Abstract
Background
Life's Simple 7 is a new metric based on modifiable health behaviors and factors that the American Heart Association uses to promote improvements to cardiovascular health (CVH). We hypothesized that better Life's Simple 7 scores are associated with lower incidence of cognitive impairment.
Methods and Results
For this prospective cohort study, we included REasons for Geographic And Racial Differences in Stroke (REGARDS) participants aged 45+ who had normal global cognitive status at baseline and no history of stroke (N=17 761). We calculated baseline Life's Simple 7 score (range, 0 to 14) based on smoking, diet, physical activity, body mass index, blood pressure, total cholesterol, and fasting glucose. We identified incident cognitive impairment using a 3‐test measure of verbal learning, memory, and fluency obtained a mean of 4 years after baseline. Relative to the lowest tertile of Life's Simple 7 score (0 to 6 points), odds ratios of incident cognitive impairment were 0.65 (0.52, 0.81) in the middle tertile (7 to 8 points) and 0.63 (0.51, 0.79) in the highest tertile (9 to 14 points). The association was similar in blacks and whites, as well as outside and within the Southeastern stroke belt region of the United States.
Conclusions
Compared with low CVH, intermediate and high CVH were both associated with substantially lower incidence of cognitive impairment. We did not observe a dose‐response pattern; people with intermediate and high levels of CVH had similar incidence of cognitive impairment. This suggests that even when high CVH is not achieved, intermediate levels of CVH are preferable to low CVH.
Keywords: cardiovascular disease prevention, cardiovascular disease risk factors, cognitive impairment, cognitive tests, lifestyle
Introduction
Cardiovascular health (CVH) plays a critical role in brain health.1 Several cardiovascular risk factors, including hypertension, diabetes, and obesity, are related to higher risk for cognitive decline.2–3 Prevention strategies targeting modifiable factors and behaviors are important for reducing risks for cognitive decline and dementia that stem from poor CVH.4
Life's Simple 7 is a new measure of CVH published by the American Heart Association (AHA) in 2010 to track health status in relation to a 2020 strategic goal to improve CVH of Americans.4 Life's Simple 7 is based on 4 modifiable health behaviors, including nonsmoking, healthy diet, physical activity, and body mass index (BMI), and 3 modifiable biological factors, including blood pressure (BP), total cholesterol, and fasting glucose.4 A score based on all 7 components represents the degree to which an individual's health behaviors and health factors are in accord with ideal CVH. Better CVH, as measured by Life's Simple 7, is associated with lower incidence of cardiovascular disease (CVD) outcomes.5–7
Determining the relationship of Life's Simple 7 with cognitive function is important to inform efforts to prevent cognitive decline and dementia through a heart‐healthy lifestyle as well as to provide information on the potential impact of the AHA's strategic goals on cognitive function in the population. We hypothesized that higher scores on Life's Simple 7, indicating better CVH, are associated with lower incidence of cognitive impairment in middle‐aged and older adults.
Methods
Design, Setting, and Participants
REGARDS is a population‐based observational cohort study of 30 239 adults aged 45 and above at baseline, enrolled between January 2003 and October 2007.8 The cohort is 55% women, 45% men, 42% blacks, 58% whites, 56% residents of stroke belt states (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee), and 44% residents of the other 40 continental United States. Institutional review boards of the collaborating institutions approved the study protocol. Participants gave written informed consent.
Data Collection
At baseline, a structured telephone interview was used to collect data on demographic characteristics, health behaviors, medical conditions, and cognitive function. After the telephone interview, a health professional conducted an in‐home visit that included body size and BP measurements, electrocardiogram (ECG), and blood sample collection. A food frequency questionnaire (FFQ) was left with the participant to complete and return by mail. Participants are contacted every 6 months by telephone to obtain additional cognitive assessments and identify strokes and other medical events.
Life's Simple 7
We measured Life's Simple 7 health behaviors and health factors according to definitions published by the AHA.4 We adapted definitions for healthy diet and physical activity components based on data available in REGARDS, as described previously5 and in Table 1. Baseline Life's Simple 7 components were each categorized as ideal, intermediate, or poor. Ideal levels of each component are defined below.
Table 1.
Ideal, Intermediate, and Poor Levels of Life's Simple 7 Components
| Component | Ideal (2 Points) | Intermediate (1 Point) | Poor (0 Points) |
|---|---|---|---|
| Smoking | Never or former >1 year | Former ≤1 year | Current |
| Healthy diet score* | 4 to 5 points | 2 to 3 points | 0 to 1 points |
| Physical activity* | ≥4 bouts per week of intense physical activity sufficient to work up a sweat | 1 to 3 bouts per week of intense physical activity sufficient to work up a sweat | No intense physical activity sufficient to work up a sweat |
| Body mass index | <25 kg/m2 | 25 to 29.9 kg/m2 | ≥30 kg/m2 |
| Blood pressure | <120/<80 mm Hg untreated | SBP 120 to 139 or DBP 80 to 89 mm Hg or treated to ideal level | SBP ≥140 or DBP ≥90 mm Hg |
| Total cholesterol | <5.18 mmol/L (<200 mg/dL) untreated | 5.18 to 6.19 mmol/L (200 to 239 mg/dL) or treated to ideal level | ≥6.22 mmol/L (≥240 mg/dL) |
| Fasting glucose | <5.55 mmol/L (<100 mg/dL) untreated | 5.55 to 6.94 mmol/L (100 to 125 mg/dL) or treated to ideal level | ≥6.99 mmol/L (≥126 mg/dL) |
DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
Diet in REasons for Geographic And Racial Differences in Stroke (REGARDS) was measured by a Block 98 Food Frequency Questionnaire. Healthy diet score was derived from the Food Frequency Questionnaire based on 5 healthy diet components, each worth 1 point: (1) fruits and vegetables: ≥4.5 servings per day; (2) fish: ≥200 g per week; (3) fiber‐to‐carbohydrate ratio: >1 g of fiber per 10 g of carbohydrate; (4) sodium: <1500 mg per day; and (5) sugar‐sweetened foods and beverages: ≤450 kcal per week. The 5 healthy diet components for REGARDS were adapted from the American Heart Association definitions of the 5 components, which were “(1) fruits and vegetables: ≥4.5 cups per day; (2) fish: ≥two 3.5‐oz servings per week (preferably oily fish); (3) fiber‐rich whole grains (≥1.1 g of fiber per 10 g of carbohydrate): ≥three 1‐oz‐equivalent servings per day; (4) sodium: <1500 mg per day; (5) sugar‐sweetened beverages: ≤450 kcal (36 oz) per week.”2
Physical activity in REGARDS was measured by asking participants “How many times per week do you engage in intense physical activity, enough to work up a sweat?” Participant responses were categorized as shown in the table. The ideal, intermediate, and poor category definitions for REGARDS were adapted from the American Heart Association definitions, which were “ideal: ≥150 min/wk moderate intensity or ≥75 min/wk vigorous intensity or ≥150 min/wk moderate+vigorous; Intermediate: 1 to 149 min/wk moderate intensity or 1 to 74 min/wk vigorous intensity or 1 to 149 min/wk moderate+vigorous; poor: none.”2
Ideal smoking status was defined as never smoking or quitting more than 1 year ago, determined by self‐report.
Ideal diet was defined as meeting ≥4 of 5 dietary recommendations, including (1) fruits and vegetables: ≥4 servings per day; (2) fish: ≥200 g per week; (3) fiber‐to‐carbohydrate ratio: >1 g of fiber per 10 g of carbohydrate; (4) sodium: <1500 mg per day; and (5) sugar‐sweetened foods and beverages: ≤450 kcal per week. Diet was assessed with a self‐administered, semiquantitative Block 98 FFQ.9
Ideal physical activity was defined as ≥4 bouts per week of intense physical activity sufficient to work up a sweat, based on self‐report.
Ideal BMI was defined as BMI <25 kg/m2, calculated from measured weight and height.
Ideal BP was defined as untreated systolic BP (SBP) <120 mm Hg and diastolic BP (DBP) <80 mm Hg, from the average of 2 measurements. Treatment with antihypertensive medication was determined by self‐report.
Ideal total cholesterol was defined as untreated total cholesterol <5.18 mmol/L (<200 mg/dL), measured by colorimetric reflectance spectrophotometry.10 Treatment with lipid‐lowering medication was determined by self‐report.
Ideal fasting glucose was defined as untreated fasting glucose <5.55 mmol/L (<100 mg/dL), measured by colorimetric reflectance spectrophotometry.10 Treatment with insulin or oral hypoglycemic medication was determined by self‐report.
In contrast to ideal health, poor health is characterized by current smoking, meeting no more than 1 of 5 dietary recommendations, engaging in no physical activity, and having elevated levels of BMI (≥30 kg/m2), BP (SBP ≥140 mm Hg or DBP ≥90 mm Hg), total cholesterol (≥6.22 mmol/L), and fasting glucose (≥6.99 mmol/L). Further details for poor and intermediate levels of each component are given in Table 1.
We calculated a Life's Simple 7 total score by assigning each component 2 points for ideal, 1 point for intermediate, or zero points for poor, then summing all 7 component scores to yield a total score ranging from zero (worst CVH) to 14 points (best CVH). For example, a participant who never smoked, met 2 of 5 dietary recommendations, engaged in 4 bouts per week of intense physical activity, had BMI of 24 kg/m2, treated BP of 125/85, untreated total cholesterol of 5 mmol/L, and untreated fasting glucose of 5 mmol/L would score 12 points, a high level of CVH. In contrast, a participant who currently smoked, met 1 dietary recommendation, engaged in 1 bout per week of intense physical activity, had BMI of 29 kg/m2, BP of 145/95, total cholesterol of 6.5 mmol/L, and treated fasting glucose of 6 mmol/L would score 3 points, a low level of CVH.
Tertiles of Life's Simple 7 score were 0 to 6 (low), 7 to 8 (middle), and 9 to 14 points (high). We also calculated a health behavior score, based on smoking, diet, physical activity, and BMI, ranging from 0 (worst) to 8 points (best), and a biological health factor score, based on BP, total cholesterol, and fasting glucose, ranging from 0 (worst) to 6 points (best).
Covariates
To adjust for potential confounding, we selected baseline demographic and CVD covariates that could be determinants of cognitive function as well as correlates of Life's Simple 7 health behaviors and health factors. Baseline age was calculated from baseline telephone interview date and self‐reported birth date. Sex, race (black, white), education, and income were self‐reported. We categorized education as less than high school, high school graduate, some college, or college graduate and above. We categorized income as <$20 000 per year (y), $20 000 to $34 999/y, $35 000 to $74 999/y, or ≥$75 000/y. Region of residence was categorized as within or outside the stroke belt based on participant's home address. Atrial fibrillation (AF) was defined as self‐reported physician diagnosis or presence of AF or atrial flutter on ECG. Left ventricular hypertrophy (LVH) was defined based on ECG.11 Baseline atherosclerotic disease was defined as having at least 1 of the following: coronary artery disease (CAD), carotid artery disease, peripheral arterial disease (PAD), or transient ischemic attack (TIA). CAD was defined as self‐reported physician diagnosis of myocardial infarction (MI), self‐reported coronary revascularization, or evidence of MI on ECG. Carotid artery disease and PAD were defined as self‐reported procedures or surgeries to repair arteries in the neck or legs. TIA was defined as self‐reported physician diagnosis.
Cognitive Function at Baseline
Beginning in December 2003, participants were administered the Six‐Item Screener (SIS) during the baseline telephone interview. The SIS is a brief measure of global cognitive status that assesses recall of 3 words and orientation to year, month, and day of the week (range, 0 to 6).12 Participants whose baseline telephone interview occurred before December 2003 received the baseline SIS during a subsequent follow‐up telephone interview. We defined baseline cognitive impairment as scoring below 5 points on the SIS based on validity studies in clinical and community samples relating the SIS to clinical diagnoses of dementia and mild cognitive impairment (MCI).12
Cognitive Function During Follow‐up
Beginning in January 2006 and every 2 years thereafter, participants were administered a 3‐test measure of cognitive function, including verbal learning, memory, and fluency. Verbal learning was assessed by a 3‐trial, 10‐item word list learning task (range, 0 to 30).13 Verbal memory was assessed by free recall of the 10‐item list after a brief delay filled with noncognitive questions (range, 0 to 10).13 Verbal fluency was assessed by animal naming, in which the participant names as many animals as possible in 60 seconds.14
For the analyses in this article, we used word list learning, word list delayed recall, and animal naming assessments that were obtained, scored, and included in the REGARDS data set as of April 2012.
We developed a definition of clinically relevant incident cognitive impairment (ICI) on the 3‐test measure using a demographically corrected, regression‐based approach. This approach was based on findings of previous research comparing methods of identifying clinically relevant cognitive impairment.15–16 First, using all participants' first assessment, we calculated mean scores on each test within 64 strata defined by age group (45 to 54, 55 to 64, 65 to 74, and 75+), sex (female, male), race (black, white), and education category (less than high school, high school graduate, some college, and college graduate and above). Next, for each test, we defined an impairment threshold as scoring ≥1.5 standard deviations below the stratum‐specific mean—a threshold conventionally used in defining MCI.17 Finally, among people who were cognitively intact according to the baseline SIS, we defined ICI on the 3‐test measure as scoring below the impairment threshold on at least 2 of 3 tests at the most recent 3‐test assessment, which was obtained a mean of 4.0 years after baseline (Figure 1).
Figure 1.
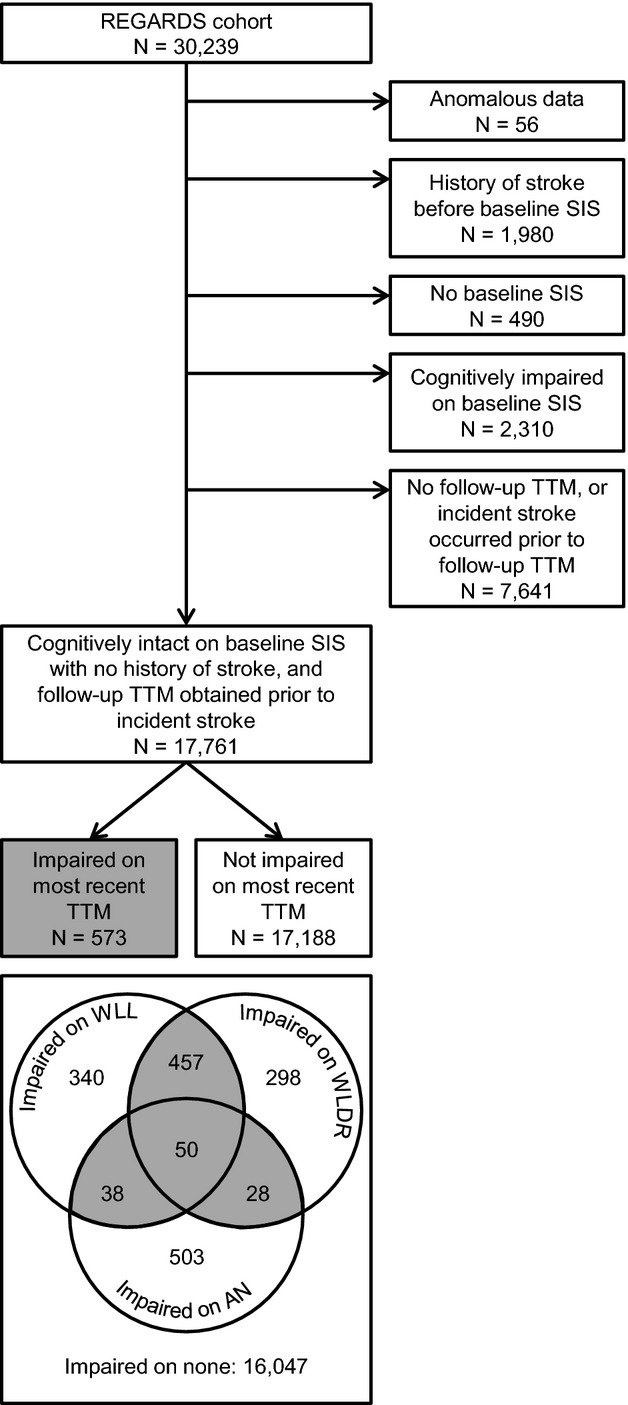
Flow chart of REGARDS participants. Participants had to score in the impaired range on at least 2 of 3 tests (gray shading) to be considered impaired on the most recent 3‐test measure. AN indicates animal naming; REGARDS, REasons for Geographic And Racial Differences in Stroke; SIS, Six‐Item Screener; TTM, 3‐test measure; WLDR, Word List Delayed Recall; WLL, Word List Learning.
Cohort for Analysis
To identify a stroke‐free cohort, baseline history of stroke was defined as self‐reported physician diagnosis. Suspected incident stroke was identified from participant reports during follow‐up telephone interviews, and medical records were retrieved and physician adjudicated using standardized criteria.18 To establish a cohort at risk for ICI on the 3‐test measure, we excluded participants who had anomalous data, a history of stroke before their baseline SIS, no baseline SIS, or cognitive impairment according to their baseline SIS. We also excluded those who had no follow‐up 3‐test measure or had an incident stroke before their follow‐up 3‐test measure, resulting in 17 761 participants (Figure 1).
Statistical Analysis
We examined participant characteristics by Life's Simple 7 score tertile and by ICI status. We compared Life's Simple 7 score distributions in blacks versus whites and within the stroke belt versus outside the stroke belt. Within each Life's Simple 7 score tertile, we calculated age group‐, sex‐, race‐, and education category–standardized proportions of participants who had ICI. Using logistic regression, we estimated odds ratios (ORs) of ICI for the middle and high tertiles, compared with the low tertile. We also estimated ORs of ICI for ideal and intermediate levels of each Life's Simple 7 component, compared with the poor level. ORs were adjusted for age, sex, race, education category, income category, region, baseline AF, LVH, atherosclerotic disease, and time interval from baseline to most recent 3‐test measure. We repeated standardized proportions and adjusted ORs for blacks and whites, as well as for stroke belt and non‐belt regions. Similarly, we explored age, sex, and education as potential effect modifiers. To test multiplicative interaction terms for race, region, age, sex, and education with Life's Simple 7 score tertiles, we used F tests for models based on multiple imputation data (see below) and likelihood ratio tests for sensitivity analysis models based on complete data (see below). Although the age‐, sex‐, race‐, education‐standardized method of determining cognitive impairment on the 3‐test measure precludes assessment of age, sex, race, and education themselves as risk factors, it does not preclude assessment of these variables as potential effect modifiers of the association of Life's Simple 7 with ICI on the 3‐test measure.
We addressed missing values for Life's Simple 7 components and baseline covariates with multiple imputation. Our rationale for using multiple imputation was to reduce bias in estimates of association that could arise from nonrandom patterns of missing data and to increase precision by retaining as many participants as possible in the analysis. We generated 5 imputed data sets using the method of chained equations.19–20 The imputation model included ICI status, Life's Simple 7 components (each coded as ideal, intermediate, or poor), age, sex, education categories, income categories, and indicators for baseline CVDs. Imputation was done separately by race and geographic region because we assessed interactions of Life's Simple 7 score with race and region.20 Each component of Life's Simple 7 was imputed for <4% of participants, except for fasting glucose, which was imputed for 15% (who were either missing glucose or were not fasting) and healthy diet score, which was imputed for 23% (who were missing the FFQ). Other covariates were each imputed for <2% of participants, except income category, which was imputed for 11%. Multiple imputation has been recommended for data in which 10% to 60% of values are missing.21 In this analysis, 44% of participants had at least 1 variable with a missing value. In a sensitivity analysis, we restricted the data set to participants who had complete data for all Life's Simple 7 components and covariates, except for income, to which we added a category of income not reported. This sensitivity analysis included 11 247 participants.
Results
Among 17 761 participants included in this analysis, baseline Life's Simple 7 scores were normally distributed with a mean of 7.5 points (SD=2.1 points). Mean Life's Simple 7 score was slightly lower among participants who were excluded from analyses because they had no follow‐up 3‐test measure assessment (7.3 points). Mean score was higher in whites (7.9 points) than in blacks (6.9 points) and was similar within (7.5 points) and outside the stroke belt (7.6 points) (Figure2). Higher scores, indicating better CVH, were more common in men and those with higher education, higher income, and absence of CVDs (Table 2). The proportion of participants classified as having ICI on the 3‐test measure was similar by age, sex, race, and education category because those variables were used to define the stratum‐specific cut‐off scores for impairment. However, ICI was more common with lower income, residence in the stroke belt, and presence of CVD (Table 2). Overall, 3.2% of 17 761 participants experienced ICI. Standardized percentages were 4.6% (95% confidence interval [CI], 4.0, 5.2) in the lowest (least healthy) tertile of Life's Simple 7 score, 2.7% (95% CI, 2.3, 3.1) in the middle tertile, and 2.6% (95% CI, 2.1, 3.1) in the highest (most healthy) tertile. Adjusted ORs for ICI were nearly the same for the middle (OR, 0.65; 95% CI, 0.52, 0.81) and highest tertile (OR, 0.63; 95% CI, 0.51, 0.79) of Life's Simple 7 score, relative to the lowest tertile (Table 3). The association was similar in whites and blacks, as well as outside and within the stroke belt (Table 3). The association was also similar for different ages, women and men, and levels of education (data not shown).
Figure 2.
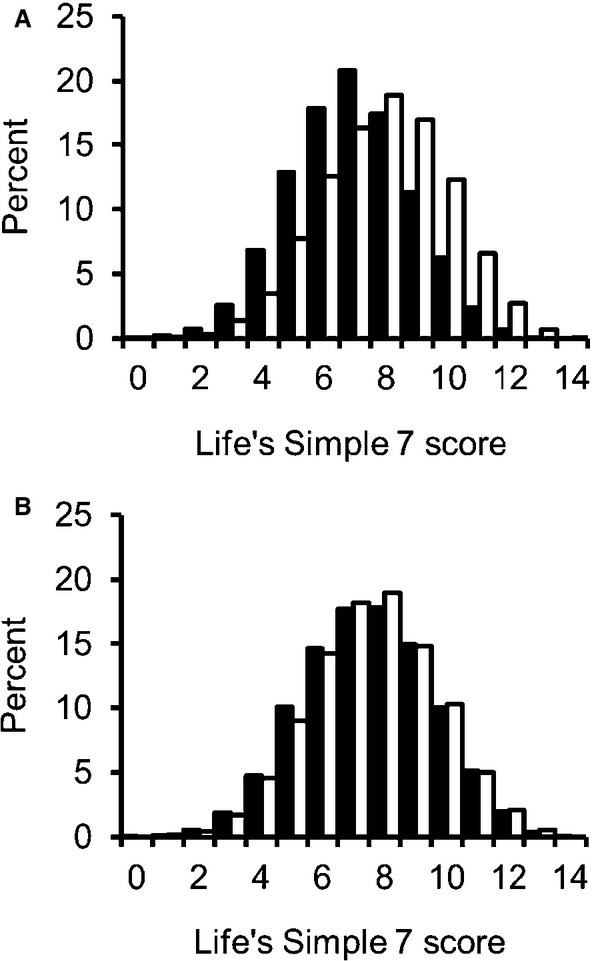
Unadjusted distributions of Life's Simple 7 score. A, Distributions by race: blacks (solid bars), whites (open bars). B, Distributions by geographic region: within the stroke belt (solid bars), outside the stroke belt (open bars).
Table 2.
Baseline Characteristics of REGARDS Participants by Life's Simple 7 Score Tertile and Incident Cognitive Impairment Status (N=17 761)
| Characteristic* | Life's Simple 7 Score Tertile* | Most Recent 3‐Test Measure | |||
|---|---|---|---|---|---|
| 0 to 6 Points (Low) N=5519 | 7 to 8 Points (Middle) N=6445 | 9 to14 Points (High) N=5797 | Impaired N=573 | Not Impaired N=17 188 | |
| Age, y, mean (SD) | 63.4 (8.3) | 64.4 (8.8) | 64.1 (9.2) | 65.1 (10.2) | 64.0 (8.7) |
| Male | 37.6 | 45.1 | 44.8 | 41.2 | 42.7 |
| Black | 47.6 | 37.7 | 23.0 | 33.5 | 36.1 |
| Education | |||||
| Less than high school | 12.8 | 8.8 | 5.2 | 8.2 | 8.9 |
| High school graduate | 29.5 | 26.2 | 19.8 | 24.3 | 25.2 |
| Some college | 29.5 | 26.6 | 25.6 | 28.3 | 27.2 |
| College graduate | 28.2 | 38.3 | 49.4 | 39.3 | 38.8 |
| Income | |||||
| <$20 000/y | 23.4 | 16.1 | 10.8 | 28.8 | 16.2 |
| $20 000 to $34 999/y | 30.0 | 26.6 | 22.3 | 26.2 | 26.2 |
| $35 000 to $74 999/y | 33.0 | 37.6 | 39.7 | 32.0 | 37.0 |
| ≥$75 000/y | 13.7 | 19.7 | 27.2 | 12.9 | 20.5 |
| Residing in stroke belt | 56.4 | 53.8 | 54.9 | 66.0 | 54.6 |
| Atrial fibrillation | 8.8 | 7.7 | 6.6 | 9.6 | 7.6 |
| Left ventricular hypertrophy | 6.2 | 4.9 | 3.0 | 6.6 | 4.6 |
| Atherosclerotic disease* | 21.7 | 19.0 | 14.8 | 25.6 | 18.2 |
REGARDS indicates REasons for Geographic And Racial Differences in Stroke; y, year.
Column percentages, unless otherwise noted.
Numbers of participants in each tertile are averaged across imputed data sets.
Atherosclerotic disease was defined as coronary artery disease, carotid artery disease, peripheral arterial disease, or transient ischemic attack.
Table 3.
Standardized Percentages and Adjusted Odds Ratios of Incident Cognitive Impairment on the 3‐Test Measure by Baseline Life's Simple 7 Score
| Life's Simple 7 Score | Incident Cognitive Impairment Cases* N=573 | Noncases* N=17 188 | Standardized Percentage* (95% CI) | Adjusted Odds Ratio* (95% CI) |
|---|---|---|---|---|
| Overall | ||||
| 0 to 6 points | 241 | 5278 | 4.6 (4.0, 5.2) | 1.00 (reference) |
| 7 to 8 points | 175 | 6270 | 2.7 (2.3, 3.1) | 0.65 (0.52, 0.81) |
| 9 to 14 points | 156 | 5641 | 2.6 (2.1, 3.1) | 0.63 (0.51, 0.79) |
| Whites | ||||
| 0 to 6 points | 146 | 2747 | 5.1 (4.3, 6.0) | 1.00 (reference) |
| 7 to 8 points | 110 | 3904 | 2.7 (2.1, 3.3) | 0.58 (0.44, 0.76) |
| 9 to 14 points | 125 | 4339 | 2.7 (2.2, 3.2) | 0.60 (0.46, 0.77) |
| Blacks | ||||
| 0 to 6 points | 95 | 2531 | 3.6 (2.7, 4.4) | 1.00 (reference) |
| 7 to 8 points | 66 | 2366 | 2.5 (1.9, 3.2) | 0.80 (0.52, 1.08) |
| 9 to 14 points | 31 | 1301 | 2.3 (1.4, 3.1) | 0.68 (0.36, 0.99) |
| P for race×LS7 tertile | 0.38 | |||
| Outside stroke belt | ||||
| 0 to 6 points | 78 | 2326 | 3.4 (2.6, 4.3) | 1.00 (reference) |
| 7 to 8 points | 65 | 2913 | 2.1 (1.6, 2.7) | 0.71 (0.50, 1.00) |
| 9 to 14 points | 52 | 2562 | 1.8 (1.2, 2.3) | 0.61 (0.42, 0.89) |
| Within stroke belt | ||||
| 0 to 6 points | 164 | 2951 | 5.5 (4.6, 6.4) | 1.00 (reference) |
| 7 to 8 points | 110 | 3357 | 3.2 (2.6, 3.9) | 0.62 (0.45, 0.79) |
| 9 to 14 points | 104 | 3078 | 3.4 (2.6, 4.2) | 0.64 (0.47, 0.82) |
| P for region×LS7 tertile | 0.77 | |||
CI indicates confidence interval.
Numbers of participants in each tertile are averaged across imputed data sets and may not sum to total as a result of rounding.
Percentages were standardized by age group, sex, race, and education category, except race‐specific percentages, which were not standardized by race.
Odds ratios were adjusted for age, sex, race, education category, income category, region, atrial fibrillation, left ventricular hypertrophy, atherosclerotic disease, and time interval from baseline to most recent 3‐test measure, except race‐specific odds ratios, which were not adjusted for race, and region‐specific odds ratios, which were not adjusted for region.
Standardized percentages of participants with ICI across tertiles of health behavior score and biological health factor score are shown in Figure3. In general, higher health behavior scores and higher biological health factor scores were both associated with lower incidence of cognitive impairment. However, patterns were not always consistent across the middle tertiles. In a model with all 7 individual Life's Simple 7 components included simultaneously, associations with ICI were strongest for smoking, BMI, and fasting glucose (Table 4).
Figure 3.
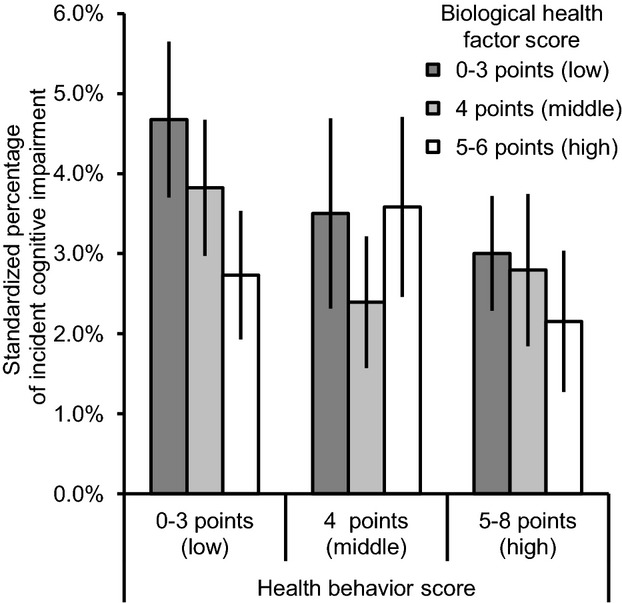
Standardized percentages of incident cognitive impairment by baseline health behavior score and biological health factor score. Health behavior score and biological health factor score were divided into tertiles. Percentages were standardized by age group, sex, race, and education category. Error bars represent 95% confidence intervals.
Table 4.
Adjusted Odds Ratios of Incident Cognitive Impairment on the 3‐Test Measure by Baseline Life's Simple 7 Components
| Life's Simple 7 Component | Adjusted Odds Ratio* (95% CI) |
|---|---|
| Smoking | |
| Poor | 1.00 (reference) |
| Intermediate or ideal* | 0.77 (0.60, 0.98) |
| Healthy diet score | |
| Poor | 1.00 (reference) |
| Intermediate or ideal* | 0.90 (0.68, 1.18) |
| Physical activity | |
| Poor | 1.00 (reference) |
| Intermediate | 0.88 (0.72, 1.08) |
| Ideal | 0.94 (0.76, 1.17) |
| Body mass index | |
| Poor | 1.00 (reference) |
| Intermediate | 0.77 (0.63, 0.95) |
| Ideal | 0.71 (0.56, 0.91) |
| Blood pressure | |
| Poor | 1.00 (reference) |
| Intermediate | 0.79 (0.64, 0.97) |
| Ideal | 0.86 (0.65, 1.14) |
| Total cholesterol | |
| Poor | 1.00 (reference) |
| Intermediate | 1.08 (0.80, 1.45) |
| Ideal | 1.04 (0.77, 1.40) |
| Fasting glucose | |
| Poor | 1.00 (reference) |
| Intermediate | 0.79 (0.59, 1.07) |
| Ideal | 0.72 (0.53, 0.97) |
CI indicates confidence interval.
Odds ratios were adjusted for the other Life's Simple 7 components shown in the table plus age, sex, race, education category, income category, region, atrial fibrillation, left ventricular hypertrophy, atherosclerotic disease, and time interval from baseline to most recent 3‐test measure.
Intermediate and ideal categories for smoking were combined because of low numbers in the intermediate category. Intermediate and ideal categories for healthy diet score were combined because of low numbers in the ideal category.
In a sensitivity analysis using complete data, adjusted ORs of ICI were lower than in the main analysis. ORs were 0.57 (0.44, 0.75) for the middle tertile of Life's Simple 7 score and 0.52 (0.39, 0.69) for the highest tertile, relative to the lowest tertile.
Discussion
In this prospective cohort study of black and white adults in the United States aged 45 and older, free of stroke and baseline cognitive impairment, mid‐range to high Life's Simple 7 scores at baseline were associated with lower incidence of clinically relevant cognitive impairment on a 3‐test measure of verbal learning, memory, and fluency. Rather than a dose‐response pattern across the range of Life's Simple 7 scores, we observed that associations with ICI were the same for the highest tertile of Life's Simple 7 score and the middle tertile, relative to the lowest tertile. This pattern suggests that even intermediate levels of CVH are preferable to low levels of CVH. This is an encouraging message for population health promotion, because intermediate CVH is a more realistic target than ideal CVH for many individuals.
The purpose of the Life's Simple 7 metric is to summarize several modifiable factors into a single score to promote and measure individual‐ and population‐level improvements in CVH.4 Previous research has shown that better CVH, according to Life's Simple 7 factors, is associated with lower risk for stroke.5–6 In the current study, middle to high CVH was also linked with lower risk for future cognitive impairment in the absence of clinical stroke. We excluded participants who experienced stroke before their follow‐up 3‐test measure of cognitive function. Therefore, our results suggest that the mechanism linking CVH with cognition is something other than clinical stroke. Our findings do not rule out covert or subclinical stroke as a potential mechanism.
Our results complement the findings of an earlier analysis in REGARDS that demonstrated an association of the Framingham Stroke Risk Profile with the risk for ICI according to the SIS.2 Although the Framingham Stroke Risk Profile and Life's Simple 7 both reflect CVH, Life's Simple 7 places a stronger emphasis on modifiable risk factors and therefore may have more direct implications for health promotion and disease prevention.
In the Coronary Artery Risk Development in Young Adults (CARDIA) study, better CVH in adults aged 18 to 30 was associated with better performance on cognitive function tests 25 years later, highlighting the importance of early life health behaviors and health factors.22 Our study, in which baseline Life's Simple 7 score was determined at an average age of 64 years, complements the findings of the CARDIA study by emphasizing the importance of mid‐ to late‐life health behaviors and health factors. Taken together, the findings from REGARDS and CARDIA suggest that both older adult and younger adult populations could be appropriately targeted for lifestyle interventions aimed at reducing risk for cognitive decline.
The distribution of Life's Simple 7 scores and association of Life's Simple 7 score with ICI were similar in stroke belt residents and non‐stroke‐belt residents. These findings suggest that there must be explanations other than poor CVH for lower cognitive function observed among residents of the stroke belt.23 Although the higher incidence of cognitive impairment in the stroke belt may result, in part, from higher rates of clinical stroke, a previous analysis from REGARDS suggested that other factors are at play, perhaps subclinical stroke.23 The distribution of Life's Simple 7 scores was slightly higher in whites than in blacks. However, race was not an effect modifier of the association of Life's Simple 7 with ICI, suggesting that the value of CVH in relation to cognitive health may be similar for both races.
A limitation of our study was a nonrandom pattern of missing follow‐up data, a type of selection bias that commonly occurs in longitudinal studies of cognitive outcomes.24 Participants who experience cognitive impairment during follow‐up are less likely to complete follow‐up cognitive assessments. As a result, observed associations of Life's Simple 7 with ICI may differ from the true associations in the population. Another limitation was that Life's Simple 7 components, cognitive function, and covariates may have been measured with error. Such errors were likely random, potentially diluting the associations of interest or the adjustment for confounding. Although we adjusted for education and income levels, these adjustment variables may not capture aspects of the underlying social determinants of health behaviors and cognitive impairment, so there could be residual confounding. Also, we modified the AHA definitions of diet and physical activity components based on data available in REGARDS, which may have affected the Life's Simple 7 score for some participants. Another limitation was that we lacked data on changes in Life's Simple 7 scores during follow‐up.
A major strength of our study was that we used a clinically meaningful definition of ICI derived from 3 cognitive tests. Our 3‐test measure accounted for age, sex, race, and educational attainment and required impairment to be pervasive across at least 2 cognitive domains17 that have been consistently associated with performance on instrumental activities of daily living.25–26 This approach allowed us to identify the most impaired participants for comparison with remaining participants. Additional strengths of our study were exclusion of participants who experienced clinical stroke, adjustment for potential confounding, large sample size that led to excellent precision, use of multiple imputation for missing data to reduce bias and increase precision, assessment of race and geographic region as possible effect modifiers, and the population‐based sample, which makes the results generalizable to middle‐aged and older blacks and whites across the United States.
In conclusion, these findings provide evidence that CVH is an important factor in cognitive health. We observed that intermediate and high levels of CVH were associated with similarly low incidence of cognitive impairment, rather than a dose response across the range of CVH. Based on these findings, we hypothesize that the AHA's strategic efforts to improve CVH from poor to intermediate or higher levels could lead to reductions in cognitive decline, and we believe further research addressing this hypothesis is warranted.
Sources of Funding
This research project is supported by a cooperative agreement (U01 NS041588) from the National Institute of Neurological Disorders and Stroke (NINDS), NIH, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH. Representatives of the funding agency have been involved in the review of the manuscript, but not directly involved in the collection, management, analysis, or interpretation of the data.
Disclosures
None.
Acknowledgments
The authors thank Aleena Mosher for assistance with data analysis. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This research was presented at the AHA Epidemiology and Prevention Conference, New Orleans, LA, March 19–22, 2013. Dr. Thacker has received a Clinical Research Loan Repayment Program award from the National Heart, Lung and Blood Institute, National Institutes of Health (NIH).
References
- 1.Sacco RL. Achieving ideal cardiovascular and brain health: opportunity amid crisis: presidential address at the American heart Association 2010 Scientific Sessions. Circulation. 2011; 123:2653-2657. [DOI] [PubMed] [Google Scholar]
- 2.Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, Kissela BM, Kelley BJ, Kennedy R, Moy CS, Howard V, Howard G. Vascular risk factors and cognitive impairment in a stroke‐free cohort. Neurology. 2011; 77:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009; 66:1210-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010; 121:586-613. [DOI] [PubMed] [Google Scholar]
- 5.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's simple 7 and risk of incident stroke: the Reasons for Geographic and Racial Differences in Stroke study. Stroke. 2013; 44:1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: The Northern Manhattan Study. Circulation. 2012; 125:2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011; 57:1690-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005; 25:135-143. [DOI] [PubMed] [Google Scholar]
- 9.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986; 124:453-469. [DOI] [PubMed] [Google Scholar]
- 10.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased c‐reactive protein for cardiovascular risk stratification in black and white men and women in the us. Clin Chem. 2009; 55:1627-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating Cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic And Racial Differences in Stroke study. J Electrocardiol. 2010; 43:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six‐item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002; 40:771-781. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39:1159-1165. [DOI] [PubMed] [Google Scholar]
- 14.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests. 20063rd edNew York, NY: Oxford University Press [Google Scholar]
- 15.Ivnik RJ, Smith GE, Petersen RC, Boeve BF, Kokmen E, Tangalos EG. Diagnostic accuracy of four approaches to interpreting neuropsychological test data. Neuropsychology. 2000; 14:163-177. [DOI] [PubMed] [Google Scholar]
- 16.Frerichs RJ, Tuokko HA. A comparison of methods for measuring cognitive change in older adults. Arch Clin Neuropsychol. 2005; 20:321-333. [DOI] [PubMed] [Google Scholar]
- 17.Schinka JA, Loewenstein DA, Raj A, Schoenberg MR, Banko JL, Potter H, Duara R. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am J Geriatr Psychiatry. 2010; 18:684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011; 69:619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007; 16:219-242. [DOI] [PubMed] [Google Scholar]
- 20.StataCorp. Stata: Release 12. Multiple‐Imputation Reference Manual. 2011College Station, TX: StataCorp [Google Scholar]
- 21.Barzi F, Woodward M. Imputations of missing values in practice: results from imputations of serum cholesterol in 28 cohort studies. Am J Epidemiol. 2004; 160:34-45. [DOI] [PubMed] [Google Scholar]
- 22.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Jr, Zhu N, Lloyd‐Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013; 73:170-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadley VG, Unverzagt FW, McGuire LC, Moy CS, Go R, Kissela B, McClure LA, Crowe M, Howard VJ, Howard G. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011; 70:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008; 19:440-447. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan L, Giovanello K. Executive function in daily life: age‐related influences of executive processes on instrumental activities of daily living. Psychol Aging. 2010; 25:343-355. [DOI] [PubMed] [Google Scholar]
- 26.Greenaway MC, Duncan NL, Hanna S, Smith GE. Predicting functional ability in mild cognitive impairment with the Dementia Rating Scale‐2. Int Psychogeriatr. 2012; 24:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]


