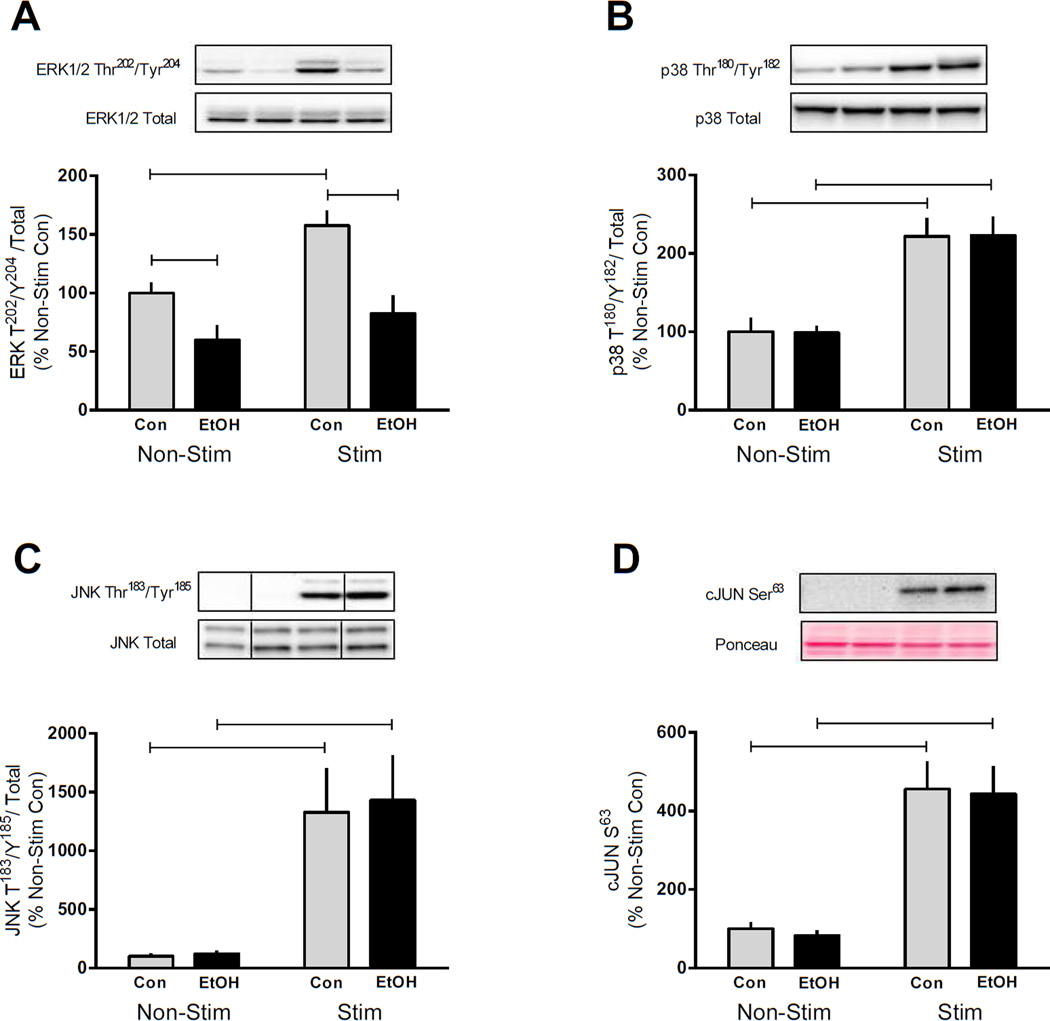
The MAPK pathway may also influence the rate of protein synthesis and have the potential to be differentially regulated by EtOH following muscle contraction (Roux et al., 2007, Anjum and Blenis, 2008). Electrically stimulated muscle contraction in control mice increased phosphorylation of ERK Thr202/Tyr204 (~60%); however, this induction was reversed by EtOH (Figure 3A). EtOH also decreased ERK phosphorylation 40% in the basal unstimulated muscle. In contrast to the suppressive actions of EtOH on ERK, muscle contraction increased the phosphorylation of p38 Thr180/Tyr182 (120%), JNK Thr183/Tyr185 (13–14-fold) and cJUN Ser63 (350%) by a similar magnitude in both the control and EtOH treated mice (Figure 3B, C and D, respectively).
Figure 3.
MAPK signaling is differentially altered by EtOH and muscle contraction. The gastrocnemius/plantaris muscle complex was removed 2 h after EtOH (3 g/kg) intoxication and 4 h after cessation of muscle contraction for measurement of ERK Thr202/Tyr204 (A), p38 Thr180/Tyr182 (B), JNK Thr183/Tyr185 (C) and cJUN Ser63 (D). Bar graphs represent quantification of Western blot images normalized to the total amount of the respective protein with the control non-contracted value set to 100%. Black bars correspond to alcohol (EtOH) treated mice (n=10) and light bars represent control (Con) mice (n=9). ‘Non-Stim’ indicates control condition, while ‘Stim’ indicates muscle which underwent electrically stimulated muscle contraction. Horizontal bars indicate statistical differences between groups (P<0.05). Values are expressed as means + SE.
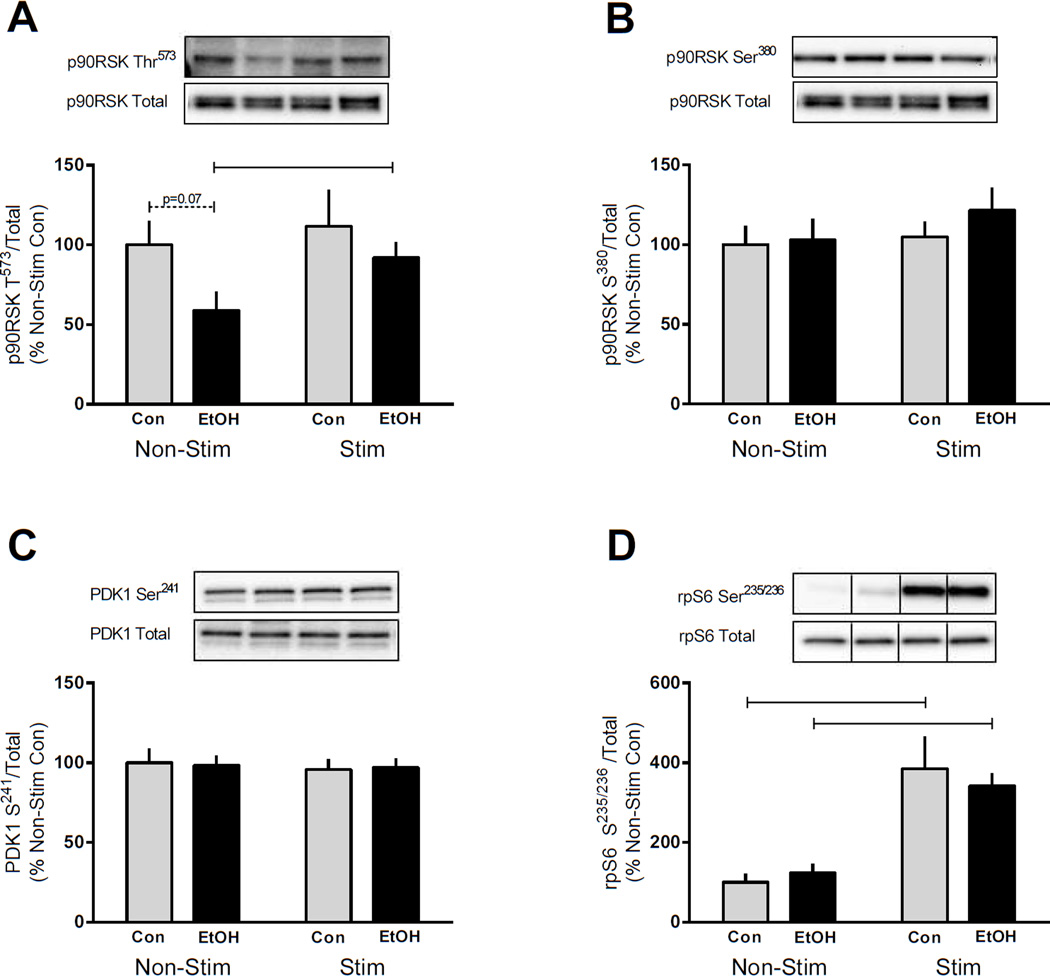
Since ERK and p38 were differentially altered by EtOH, we measured the phosphorylation of their downstream substrate, p90RSK. ERK phosphorylates p90RSK on Thr573 however, unlike ERK no change in Thr573 phosphorylation was detected in control mice following muscle contraction (Figure 4A). EtOH tended to decrease the phosphorylation of p90RSK Thr573 in the non-stimulated muscle compared with controls (p=0.07), while muscle contraction ameliorated this change and no difference between control and EtOH was detected in the stimulated muscle (Figure 4A). Phosphorylation of Thr573 leads to Ser380 phosphorylation which then serves as a docking site for PDK1 and p90RSK activation (Anjum and Blenis, 2008). No differences were detected after stimulated muscle contraction and/or EtOH administration for either p90RSK Ser380 or PDK1 Ser214 (Figure 4B and C). Lastly, rpS6 Ser235/236, a p90RSK substrate, was increased by stimulated muscle contraction in both the control and EtOH treated mice (200–300%) contrasting those changes seen in p90RSK signaling.
Figure 4.
p90RSK signaling in response to EtOH and muscle contraction. p90RSK Thr573 (A), p90RSK Ser380 (B), PDK1 Ser241 (C), and rpS6 Ser235/236 (D) were assessed in the gastrocnemius/plantaris muscle complex 4 h after cessation of muscle contraction and 2 h after EtOH (3 g/kg) intoxication. Bar graphs represent quantification of Western blot images normalized to the total amount of the respective protein with the control non-contracted value set to 100%. Black bars correspond to alcohol (EtOH) treated mice (n=10) and light bars represent control (Con) mice (n=9). ‘Non-Stim’ indicates control condition, while ‘Stim’ indicates muscle which underwent electrically stimulated muscle contraction. Horizontal bars indicate statistical differences between groups (P<0.05). Values are expressed as means + SE.
Acute EtOH intoxication antagonizes protein elongation
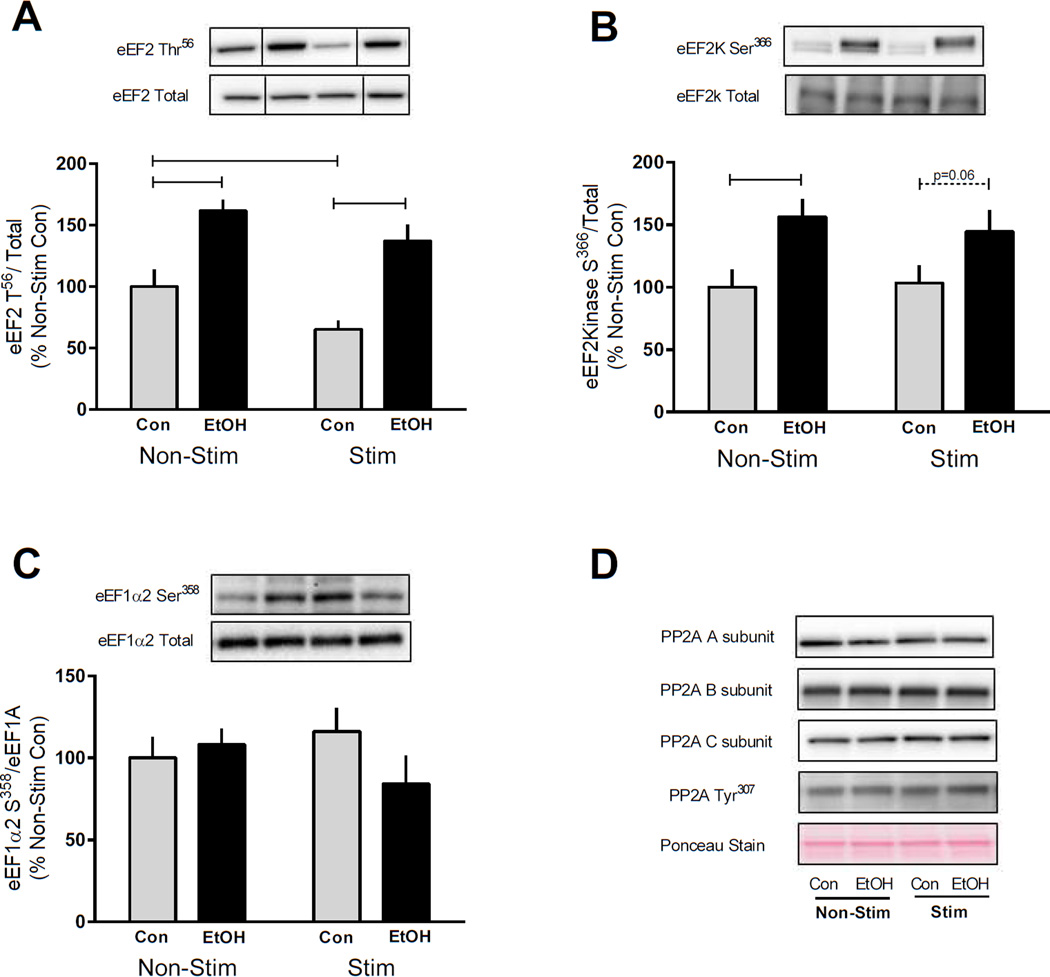
Elongation of the mRNA at the ribosome also regulates the rate of protein synthesis and is controlled by the expression of several elongation factors including eEF2. Elongation is suppressed following phosphorylation of eEF2 on Thr56 by eEF2k (Browne and Proud, 2002). Similar to the suppression of protein synthesis by EtOH, we found that phosphorylation of eEF2 Thr56 was increased by EtOH in both the non-contracted and contracted muscle compared with controls (Figure 5A). In contrast, phosphorylation of eEF2 Thr56 was decreased by contraction in control but not EtOH treated mice, indicating EtOH inhibited elongation following muscle contraction (Figure 5A). eEF2 is specifically phosphorylated by eEF2k which is a substrate of both S6K1 and RSK (Wang et al., 2001). Phosphorylation of eEF2k impairs its ability to phosphorylate and inhibit eEF2 to subsequently decrease elongation during times of low energy availability. However, contrary to expectations, the phosphorylation of eEF2K Ser366 following EtOH intoxication was increased in both the non-stimulated and stimulated muscle (Figure 5B). To assess an additional elongation factor eEF1A2 Ser358 was measured but values did not differ among the four experimental conditions (Figure 5C). Lastly, the total amount of each of these elongation factors (eEF2 kinase, eEF2 and eEF1A) was not changed by muscle contraction and/or EtOH (data not shown). Protein phosphatase 2A expression was also measured as a possible explanation for the variable signaling observed between elongation factors (Hong-Brown et al., 2007); however no changes were detected in any of the 3 PP2A subunits (A, B or C) nor its phosphorylation on Tyr307 which is inversely related to its activity (Figure 5D).
Figure 5.
EtOH increases phosphorylation of eukaryotic elongation factors (eEF) to inhibit protein elongation. Four hours after cessation of muscle contraction and 2 h following EtOH (3 g/kg) intoxication eEF2K Ser366 (A), eEF2Thr56 (B) and eEF1α2 Ser358 (C) were assessed in the gastrocnemius/plantaris muscle complex. Bar graphs represent quantification of Western blot images normalized to the total amount of the respective protein with the control non-contracted value set to 100%. Black bars correspond to alcohol (EtOH) treated mice (n=10) and light bars represent control (Con) mice (n=9). ‘Non-Stim’ indicates control condition, while ‘Stim’ indicates muscle which underwent electrically stimulated muscle contraction. Horizontal bars indicate statistical differences between groups (P<0.05). Values are expressed as means + SE. Expression of PP2A including the A, B and C subunit as well as phosphorylation on Tyr307 was also measured and representative images are shown (D). Ponceau S Stain was used as a loading control. No statistically significant differences were detected between the groups for PP2A signaling in the gastrocnemius/plantaris muscle complex at this time point.
Discussion
EtOH has well documented suppressive effects on mTORC1-mediated rates of protein synthesis within skeletal muscle (Lang et al., 2004, Lang et al., 2009); however, it is yet to be shown whether EtOH will reverse the elevation in mTORC1-mediated protein synthesis induced by muscle contraction. The current findings show that acute EtOH intoxication completely eliminates the contraction-induced increase in skeletal muscle protein synthesis in addition to suppressing basal rates. However, the suppression of protein synthesis by EtOH was not due to the abrogation of mTORC1 or MAPK signaling. Instead, EtOH appeared to inhibit translocation of the tRNA within the ribosome and therefore mRNA elongation as determined by increased phosphorylation of eEF2. Therefore, despite maintenance of contraction-induced increases in MAPK and mTORC1 signaling, global protein synthesis increased by electrically stimulated muscle contraction was reversed by acute EtOH intoxication. As a consequence, high levels of alcohol repeatedly consumed after exercise might be expected to interfere with the benefits of resistance exercise/muscle contraction.
Contraction or increased loading stimulates skeletal muscle protein synthesis leading to hypertrophy in an mTORC1-dependent manner (Bodine et al., 2001, Baar and Esser, 1999, Goodman et al., 2011). For example, muscle hypertrophy and S6K1 Thr389 phosphorylation in response to overload (via synergistic ablation) was prevented by treatment with the allosteric inhibitor of mTOR kinase activity, rapamycin, as well as in muscle-specific rapamycin resistant and rapamycin resistant kinase dead mutant mice (Goodman et al., 2011, Bodine et al., 2001). Further, rapamycin inhibits muscle protein synthesis induced by ex vivo passive stretch and in vivo resistance exercise concordantly with decreased S6K1 phosphorylation providing support for a causal relationship between mTORC1 signaling, protein synthesis and muscle hypertrophy (Kubica et al., 2005, Hornberger et al., 2004). Despite the evidence linking synthesis and mTORC1 signaling, our current data suggest otherwise in that we found synthesis rates to be decreased concurrently with increases in S6K1 and 4E-BP1 phosphorylation. This disconnect between mTORC1 signaling and the rate of synthesis following EtOH treatment exemplifies the potential shortcomings of experiments in which rates of synthesis are inferred from studies solely using S6K1 Thr389 phosphorylation as a surrogate marker of mTORC1 activity. Ours is not the first work to demonstrate a lack of synchronization between synthesis and signaling as Areta et al. (2013) showed that increases in S6K1 Thr389 stimulated by a bout of resistance exercise and protein intake were not correlated with elevations in myofibrillar FSR in human skeletal muscle over a 12 h recovery period (Areta et al., 2013).
Previous reports have revealed that pretreatment with EtOH prevents hormonal (IGF-1, insulin), and nutrient (feeding, leucine) induced stimulation of protein synthesis and mTORC1 signaling in skeletal muscle (Kumar et al., 2002, Lang et al., 2003, Sneddon et al., 2003). These findings, in combination with those showing that EtOH impairs mTOR-mediated translation initiation under basal conditions, led to the speculation that the suppressive effects of EtOH were primarily mTORC1-mediated (Lang et al., 2009). The current data alternatively show that EtOH does not decrease mTORC1 signaling after anabolic stimulation and instead provide evidence that EtOH potentially decreases stimulated rates of synthesis via inhibition of peptide chain elongation. A similar inhibitory effect of EtOH on elongation factors has been reported previously in C2C12 myocytes as 24 h of EtOH exposure (100 mM) increased eEF2 Thr56 phosphorylation in accordance with decreased rates of protein synthesis (Hong-Brown et al., 2007). In contrast, EtOH-induced phosphorylation of eEF2 Thr56 in C2C12 cells was associated with the expected decrease in phosphorylation of eEF2K (compared to our contradictory increase) (Hong-Brown et al., 2007). Therefore, the increased phosphorylation of eEF2 Thr56 following in vivo administered EtOH could have instead been the result of decreased activity of protein phosphatase 2 (PP2A) similar to that described in C2C12 myoblasts (Hong-Brown et al., 2007); however, our data indicate otherwise as no changes were observed in the expression of PP2A. In addition to eEF2, eEF1A may also contribute to peptide chain elongation as it plays a role in the transfer of aminoacyl-tRNAs to the A site of the ribosome, an event which precedes the eEF2 directed movement of the tRNA to the P site. However, no difference was detected in response to EtOH compared with control values, thus implicating specific inhibition of tRNA translocation within the ribosome (A to P site) as a potential mechanism for the decreased protein synthesis.
Proteins within the MEK/ERK pathway may influence the phosphorylation status of several components both upstream and downstream of mTORC1 and accordingly contribute to the regulation of translation initiation and elongation (Anjum and Blenis, 2008). Presently, ERK1/2 phosphorylation was decreased in EtOH treated muscle irrespective of muscle contraction. Similarly, EtOH tended to decrease the phosphorylation of p90RSK Thr573 in control muscle which is a site phosphorylated by ERK1/2 and the first step enroute to p90RSK multisite-dependent activation (Anjum and Blenis, 2008). However, there was variability in the phosphorylation of other p90RSK residues and proteins (PDK1) within the p90RSK signaling pathway thereby suggesting that p90RSK-dependent signaling does not significantly contribute to EtOH-induced suppression of protein synthesis. RSK activity may also be regulated by p38 via MAPK-activated kinase-2 (MK2) or MK3 but this was not evident either in the current data as basal- and contraction-induced phosphorylation of p38 did not differ between EtOH and control muscle.
Suppression of mTORC1 activity, phosphorylation of its substrates and impaired translation initiation following acute and chronic EtOH exposure under basal conditions is a well reported phenomenon (Lang et al., 2004, Kumar et al., 2002, Lang et al., 2009, Lang et al., 2010b, Lang et al., 2003). Presently though, only modest decreases in the phosphorylation of S6K1 (20–30% decrease) and 4E-BP1 (30–35% decrease) were observed in the non-stimulated leg after EtOH intoxication. However, the experimental conditions and statistical model were not optimized to make this comparison and detection of potential differences induced by EtOH may have been missed. For example, all mice were fasted for 20–24 h prior to tissue collection which is in contrast to previous studies performed on rats with only a 12 h fast prior to EtOH intoxication and tissue collection (Lang et al., 2004, Kumar et al., 2002, Lang et al., 2009). The purpose of the present fast was to maximize changes observed with muscle contraction as well as allow for metabolically matched sampling since mTOR signaling is nutrient sensitive. It is also recognized that such a prolonged fast in mice can cause weight loss and decrease mTORC1 activity as a means to conserve energy and therefore our results may only be applicable under fasted conditions. Lastly, the method of sample analysis may have also limited the detection of alcohol related changes as our primary purpose was to compare the contracted muscle with the non-contracted muscle of the same animal (both control and EtOH), meaning that samples from both conditions (contracted and non-contracted) were analyzed on the same gel to maintain consistency of experimental conditions. Therefore, lower levels of phosphorylation in the non-contracted muscle may have been masked as image band density was optimized to expose the increased levels observed in the contracted muscle (e.g. S6K1, JNK).
Of additional interest to the current work is that during the analysis of the data presented herein, Parr et al. (2014) published work in humans showing that the combination of alcohol (1.5 g/kg) and carbohydrate intake following an intensive exercise bout (resistance exercise and aerobic exercise) blunted the increase in mTORC1 signaling and protein synthesis compared to those athletes receiving 2 doses of protein (25 g each) in place of alcohol. While these findings are in contrast to the present work, they are in agreement with previous data showing that EtOH suppresses anabolic stimulation of protein synthesis and mTORC1 signaling in skeletal muscle of rodents (Sneddon et al., 2003, Lang et al., 2004, Lang et al., 2010a, Lang et al., 2003, Kumar et al., 2002). Reasons for these discordant findings could include differences in the exercise stimuli (muscle contraction vs. combination of resistance and aerobic exercise) and nutrient availability/nutrition status before, during and after the exercise (Parr et al., 2014). Further, blood alcohol levels of the subjects remained relatively low throughout the 8 h recovery period (~0.02–0.06 g/100mL) while they were elevated throughout the protocol in our mouse study. Therefore, future work may be necessary to establish a dose-response curve for the suppressive effects of EtOH on muscle health and recovery from exercise.
In summary, this work provides evidence of mTORC1 and MAPK-independent effects of EtOH on the rate of protein synthesis within skeletal muscle following contraction in fasted mice. It also shows that EtOH is able to reverse the anabolic effect of muscle contraction on protein synthesis, potentially via inhibition of peptide chain elongation. It remains to be determined whether repeated exposures to high levels of EtOH following muscle contraction would impair muscle hypertrophy over a longer period of time and whether lower doses of EtOH would have similar detrimental effects. Based on the current evidence showing that high levels of EtOH may have negative effects on muscle anabolism, we speculate that to obtain the greatest anabolic effect of exercise, alcohol should not be consumed in the immediate post-exercise period.
Acknowledgement
The authors thank Dr. Chris Proud for the generous gift of the antibody which recognizes T56-phosphorylated eEF2.
We thank Maithili Navaratnarajah, Anne Pruznak, and Gina Deiter for their excellent technical assistance and Dr. Sean Stocker for use of his current stimulator.
Funding: This work was supported by a grant from NIAAA R37 AA011290 (C.H.L) and F32 AA023422 (J.L.S).
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Contributor Information
Jennifer L. Steiner, Email: jls1075@psu.edu.
Charles H. Lang, Email: clang@psu.edu.
References
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. 2013;591:2319–2331. doi: 10.1113/jphysiol.2012.244897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Carrière A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Chen MM, Palmer JL, Ippolito JA, Curtis BJ, Choudhry MA, Kovacs EJ. Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation. Mediators Inflamm. 2013;2013:971481. doi: 10.1155/2013/971481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig J, Griffiths LA, Peberdy D, Dharmasaroja P, Vera M, Davies FJ, Newbery HJ, Brownstein D, Abbott CM. In vivo characterization of the role of tissue-specific translation elongation factor 1A2 in protein synthesis reveals insights into muscle atrophy. FEBS J. 2013;280:6528–6540. doi: 10.1111/febs.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589:5485–5501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol regulates eukaryotic elongation factor 2 phosphorylation via an AMP-activated protein kinase-dependent mechanism in C2C12 skeletal myocytes. J Biol Chem. 2007;282:3702–3712. doi: 10.1074/jbc.M606593200. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CJ, Buch MB, Krag TO, Hemmings BA, Gammeltoft S, Frödin M. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 1999;274:27168–27176. doi: 10.1074/jbc.274.38.27168. [DOI] [PubMed] [Google Scholar]
- Jørgensen R, Merrill AR, Andersen GR. The life and death of translation elongation factor 2. Biochem Soc Trans. 2006;34:1–6. doi: 10.1042/BST20060001. [DOI] [PubMed] [Google Scholar]
- Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280:7570–7580. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E917–E928. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–299. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E1205–E1215. doi: 10.1152/ajpendo.00177.2003. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Kumar V, Wu D, Vary TC. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res. 2000;24:322–331. [PubMed] [Google Scholar]
- Lang CH, Kimball SR, Frost RA, Vary TC. Alcohol myopathy: impairment of protein synthesis and translation initiation. Int J Biochem Cell Biol. 2001;33:457–473. doi: 10.1016/s1357-2725(00)00081-9. [DOI] [PubMed] [Google Scholar]
- Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr. 2010a;140:932–938. doi: 10.3945/jn.109.120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Lynch CJ, Vary TC. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am J Physiol Regul Integr Comp Physiol. 2010b;299:R935–R944. doi: 10.1152/ajpregu.00297.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res. 2004;28:1758–1767. doi: 10.1097/01.alc.0000145787.66405.59. [DOI] [PubMed] [Google Scholar]
- Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats. Nutr Metab (Lond) 2009;6:4. doi: 10.1186/1743-7075-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SM, Kazi AA, Hong-Brown L, Lang CH. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS One. 2012;7:e38910. doi: 10.1371/journal.pone.0038910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Mothe-Satney I, Brunn GJ, McMahon LP, Capaldo CT, Abraham RT, Lawrence JC. Mammalian target of rapamycin-dependent phosphorylation of PHAS-I in four (S/T)P sites detected by phospho-specific antibodies. J Biol Chem. 2000;275:33836–33843. doi: 10.1074/jbc.M006005200. [DOI] [PubMed] [Google Scholar]
- Parr EB, Camera DM, Areta JL, Burke LM, Phillips SM, Hawley JA, Coffey VG. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One. 2014;9:e88384. doi: 10.1371/journal.pone.0088384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J Biol Chem. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–2791. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon AA, Koll M, Wallace MC, Jones J, Miell JP, Garlick PJ, Preedy VR. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E874–E882. doi: 10.1152/ajpendo.00209.2002. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Campbell DG, Cohen P. Identification of insulin-stimulated protein kinase-1 as the rabbit equivalent of rskmo-2. Identification of two threonines phosphorylated during activation by mitogen-activated protein kinase. Eur J Biochem. 1993;212:581–588. doi: 10.1111/j.1432-1033.1993.tb17696.x. [DOI] [PubMed] [Google Scholar]
- Vary TC, Lang CH. Differential phosphorylation of translation initiation regulators 4EBP1, S6k1, and Erk 1/2 following inhibition of alcohol metabolism in mouse heart. Cardiovasc Toxicol. 2008;8:23–32. doi: 10.1007/s12012-008-9012-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172:227–238. doi: 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]