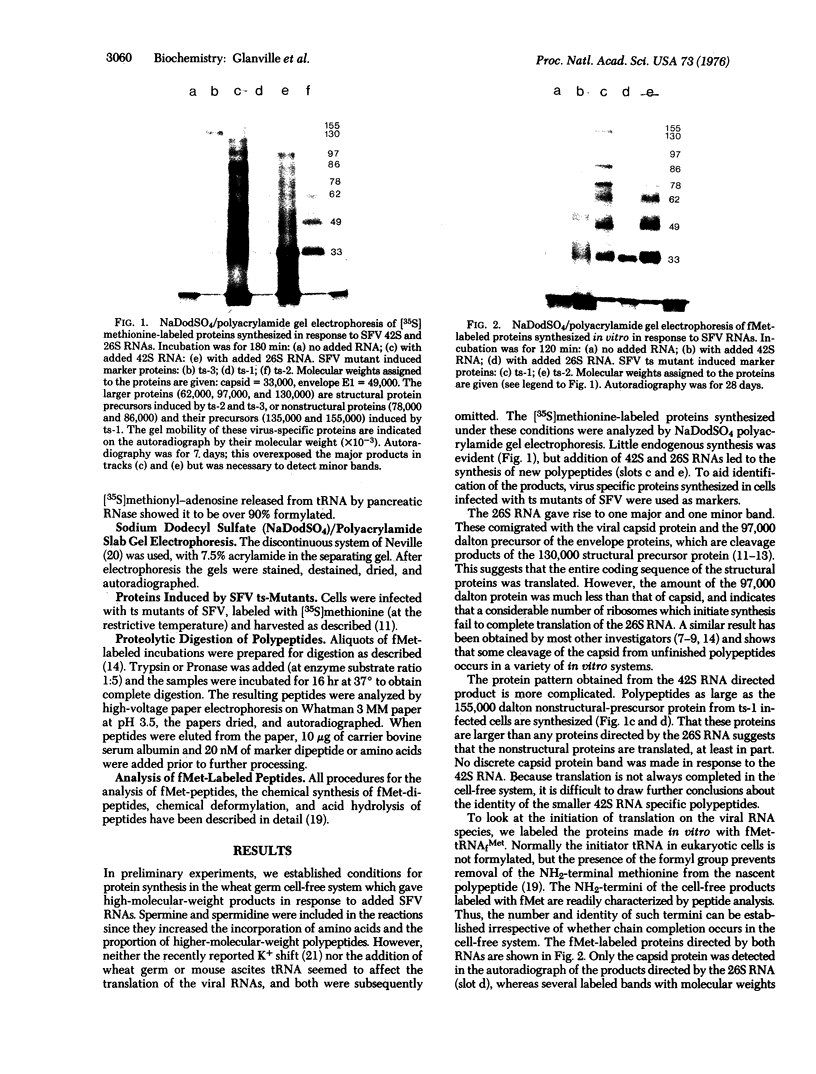
Abstract
The proteins synthesized in vitro in response to 42S and 26S RNAs from Semliki Forest virus were labeled with formyl-[35S]methionine from initiator tRNA. One protein which comigrated with viral capsid protein was labeled under the direction of 26S RNA, and only one labeled peptide was detected after digestion with trypsin. Further digestion with pronase gave rise to the dipeptide fMet-AsN. Several labeled polypeptides were found in the 42S RNA directed product and these had molecular weights of up to 150,000. However, tryptic digestion of the product yielded only one formylmethionyl-labeled peptide, which had a different mobility from that directed by the 26S RNA. Further digestion with pronase gave a single dipeptide, fMet-Ala. This indicates that nonstructural proteins as large as 150,000 daltons are probably synthesized from one initiation site on the 42S RNA. Translation starting from the internal initiation site on the 42S RNA, which is equivalent to that on the 26S RNA, could not be detected under the conditions used. Internal initiation sites which are similarly inactive have also been detected in other viral RNAs (e.g., brome mosaic virus, tobacco mosaic virus, and polyoma 19S RNA) and this suggests that, although eukaryotic mRNAs can contain more than one initiation site for protein synthesis, only the site nearer the 5' terminus is active in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda R., Villa-Komaroff L., Lodish H. F., Schlesinger M. Initiation sites for translation of sindbis virus 42S and 26S messenger RNAs. Cell. 1975 Oct;6(2):215–222. doi: 10.1016/0092-8674(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. In vitro synthesis of structural proteins of Semliki Forest virus directed by isolated 26 S RNA from infected cells. FEBS Lett. 1974 Jun 15;42(3):327–330. doi: 10.1016/0014-5793(74)80757-x. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Initiation of synthesis of the structural proteins of Semliki Forest virus. J Mol Biol. 1975 Oct 5;97(4):401–411. doi: 10.1016/s0022-2836(75)80050-7. [DOI] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H., Dubin D. T., Stollar V. 5' nucleotide sequence of sindbis viral RNA. J Virol. 1975 Jan;17(1):149–159. doi: 10.1128/jvi.17.1.149-159.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Proteins synthesized by Semliki Forest virus and its 16 temperature-sensitive mutants. J Virol. 1975 Aug;16(2):388–396. doi: 10.1128/jvi.16.2.388-396.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmi B. E., Glanville N., Keränen S., Läriäinen L. Tryptic peptide analysis on nonstructural and structural precursor proteins from Semliki Forest virus mutant-infected cells. J Virol. 1975 Dec;16(6):1615–1629. doi: 10.1128/jvi.16.6.1615-1629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmi B. E., Käriäinen L. Sequential translation of nonstructural proteins in cells infected with a Semliki Forest virus mutant. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1936–1940. doi: 10.1073/pnas.73.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. V. Polyribosomes and mRNA in infected cells. J Virol. 1974 Sep;14(3):552–559. doi: 10.1128/jvi.14.3.552-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Smith A. E. The initiation of protein synthesis directed by the RNA from encephalomyocarditis virus. Eur J Biochem. 1973 Mar 1;33(2):301–313. doi: 10.1111/j.1432-1033.1973.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Wheeler T., Glanville N., Käriäinen Translation of Semliki-Forest-virus 42-S RNA in a mouse cell free system to give virus-coat proteins. Eur J Biochem. 1974 Nov 1;49(1):101–110. doi: 10.1111/j.1432-1033.1974.tb03815.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Bollen T. H., Bloemedal H. Formyl-Met-tRNA f Met, a questionable initiator tRNA in eukaryotic systems. Mol Biol Rep. 1974 Dec;1(8):471–475. doi: 10.1007/BF00360674. [DOI] [PubMed] [Google Scholar]
- Tuomi K., Kädäridäinen L., Söderlund H. Quantitation of Semlike Forest virus RNAs in infected cells using 32-P equilibrium labelling. Nucleic Acids Res. 1975 Apr;2(4):555–565. doi: 10.1093/nar/2.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Beato M., Hackemack B. A. Translation of 26 S virus-specific RNA from Semliki Forest virus-infected cells in vitro. Virology. 1974 Sep;61(1):120–128. doi: 10.1016/0042-6822(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Wengler G. Comparative studies on polyribosomal, nonpolyribosome-associated and viral 42 S RNA from BHK 21 cells infected with Semliki Forest virus. Virology. 1975 Jun;65(2):601–605. doi: 10.1016/0042-6822(75)90068-9. [DOI] [PubMed] [Google Scholar]