Abstract
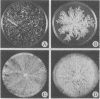
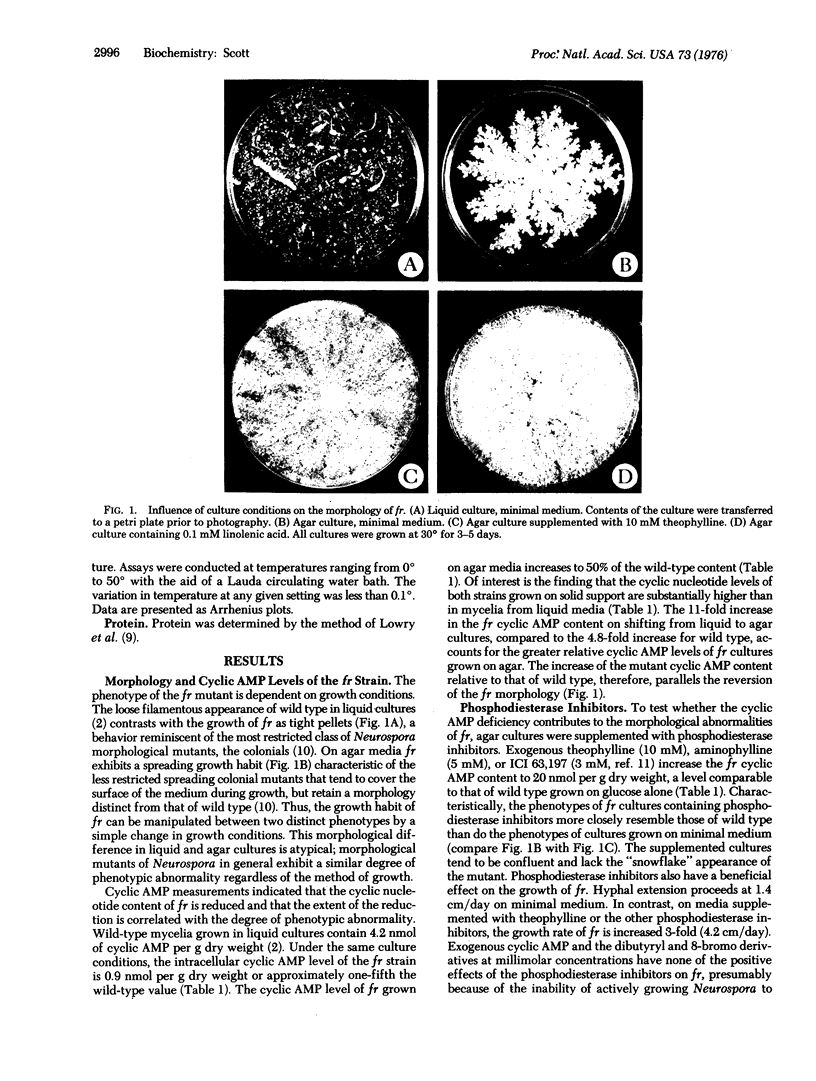
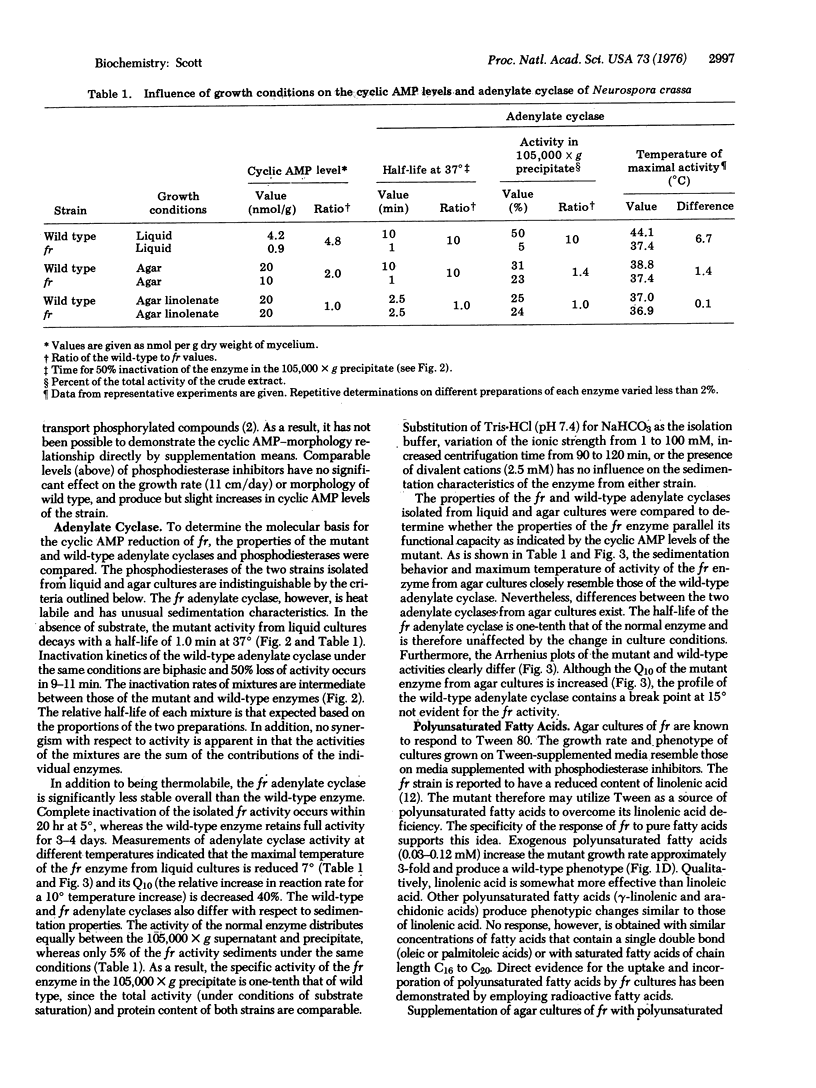
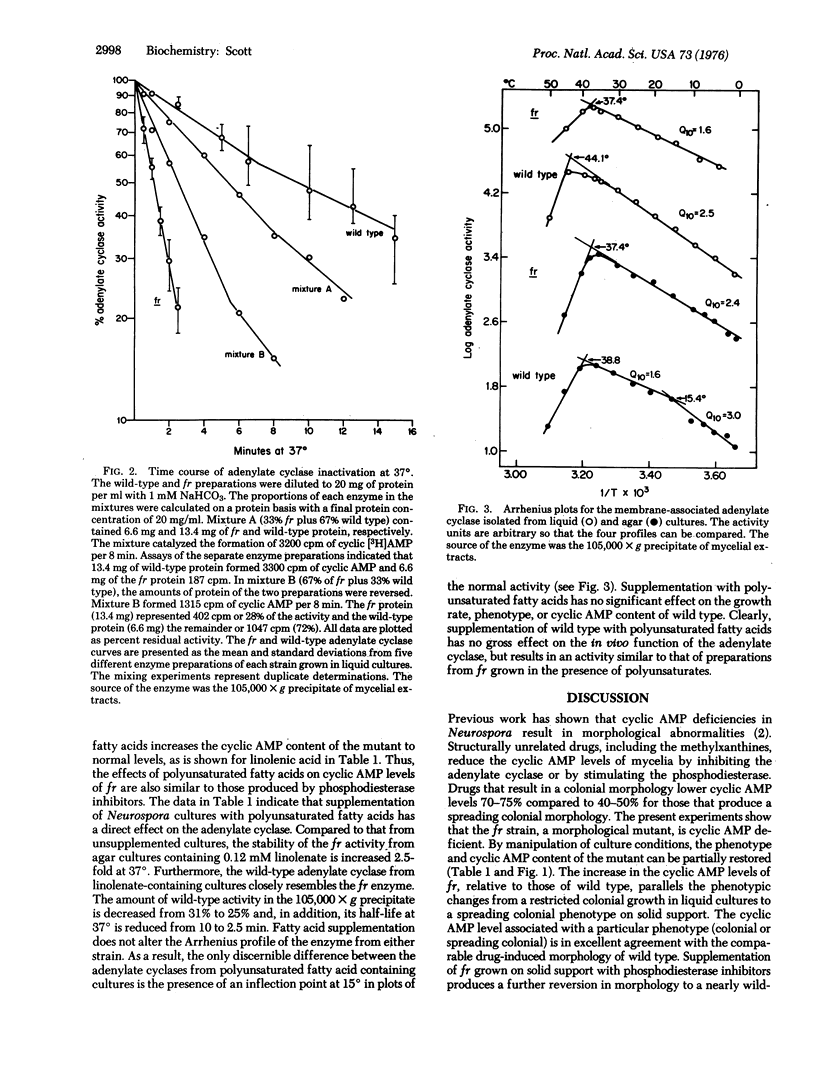
Depending on growth conditions, the adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels of the fr mutant, a morphologically aberrant strain of Neurospora crassa, are reduced 2- to 5-fold. By taking advantage of the differences in phenotype of fr in liquid and agar cultures and the positive response of fr grown on solid support to exogenous theophylline, a relationship between the degree of morphological abnormality and intracellular cyclic AMP levels of the mutant is observed. Progressive restoration of the fr phenotype toward a normal state is paralleled by increases in cyclic nucleotide content. Striking differences in the sedimentation and thermal characteristics of the fr and wild-type adenylate cyclases [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] are observed. Approximately 50% of the normal activity sediments at 105,000 X g compared to 5% of the mutant enzyme. In addition, the overall stability of the fr adenylate cyclase is significantly decreased and its rate of inactivation at 37 degrees in the absence of substrate is 10-fold greater than the wild-type adenylate cyclase. Arrhenius plots also indicated that the Q10 (increase in rate per 10 degrees temperature increase) and the temperature of maximal activity of the fr enzyme are reduced. Supplementation of fr agar cultures with linolenic acid results in an elevated cyclic AMP content and a wild-type-like morphology similar to that obtained with inhibitors of phosphodiesterase (3':5'-cyclic AMP 5'-nucleotidohydrolase, EC 3.1.4.17). An increased thermostability of the fr adenylate cyclase occurs on linolenate enrichment of the mutant. It is concluded that the cyclic AMP deficiency is at least partially responsible for the fr phenotype and that this reduction results from a membrane defect that affects adenylate cyclase function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner J. T. Aggregation and differentiation in the cellular slime molds. Annu Rev Microbiol. 1971;25:75–92. doi: 10.1146/annurev.mi.25.100171.000451. [DOI] [PubMed] [Google Scholar]
- Brody S., Nyc J. F. Altered fatty acid distribution in mutants of Neurospora crassa. J Bacteriol. 1970 Nov;104(2):780–786. doi: 10.1128/jb.104.2.780-786.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Adenylate cyclase activity in Neurospora crassa. I. General properties. J Biol Chem. 1972 Nov 10;247(21):6873–6879. [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Leakage of cyclic AMP from human diploid fibroblasts in tissue culture. Nat New Biol. 1973 Nov 28;246(152):119–120. doi: 10.1038/newbio246119a0. [DOI] [PubMed] [Google Scholar]
- Garnjobst L., Tatum E. L. A survey of new morphological mutants in Neurospora crassa. Genetics. 1967 Nov;57(3):579–604. doi: 10.1093/genetics/57.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner P. W., Keirns J. J., Bitensky M. W. A temperature-sensitive change in the energy of activation of hormone-stimulated hepatic adenylyl cyclase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1785–1789. doi: 10.1073/pnas.70.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey G. S. Restoration of glucagon responsiveness of solubilized myocardial adenyl cyclase by phosphatidylserine. Biochem Biophys Res Commun. 1971 Apr 2;43(1):108–113. doi: 10.1016/s0006-291x(71)80093-1. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Kozyreff V., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. VI. Evidence for a role of membrane lipids. J Biol Chem. 1971 Jul 25;246(14):4447–4454. [PubMed] [Google Scholar]
- Réthy A., Tomasi V., Trevisani A. The role of lipids in the activity of adenylate cyclase of rat liver plasma membranes. Arch Biochem Biophys. 1971 Nov;147(1):36–40. doi: 10.1016/0003-9861(71)90306-7. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Solomon B. Adenosine 3',5'-cyclic monophosphate and morphology in Neurospora crassa: drug-induced alterations. J Bacteriol. 1975 May;122(2):454–463. doi: 10.1128/jb.122.2.454-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Solomon B. Cyclic 3',5'-AMP phosphodiesterase of Neurospora crassa. Biochem Biophys Res Commun. 1973 Aug 6;53(3):1024–1030. doi: 10.1016/0006-291x(73)90194-0. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Liepkalns V. A., Spector A. A. Changes in (Na+ + K+)-ATPase activity of Ehrlich ascites tumor cells produced by alteration of membrane fatty acid composition. Biochemistry. 1976 Feb 24;15(4):892–897. doi: 10.1021/bi00649a026. [DOI] [PubMed] [Google Scholar]