Abstract
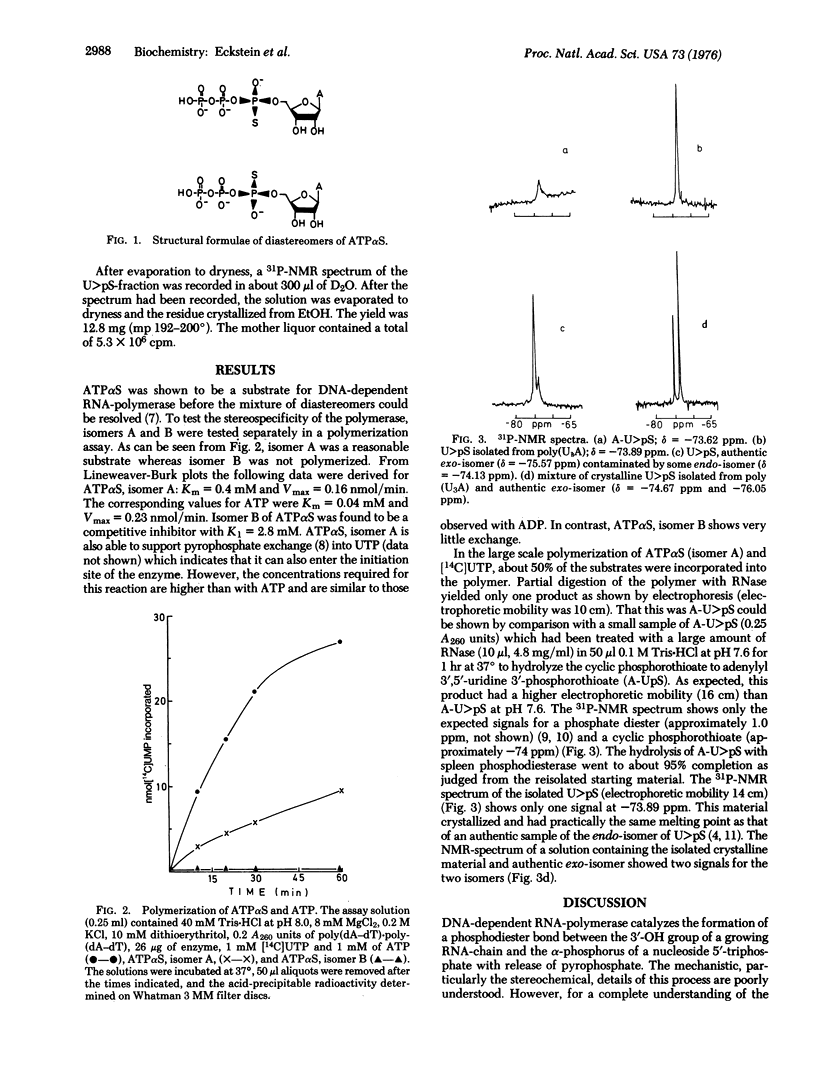
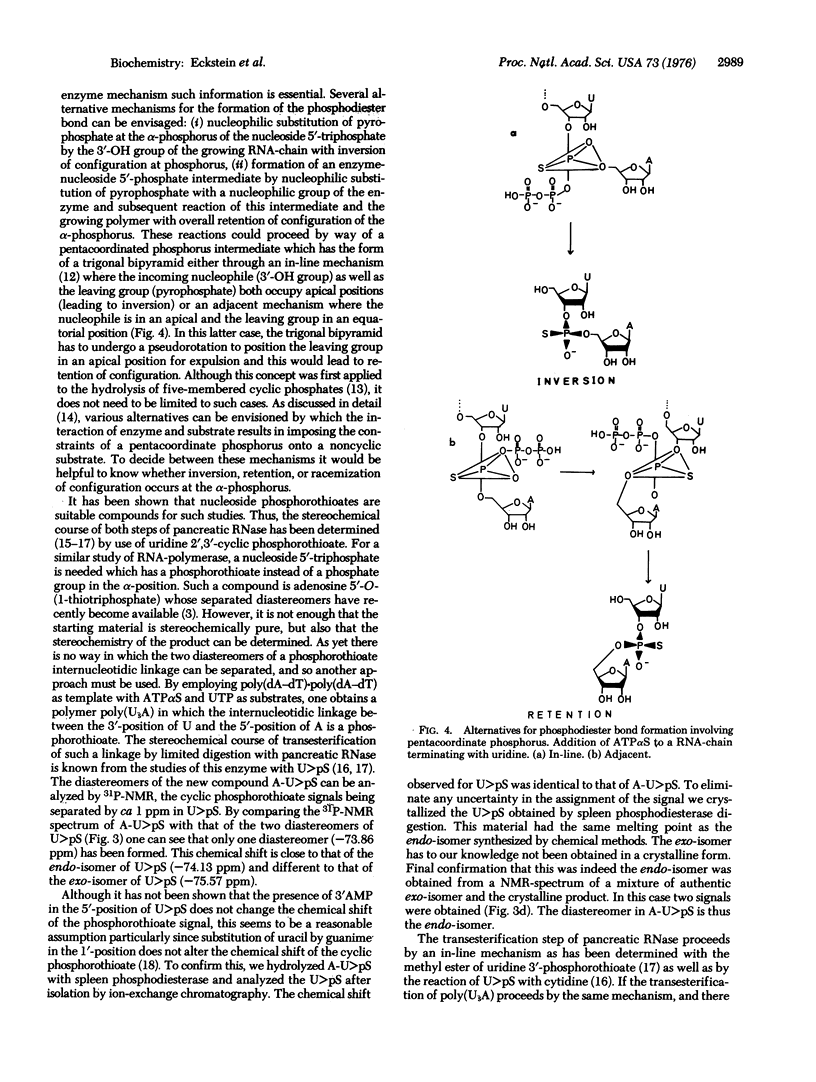
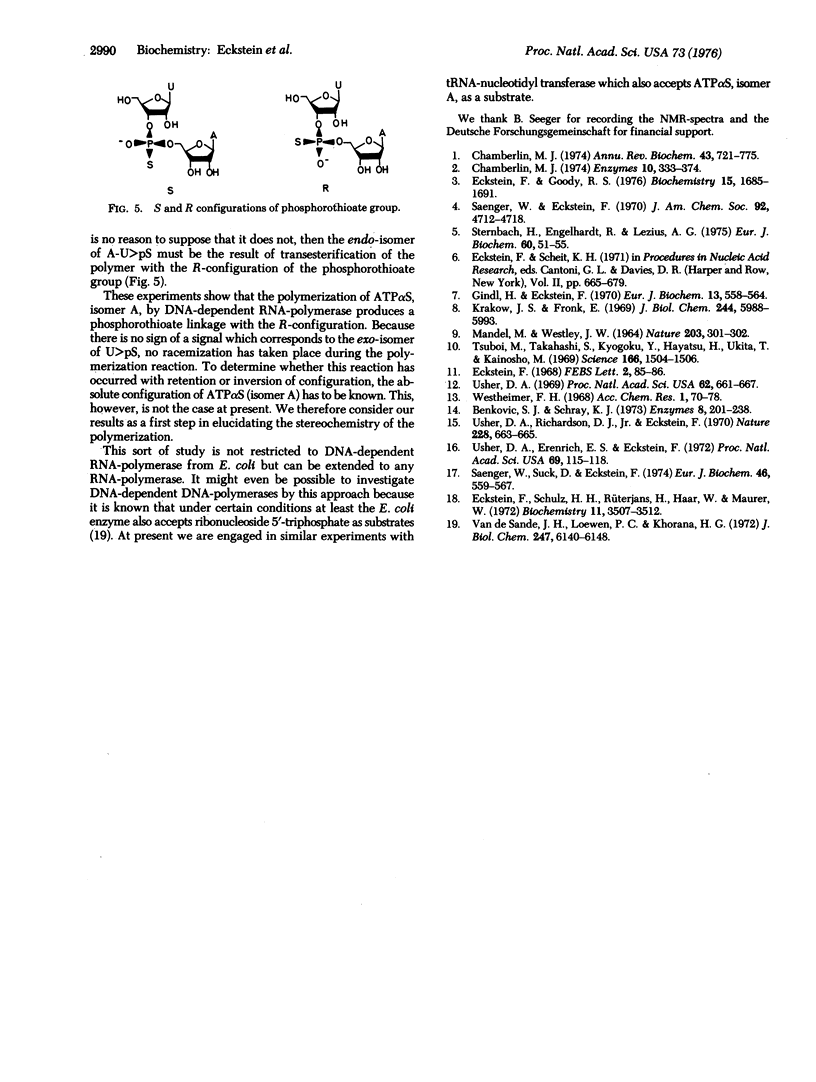
The phosphodiester bond formation by DNA-dependent RNA-polymerase (RNA nucleotidyltransferase, nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) can in principle result in retention, inversion, or racemization of configuration at the alpha-phosphorus of the nucleoside 5'-triphosphate being polymerized. As a first step in elucidating the stereochemistry of this reaction, one diastereomer (A) of adenosine 5'-O-(1-thiotriphosphate) (ATPalphaS) was polymerized with UTP in the presence of poly(dA-dT)-poly(dA-dT). The resulting polymer was enzymatically cleaved to uridine 2',3'-cyclic phosphorothioate which was determined to be the endo-isomer by comparison with an authentic sample. This shows that no reacemization had occurred and that isomer A of ATPalphaS gives a phosphorothioate diester bond with the R-configuration. Whether this represents inversion of retention of configuration awaits elucidation of the absolute configuration of isomer A for ATPalphaS.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Gindl H. Polyribonucleotides containing a phosphorothioate backbone. Eur J Biochem. 1970 Apr;13(3):558–564. doi: 10.1111/j.1432-1033.1970.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Goody R. S. Synthesis and properties of diastereoisomers of adenosine 5'-(O-1-thiotriphosphate) and adenosine 5'-(O-2-thiotriphosphate). Biochemistry. 1976 Apr 20;15(8):1685–1691. doi: 10.1021/bi00653a015. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. J Am Chem Soc. 1970 Jul 29;92(15):4718–4723. doi: 10.1021/ja00718a039. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Schulz H. H., Rüterjans H., Haar W., Maurer W. Stereochemistry of the transesterification step of ribonuclease T 1 . Biochemistry. 1972 Sep 12;11(19):3507–3512. doi: 10.1021/bi00769a002. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Uridine 2',3'-O,O-cyclophosphorothioate as substrate for pancreatic ribonuclease (I). FEBS Lett. 1968 Dec;2(2):85–86. doi: 10.1016/0014-5793(68)80108-5. [DOI] [PubMed] [Google Scholar]
- Krakow J. S., Fronk E. Azotobacter vinelandii ribonucleic acid polymerase. 8. Pyrophosphate exchange. J Biol Chem. 1969 Nov 10;244(21):5988–5993. [PubMed] [Google Scholar]
- MANDEL M., WESTLEY J. W. NUCLEAR MAGNETIC RESONANCE OF PHOSPHORUS IN DEOXYTHYMIDINE POLYNUCLEOTIDES. Nature. 1964 Jul 18;203:301–302. doi: 10.1038/203301b0. [DOI] [PubMed] [Google Scholar]
- Saenger W., Suck D., Eckstein F. On the mechanism of ribonuclease A. Crystal and molecular structure of uridine 3'-O-thiophosphate methyl ester triethylammonium salt. Eur J Biochem. 1974 Aug 1;46(3):559–567. doi: 10.1111/j.1432-1033.1974.tb03650.x. [DOI] [PubMed] [Google Scholar]
- Sternbach H., Engelhardt R., Lezius A. G. Rapid isolation of highly active RNA polymerase from Escherichia coli and its subunits by matrix-bound heparin. Eur J Biochem. 1975 Dec 1;60(1):51–55. doi: 10.1111/j.1432-1033.1975.tb20974.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi M., Takahashi S., Kyogoku Y., Hayatsu H., Ukita T., Kainosho M. Phosphorus-proton spin-spin coupling and conformation of a dinucleoside phosphate. Science. 1969 Dec 19;166(3912):1504–1505. doi: 10.1126/science.166.3912.1504. [DOI] [PubMed] [Google Scholar]
- Usher D. A., Erenrich E. S., Eckstein F. Geometry of the first step in the action of ribonuclease-A (in-line geometry-uridine2',3'-cyclic thiophosphate- 31 P NMR). Proc Natl Acad Sci U S A. 1972 Jan;69(1):115–118. doi: 10.1073/pnas.69.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher D. A. On the mechanism of ribonuclease action. Proc Natl Acad Sci U S A. 1969 Mar;62(3):661–667. doi: 10.1073/pnas.62.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher D. A., Richardson D. I., Jr, Eckstein F. Absolute stereochemistry of the second step of ribonuclease action. Nature. 1970 Nov 14;228(5272):663–665. doi: 10.1038/228663a0. [DOI] [PubMed] [Google Scholar]
- Van de Sande J. H., Loewen P. C., Khorana H. G. Studies on polynucleotides. 118. A further study of ribonucleotide incorporation into deoxyribonucleic acid chains by deoxyribonucleic acid polymerase I of Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6140–6148. [PubMed] [Google Scholar]


