Abstract
Human immunodeficiency virus-1 (HIV-1) infection affects the striatum resulting in gliosis and neuronal losses. To determine whether HIV-1 proteins induce striatal neurotoxicity through an apoptotic mechanism, mouse striatal neurons isolated on embryonic day 15 and the effects of HIV-1 Tat1-72 and gp120 on survival were assessed in vitro. Mitochondrial release of cytochrome c, caspase-3 activation, and neuron survival, as well as an alternative apoptotic pathway involving endonuclease G (endo G), were assessed at 4 h, 24 h, 48 h and/or 72 h using enzyme assays and immunoblotting. Both HIV-1 Tat and gp120 significantly increased caspase-3 activation in a concentration-dependent manner in striatal neurons at 4 h following continuous exposure in vitro. Tat1-72 and gp120 caused significant neuronal losses at 48 h and/or 72 h. Tat1-72 increased cytochrome c release, and caspase-3 and endo G activation at 4 h, 24 h, and/or 72 h. By contrast, gp120 increased caspase-3 activation, but failed to increase cytochrome c or endo G levels in the cytoplasm at 4 h, 24 h, and/or 72 h. The cell permeant caspase inhibitor Z-DEVD-FMK significantly attenuated gp l20-induced, but not Tat1-72-induced, neuronal death suggesting that gp 120 acts in large part through the activation of caspase(s), while Tat1-72 induced neurotoxicity was accompanied by activating an alternative pathway involving endo G. Thus, although Tat1-72 and gp 120 induced significant neurotoxicity, the nature of the apoptotic events preceding death differed. Collectively, our findings suggest that HIV-1 proteins are intrinsically toxic to striatal neurons and the pathogenesis is mediated through separate actions involving both caspase-3 and endo G.
Keywords: Caspase-3, endonuclease G, cytochrome c, neurotoxicity
INTRODUCTION
Human immunodeficiency virus-1 (HIV-1) dementia is a neurodegenerative syndrome characterized by cognitive decline, personality change, and motor deficits in humans infected with HIV-1 (McArthur et al, 1993; Lipton and Gendelman, 1995). HIV encephalitis (HIVE) often accompanies HIV dementia and is characterized by prominent microglial activation, neuronal losses, dendritic pruning, and decreased density of synapses (Masliah et al, 1996; Lipton, 1997). Apoptotic changes are seen with HIVE in both neurons and non-neuronal cells (Ramirez et al, 2001; Bonavia et al, 2001; Corasaniti et al, 2001; Adle-Biassette et al, 1995; Gelbard et al, 1995; Petito and Roberts, 1995; Shi et al, 1996; Kaul et al, 2001; Shi et al, 1998; Park et al, 2001). HIV-1 is neurotoxic by inducing inflammation and through the direct release of toxic viral proteins such as Nef, Vpr, gp120 and Tat (Haughey et al, 2001; Brenneman et al, 1988; Dreyer et al, 1990; Adamson et al, 1996; New et al, 1997; Kruman et al, 1998; Yeung et al, 1998; Huang and Bond, 2000; Trillo-Pazos et al, 2000; Nath, 2002). The neurotoxicity of gp120 has been demonstrated both in primary human neuronal cultures (Lannuzel et al, 1997; Yeung et al, 1995) and in transgenic mice (Toggas et al, 1994). Gp120 is thought to be intrinsically neurotoxic through an apoptotic mechanism (Barillari et al, 1993; Albini et al, 1996a,b; Bagetta et al, 1996b; Hesselgesser et al, 1998; Marzo et al, 1998; Meucci et al, 1998; Zheng et al, 1999) and may also act via inflammatory cytokines (Bagetta et al, 1996a, 1999; Lipton, 1997).
HIV-1 Tat protein is not only an intracellular transcriptional activator, but is also secreted by infected cells and is capable of acting as a proto-cytokine. HIV-1 Tat can bind to and activate specific tyrosine kinase receptors including the Flk-1/kinase insert domain (Flk-1/KDR) receptor for vascular endothelial growth factor (VEGF) (Albini et al, 1996b) and several classes of integrins (Albini et al, 1996a; Barillari et al, 1993; Ganju et al, 1998; Vogel et al, 1993). HIV-1 Tat can trigger apoptosis in PC-12 neuronal cells through the induction of tumor necrosis factor-a release (New et al, 1998; Shi et al, 1998). Tat is important in the pathogenesis of Kaposi’s sarcoma and in the neuroinflammatory changes seen in patients with HIV dementia (Deregibus et al, 2002; Kaaya et al, 1996).
Apoptosis is often accompanied by the activation of caspases (Creagh and Martin, 2001; Schimmer et al, 2001; Stennicke and Salvesen, 2000). Caspase-3 is a member of the CED-3 subfamily of caspases that is activated in several neurodegenerative disorders (Namura et al, 1998; Hartmann et al, 2000; Su et al, 2000). Active caspase-3 has been detected by Western blot in human fetal neural cultures exposed to gpl20 (Zheng et al, 1999); however, the specific cell population undergoing caspase activation was not identified. Postmortem studies on the brains of pediatric patients with HIV dementia showed increased procaspase-3 immunoreactivity in neurons (James et al, 1999).
The basal ganglia are especially vulnerable to HIV infection (Berger and Nath, 1997; Nath et al, 2001) and this may result from unique phenotypic characteristics of striatal neurons compared to other neuron types. For example, striatal neurons are quite susceptible to AMPA-receptor-mediated excitotoxic stimuli, but far less sensitive to NMDA-induced excitotoxicity (Goody et al, 2003; Singh et al, 2003). The basal ganglia are also a principal target for drug abuse and may be preferentially susceptible to interactions between HIV and drug abuse (Nath et al, 2001; Nath et al, 2002; Nath, 2002). For this reason, we examined the mechanisms underlying Tat and gp120 toxicity in striatal neurons and found that Tat1-72 and gp120 induced apoptotic cell death in mouse striatal neurons.
Materials and Methods
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM:F-12), B27, antibiotic-antimycotic (penicillin/streptomycin/amphoterin) were purchased from Gibco/Life Technologies, Grand Island, NY, USA. Purified mouse anti-cytochrome c monoclonal antibody was obtained from BD PharMingen, USA. Insulin, linoleic acid, and protein G beads were purchased from Sigma-Aldrich (St. Louis, MO, USA). Caspase-3 substrates, including Ac-Asp-Glu-Val-Asp- 7-amino-4methylcoumarin (Ac-DEVD- AMC) and a reversible aldehyde inhibitor, Ac-DEVD-CHO (Ac-Asp-Glu-Val-Asp-aldehyde), were obtained either from Bachem Bioscience, Inc. (King of Prussia, PA, USA) or from Molecular Probes, Inc. (Eugene, OR, USA). A cell-permeable pan-caspase inhibitor II (Z-DEVD-FMK) was purchased from Calbiochem-Novabiochem Corporation (San Diego, CA, USA) and used at 30 or 50 µM concentrations. The BCA protein assay kit and reagents were purchased from Pierce (Rockford, IL, USA). Bio-Rad Ready gels (10% and 12%) and Kaleidoscope prestained standards were obtained from Bio-Rad Laboratories (Hercules, CA, USA). An ECL Western blot analysis system containing peroxidase-labeled anti-mouse or anti-rabbit antibodies was purchased from Amersham Pharmacia Biotech (Buckinghamshire, England). Timed, pregnant ICR mice were obtained from Charles River (Charles River, MA, USA).
Viral Proteins
The tat gene encoding amino acids 1–72 was amplified from HIVbru obtained from Dr. Richard Gaynor through the AIDS repository at the NIH and inserted into an E. coli (PinPoint Xa-2) vector (Promega). Recombinant Tat1-72 was prepared as described previously (Ma and Nath, 1997) with minor modifications (Gurwell et al, 2001). Recombinant gp120 from HIV-SF2 made in CHO cells was a gift from Chiron Inc. (Emeryville, CA, USA), and purified and handled as previously described (Holden et al, 1999; Haughey, 2002).
Striatal Neuronal Cultures
Striatal neuronal cultures from embryonic day 15 (E 15) ICR mice were prepared as described before (Goody et al, 2003). Striata were dissected from ICR pups, dissociated, and striatal neurons were grown for 5–7 days in vitro (DIV) in serum-free DMEM/F-12 medium supplemented with 2% B-27, 1 µg/ml linoleic acid, 25-µg/ml insulin, and 1% antibiotic/antimycotic. Striatal neurons were seeded at identical densities (4 × 105 cells/cm2) and grown on poly-D-lysine (0.1 mg/ml) coated Costar-24 well-plates in an incubator maintained at 35–36 °C in 5% CO2 95% air and high humidity. The cultures described in the present study are enriched in medium spiny neurons, which comprise about 85–95% of neurons in the mouse striatum, and contain about 3–8% astroglia and about 0.4% monocytes/microglia (Hauser, unpublished), but which differ from an alternative mixed neuronal-glial striatal culture system previously characterized by us (Gurwell et al, 2001).
Neuronal Viability
To assess neuronal losses following continuous Tat1-72 (100 nM) or gp120 (500 pM) exposure, time-lapse, repeated measures analyses of digital photomicrographs of individual neurons were performed as previously reported (Hauser et al, 1999; Goody et al, 2003). Briefly, neuronal viability was assessed by repeatedly photographing the same neurons at 24-h intervals. Glass coverslips were lightly scored with a diamond marker, before placing into 12-well plates, to aid in finding the same field of neurons (neurons were plated on the opposite side from the score). This method of sampling is systematic, but arbitrary, since all neurons within a region predetermined before the onset of the experiment are assessed. The procedure also selects against background cell death that occurs in many primary neural culture systems, since the small proportion of dying cells are identified before the onset of treatment and are excluded from further study a priori without introducing experimenter bias. Neurons were photographed digitally using a Spot 2 camera (Diagnostic Instruments, Sterling Heights, MI) and Nikon Diaphot inverted microscope with phase contrast optics and a 20× objective. About 50–75 neurons were arbitrarily sampled per culture. At least 4–6 cultures, each consisting of cells isolated and maintained from separate mice were assessed per experimental group. Dying neurons were identified by the fragmentation and destruction of the cell body and neurites, as well as the loss of nuclear structure and/or shrinkage (pyknosis) (Fig. 4); detailed criteria for neuronal losses have been previously described (Hauser et al, 1999; Goody et al. 2003).
Figure 4.
Fluorescent photomicrographs showing the co-localization neuronal nuclear (NeuN) marker and ethidium monoazide (EMA) in striatal neuron cultures at 24 h following exposure to medium alone (A), gp120 (500 pM) with or without Z-DEVD-FMK (DEVD) (50 µM) (B,C), or DEVD alone (50 µM) (D). Cell cultures were incubated with DEVD for 4 h prior to exposure to gp120. gp120 treatment increased the proportion of dying neurons and the toxicity was prevented by co-administering DEVD (see Table 1). Viable NeuN immunofluorescence neurons (arrowheads) appear green and exclude EMA (red fluorescence) from their nuclei. Non-viable neurons (arrows) fail to exclude EMA (red) and their nuclei appear yellow. Tat (100 nM) was also assayed but showed no effect at 24 h (see Table 1). The large photomicrographs (right-side) are composite images of NeuN reactivity (upper left insets) and EMA (lower left insets); scale bar = 20 µm.
The neuronal identity of the dying cells was further confirmed by exposing cells to 0.5 µg/ml ethidium monoazide (EMA) in Dulbecco’s PBS for 30 min, permanently binding the EMA to the dying cells through photoaffinity labeling, and co-detecting EMA-positive cells with neuron-specific antigenic markers as previously described (Gurwell et al, 2001). Briefly, EMA is excluded from living cells, but intercalates to the DNA of dying cells, where it can be permanently linked via photoaffinity by exposure to a 45 W fluorescent light (15 cm distance) for 30 minutes at room temperature. Cells were then fixed with Zamboni’s fixative containing 3% paraformaldehyde. Cell cultures were characterized by immunoreactivity to the neuronal markers, i.e., neuronal nuclear protein (NeuN) (mouse monoclonal anti-NeuN IgG, Chemicon, Temecula, CA, USA; at 1:500 dilution) and PGP 9.5 (rabbit polyclonal anti-PGP 9.5 IgG, Chemicon; 1:1800 dilution).
Caspase-3 Activity
Caspase-3 activity was measured as previously described (Rigamonti et al, 2000). Briefly, embryonic mouse striatal neurons grown for 5–7 DIV in serum-free DMEM/F12 medium were treated with vehicle-treated media alone, Tat1-72 (100 nM), or gp120 (500 pM). Cells were harvested at 4 h, 24 h, or 72 h after the treatments in ice-cold harvesting buffer (25 mM HEPES, pH 7.5, 5 mM EDTA, 1 mM EGTA, 5 mM magnesium chloride, 10 mM sucrose, 5 mM dithiothreitol, 1% 3-[-(3-chloramidopropyl) dimethylammonio]-l-propanesulfonic acid (CHAPS), 10 µg/ml pepstatin, 10 µg/ml leupeptin and 1 mM PMSF). After freezing and thawing 3 times, the cell lysates were centrifuged for 10 min at 5000 rpm, and the supernatants were centrifuged at 10,000 g for 60 min. The cell lysates thus obtained were stored at −80 °C. Lysates were incubated at 37 °C in a buffer containing 25 mM HEPES, pH 7.5, 10% sucrose, 0.1% CHAPS, and 1 mM dithiothreitol supplemented with 50 µM Ac-DEVD-7- amino-4- methylcoumarin (AMC) in 96-well Costar plates. As a negative control, DEVD-CHO, a caspase-3 specific inhibitor, was added to the cell lysates 30 min before incubation with caspase-3 substrate, Ac-DEVD-AMC. The increase in fluorescence after the cleavage of the fluorogenic AMC moiety was monitored in a Cytofluor 4000 fluorimeter (Perspective Biosystems, Framingham, MA, USA) using 360 nm excitation and 460 nm emission wavelengths. Caspase-3 activity was expressed as fluorescence units per milligram of total cytosolic protein.
Immunoprecipitation, Immunoblot Analysis, and Cytochrome c Detection
Release of cytochrome c from mitochondria was measured as previously described (Yang et al, 1997). After incubation with vehicle-treated medium alone, 100 nM Tat1-72, or 500 pM gp120, striatal neurons were harvested with ice-cold buffer A containing 20 mM Hepes-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, and 250 mM sucrose. Cell homogenates were centrifuged first at 750 g for 10 min at 4 °C, and the resulting supernatant was centrifuged at 12,000 g for 30 min at 4 °C. The supernatant (cytosolic) fraction and the mitochondrial pellet fraction were used for the detection of cytochrome c. Equal amounts of cytosolic and mitochondrial protein from control and treated cultures were immunoprecipitated with 1 µg of purified mouse anti-cytochrome c monoclonal antibody (clone.6H2.B4 mouse IgG from BD PharMingen) for 3 h at 4 °C with constant rocking. Ten µl of a 50% slurry of protein G-agarose beads in PBS was added, and the tubes were incubated overnight at 4 °C. The beads were pelleted, washed three times with PBS-1% Triton X-100, and suspended in 30 µL of Laemmli sample buffer (Laemmli, 1970). After boiling for 5 min and centrifuging briefly, equal amounts of immunoprecipitated proteins (20 µg protein/lane) in the supernatant were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) on 12% polyacrylamide gels under reducing conditions (4% ß-mercaptoethanol). The proteins were electrophoretically transferred to a polyvinylidenedifluoride (PVDF) membrane. After blocking in TTBS buffer (10 mM Tris/HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20) containing 5% skimmed milk powder, the membranes were incubated with primary monoclonal antibodies against cytochrome c (7H8.2C12 mouse IgG, PharMingen, 1:333 dilution) at room temp for 3 h. Finally, proteins were incubated 1 hr at room temperature with a peroxidase-conjugated goat anti-mouse antibody. The blots were developed on Hyperfilm ECL film using Amersham ECL™ reagent (Amersham Pharmacia Biotech, Piscataway, NJ, USA) according to manufacturers’ instructions and detected using a phosphoimager (Molecular Dynamics Storm, Amersham Biosciences, Piscataway, NJ, USA) or cooled CCD camera (Kodak Image Station 440 CF, Rochester, NY, USA). The optical density of the band in each lane was expressed relative to the vehicle-treated control in the same blot.
Protein Determination
Protein concentrations were determined by the BCA-method using a commercially available kit (Pierce; Rockford, IL, USA).
Statistics
Data were reported as the mean ± SEM of the average values from replicate samples obtained from at least n = 4 separate experiments. Significant overall differences among experimental groups were assessed using ANOVA (Statistica, StatSoft, Inc., Tulsa, OK, USA). If statistically significant differences were noted using ANOVA (P < 0.05), multiple group differences were compared post hoc using Duncan’s test. Paired group differences were assessed using Student’s t test. Homogeneity of variances was screened for all data using Levene’s test.
RESULTS
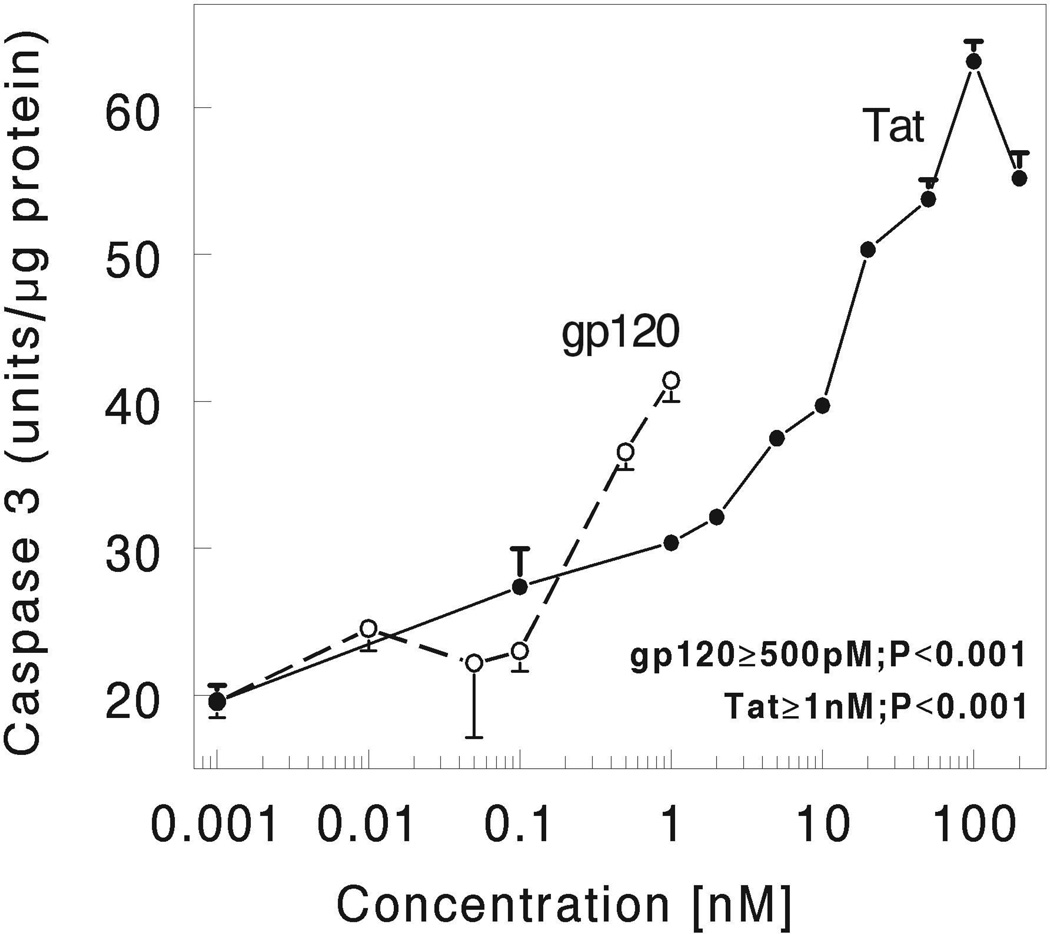
We first studied the concentration-dependence of HIV-1 Tat and gp120-induced activation of caspase-3 in striatal neurons at 4 h following continuous exposure in vitro. As shown in Figure 1, both HIV-1 Tat and gp120 caused significant increases in caspase-3 activation in a concentration-dependent manner (p<0.0001 versus untreated cultures) at 4 h following continuous exposure in vitro. The optimum concentration of HIV-1 Tat used being 100 nM whereas the optimum concentration of gp120 used being 500 pM for the measurement of neuronal viability and other biochemical studies described in this paper (Fig. 1).
Figure 1.
HIV-1 Tat and gp120 caused concentration-dependent increases in caspase-3 activation in striatal neurons at 4 h following continuous exposure in vitro. Striatal neurons were grown for 7 days in culture and incubated with varied concentrations of HIV-1 Tat (closed circles with solid line) and gp120 (open circles with dotted lines) for 4 h. Caspase-3 activity was measured as described in the Methods section. Results are the mean ± the S.E.M from n=4 experiments.
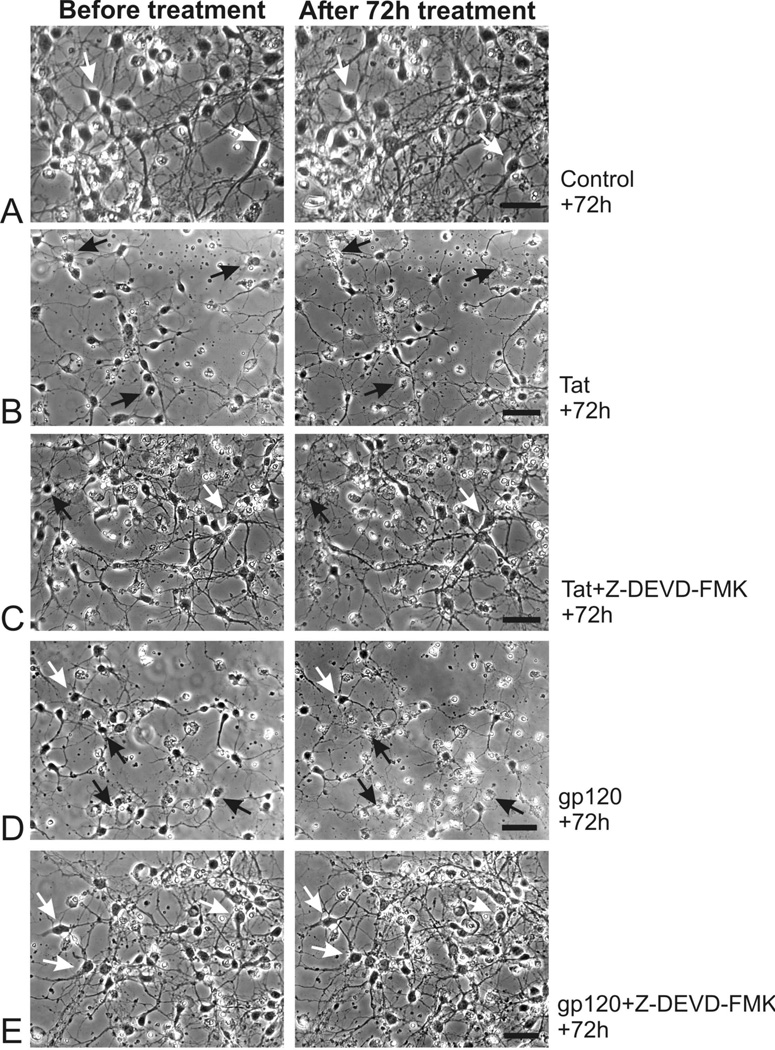
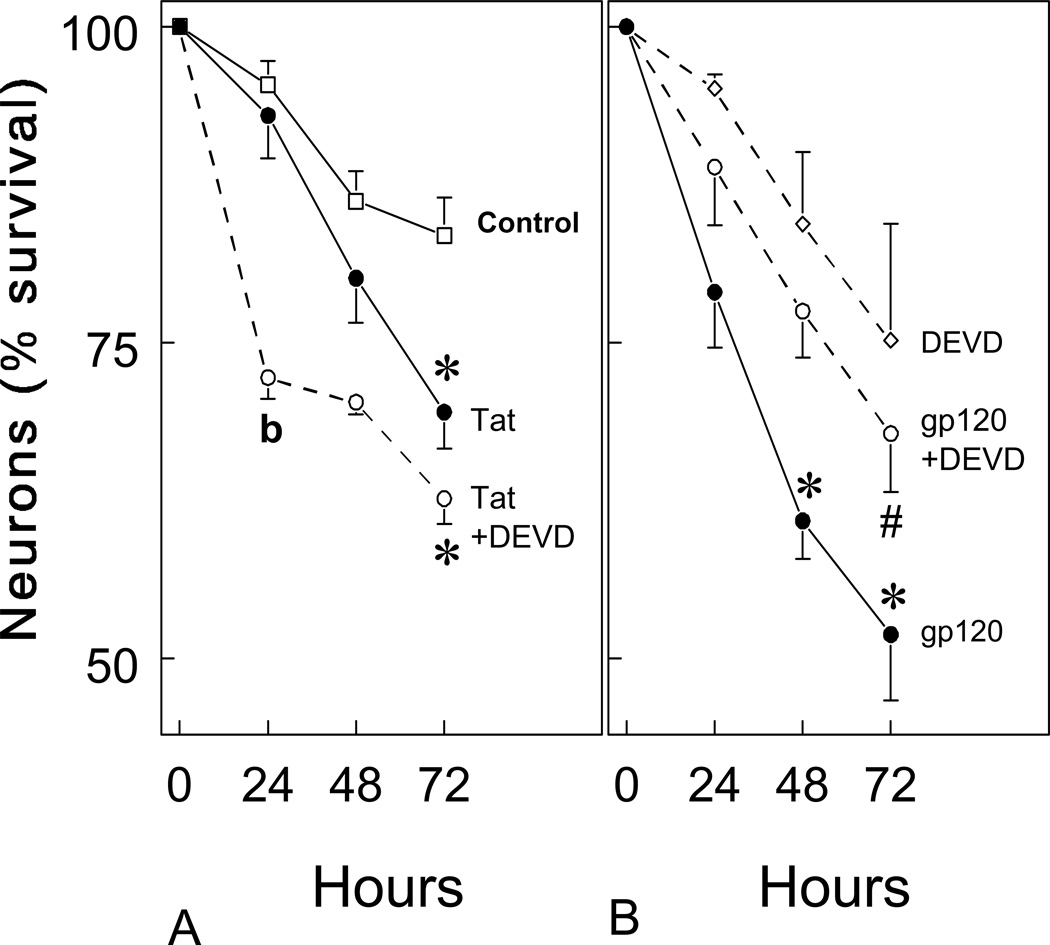
When Tat1-72 and gp120 toxicity were assessed by time-lapse photomicrography in primary cultures derived from embryonic mouse striatum, both viral proteins were found to be intrinsically toxic to striatal neurons using time-lapse photomicroscopy (Fig. 2). High rates of death were seen 72 hours after exposure to either Tat1-72 (100 nM) (Fig. 2B) or gp120 (500 pM) (Fig. 2D). Although some background neuronal death is normally present in striatal neuron cultures at 7–10 days in vitro, increased rates of cytotoxicity were clearly evident following exposure to Tat or gp120 (see Fig. 2A,B). To assess the extent and time-course of viral protein-induced neuronal losses, the effects of Tat1-72 and gp120 on neuronal death were quantified before and at 24-h intervals following continuous exposure to viral proteins (Fig. 3). The findings showed that gp120 caused rapid neuronal losses with significant cytotoxicity occurring at 48 h, while Tat1-72 caused significant toxicity after 72 h (Fig. 3) (*P < 0.05 versus controls treated with medium-vehicle alone).
Figure 2.
Time-lapse digital photomicrographs showing the effects of HIV-1 Tat1-72 or gp120 on striatal neuron survival prior to (Before treatment; left column) and at 72 h following exposure (right column) in vitro (A–F). The effects of Tat or gp120 were additionally assessed in the presence or absence of Z-DEVD-FMK (30 µM) (DEVD) a caspase inhibitor applied 4 hours prior to viral protein exposure. Tat1-72 (100 nM) (B) and gp120 (500 pM) (D) were neurotoxic compared to treatment with media alone (A). However, only gp120 (E), but not Tat (C), induced neuronal losses were significantly attenuated by DEVD. Black arrows represent dying neurons, white arrows show viable neurons; scale bars = 25 µm.
Figure 3.
HIV-1 Tat1-72 and gp120 reduced striatal neuron survival at 24, 48, and 72 hours in vitro. Both Tat (100 nM) (A) and gp120 (500 pM) (B) significantly increased the proportion of dying neurons at 48 and/or 72 h (*P < 0.05 versus vehicle-treated controls). The cytotoxic effects of gp120, but not Tat1-72, were significantly attenuated by the caspase inhibitor Z-DEVD-FMK (30 µM; #P < 0.01 versus gp120 treatment alone). Interestingly, Z-DEVD-FMK exposure appeared to enhance Tat toxicity at 24 h (bP < 0.05 versus Tat-treated or vehicle-treated cultures). About 50–75 neurons were arbitrarily sampled per culture. At least 4–6 separate cultures, each consisting of cells isolated and maintained from separate mice were assessed per experimental group. Rates of neuronal death in DEVD-FMK treated cultures did not differ significantly from vehicle-treated controls; DEVD = Z-DEVD-FMK.
Because Tat and gp120 toxicity has been associated with activation of caspases and apoptosis in astrocytes and neurons in other brain regions (Kruman et al, 1998; Su et al 2000; Garden et al, 2002; Haughey and Mattson, 2002), we examined the effect of the cell permeant pan-caspase inhibitor Z- DEVD-FMK on rates of Tat and gp120 toxicity in striatal neurons. Tat-induced neuronal death was unaffected in cultures co-incubated with DEVD-FMK (30 µM) (Fig. 2C), while pretreatment with DEVD-FMK protected neurons exposed to 500 pM gp120 (Fig. 2E). The rate of neuronal death in DEVD-FMK treated cultures did not differ significantly from controls treated with medium alone. Incubation with Z-DEVD-FMK (30 µM) significantly attenuated gp120-induced neuronal death (Fig. 3B) (#P < 0.01 versus gp120 treatment alone), but not Tat1-72-induced neuronal death (Fig. 3A). These results suggested that gp120-induced neurotoxicity is mediated by caspase-3, while Tat1-72 is neurotoxic through an alternative pathway independent of caspase-3.
To validate the time-lapse studies, striatal neuron death was assessed by co-localized immunofluorescent reactivity for the neuronal nuclear marker (NeuN) in dying cells that cannot exclude EMA at 24 h (Table 1; Fig. 4). The inability to exclude EMA precedes overt pathological changes in neuronal morphology that accompany cell death. Cell cultures were exposed to EMA and assayed at 24 h following Tat or gp120 exposure. Moreover, it was anticipated that the proportion of dying neurons labeled by EMA would be less than that counted by repeated measures, since cells are incubated in EMA for a relatively short duration (30 min) and our experience from time-lapse studies is striatal neurons undergo death quite rapidly (< 24 h). In addition, to similarly assess the role of caspase-3 in Tat and gp120-induced neuronal death, viral protein-toxicity was assessed in the presence or absence of the pan-caspase inhibitor Z-DEVD-FMK (50 µM). The results indicated significant gp120-induced neuronal losses at 24 h that were significantly attenuated by co-treating neurons with Z-DEVD-FMK (Table 1, Fig. 4B,C). In contrast, Tat1-72 did not increase the proportion of EMA-positive NeuN-identified neurons at 24 h (Table 1), which agreed with findings that Tat toxicity progresses more slowly than gp120-induced neuronal losses (Fig. 3).
TABLE 1.
Effects of Tat1-72 or gp120 and caspase inhibition on striatal neuron viability at 24 h following viral protein exposure. gp120 treatment caused significant neuronal losses at 24 h that could be significantly attenuated by pretreating cultures with the caspase inhibitor, Z-DEVD-FMK (DEVD)a
| Treatment | Non-viable neurons (%)b |
|---|---|
| Vehicle-treated controls | 0.84 ± .37 |
| DEVD (50 µM) | 0.67 ± .41 |
| HIV-1 Tat1-72 (100nM) | 1.00 ± .38 |
| HIV-1 Tat1-72 (100 nM) + DEVD (50 µM) | 1.17 ± .24 |
| HIV-1 gp120 (500 pM) | 1.71 ±.31* |
| HIV-1 gp120 (500 pM) + DEVD (50 µM) | 0.60 ±.33 |
Striatal neurons were continuously exposed to HIV-1 proteins and/or the soluble caspase inhibitor Z-DEVD-FMK (DEVD) and assayed at 24 h in vitro.
Neuronal viability was assessed by determining the proportion of neuronal nuclear (NeuN) immunoreactive neurons that failed to exclude ethidium monoazide (EMA) following 30 min incubation in EMA (see Fig. 3); percentage non-viable neurons = [(EMA and NeuN-positive neurons)/(total NeuN-positive neurons) * 100].
P < 0.05 versus vehicle-treated control or gp120-treated cultures.
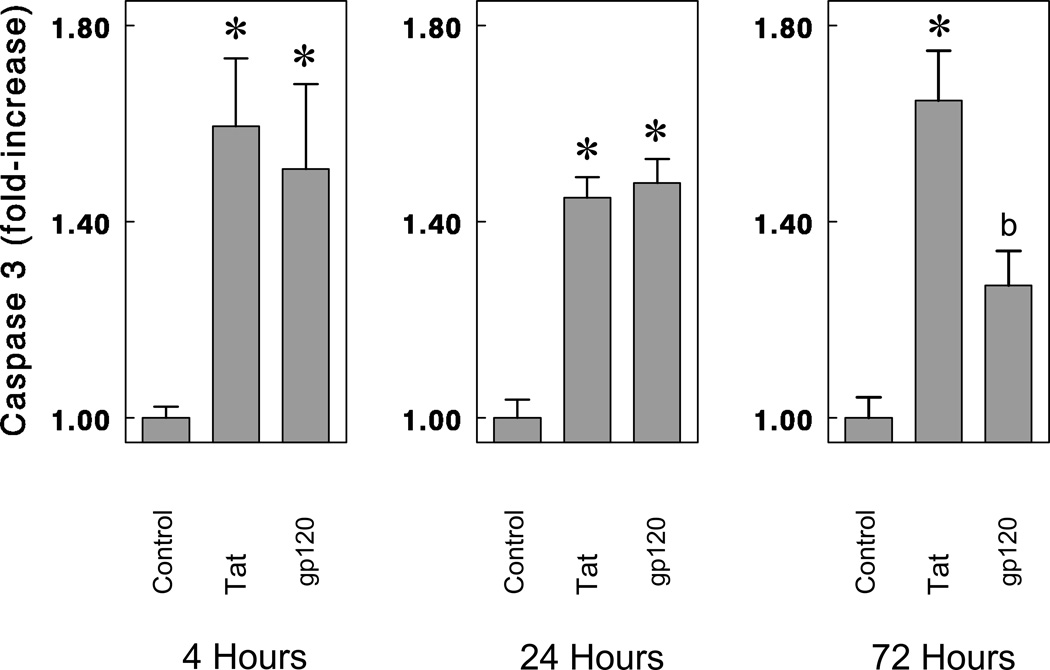
To assess the extent and time-course of viral protein-induced caspase-3 activation, the effects of Tat1-72 and gp120 on caspase-3 activation were examined at 4 h, 24 h, and 72 h (Fig. 5). Caspase-3 was significantly elevated following Tat1-72 or gp120 exposure at 4 h, 24 h, and 72 h. At 72 h, caspase-3 activity was significantly greater with Tat treatment than gp120 treatment (Fig. 5); however, at this time fewer viable neurons remain following gp120 treatment. Interestingly, despite findings that Z-DEVD-FMK failed to inhibit Tat-induced striatal neuron death (Fig. 4B), Tat nevertheless caused significant increases in caspase-3 activity at 4 h, 24 h, and 72 h (Fig. 5). Lastly, to assure that our assay was specifically assessing caspase-3, Ac-DEVD-CHO, a highly selective (but cell impermeant) caspase-3 inhibitor blocked caspase-3 activity when added to the cell lysates 30 min before incubation with caspase-3 substrate (Ac-DEVD-AMC; data not shown).
Figure 5.
Effects of Tat1-72 and gp120 on caspase-3 activation in striatal neurons at 4 h, 24 h, and 72 h following continuous exposure in vitro. Both Tat (100 nM) and gp120 (500 pM) significantly increased caspase-3 activity at 4 h, 24 h, and 72 h. Caspase-3 activity was expressed as fluorescence units per µg of cytosolic protein and averaged from triplicate determinations each from at least n = 4 separate experiments. Mean caspase-3 activity in vehicle-treated (control) cultures was 69.71 units/µg protein (*P < 0.015 versus vehicle-treated cultures; ANOVA, post hoc Duncan’s test; bP< 0.05 versus vehicle-treated or Tat-treated cultures; ANOVA, post hoc Duncan’s test).
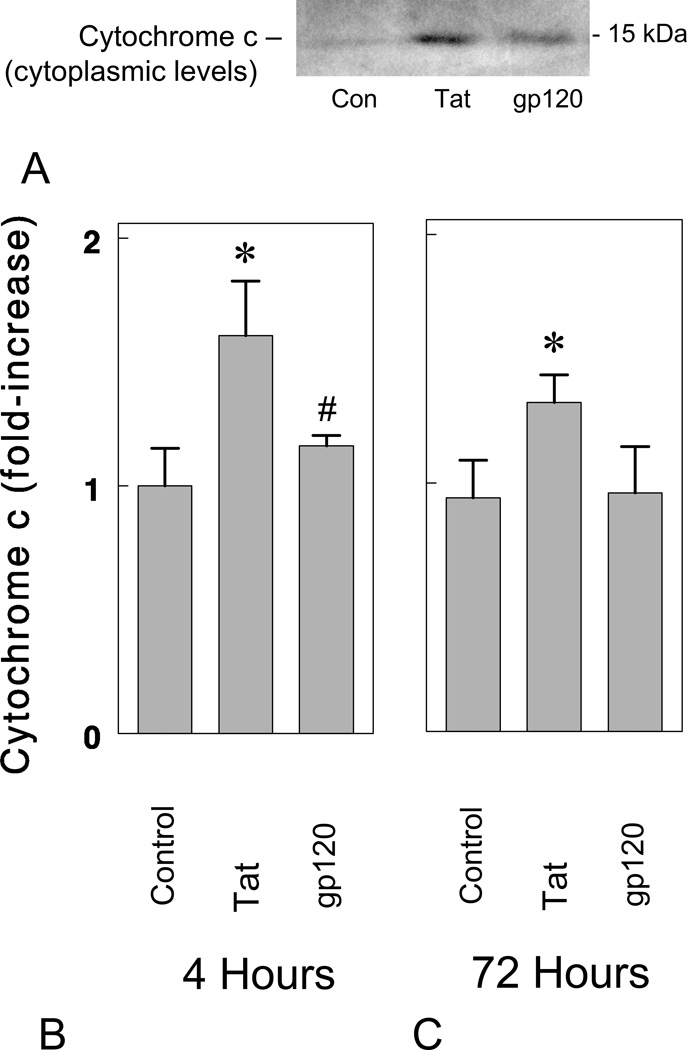
A key event in the mitochondrial-mediated pathway for caspase-3 activation is the release of cytochrome c into the cytoplasm (Kluck et al, 1997). Increases in cytoplasmic levels of cytochrome c following HIV-1 Tat1-72 and gp120 exposure were investigated in striatal neurons at 4 h and 72 h (Fig. 6). As shown in Fig. 6A–C, Tat1-72 (100 nM), but not gp120 (500 pM), significantly increased levels of cytochrome c in the cytoplasm (*P < 0.05 versus vehicle-treated control cultures; #P < 0.05 versus Tat1-72-treated neurons). Tat-induced increases in cytoplasmic levels coincided with declines in mitochondrial levels as expected and β-actin levels in the cytoplasm did not differ among treatments (data not shown). Although gp120 did not increase cytosolic levels of cytochrome c at 4 h or 72 h, this does not exclude the possibility that cytochrome c was involved at other times. The accelerated loss of neurons following gp120 compared to Tat exposure (see Fig. 4), prompts speculation that gp120 might similarly hasten proapoptotic signaling events and induce cytochrome c release prior to 4 h; this notion warrants further investigation.
Figure 6.
HIV-1 Tat1-72 increases cytoplasmic levels of cytochrome c in striatal neurons in vitro. Tat1-72 (100 nM), but not gp120 (500 pM), significantly increased levels of cytochrome c in the cytoplasm at 4 (A & B) and 72 hours (C) (*P < 0.05 versus vehicle-treated control (Con) cultures; #P < 0.05 versus Tat-treated neurons; n = 4 experiments).
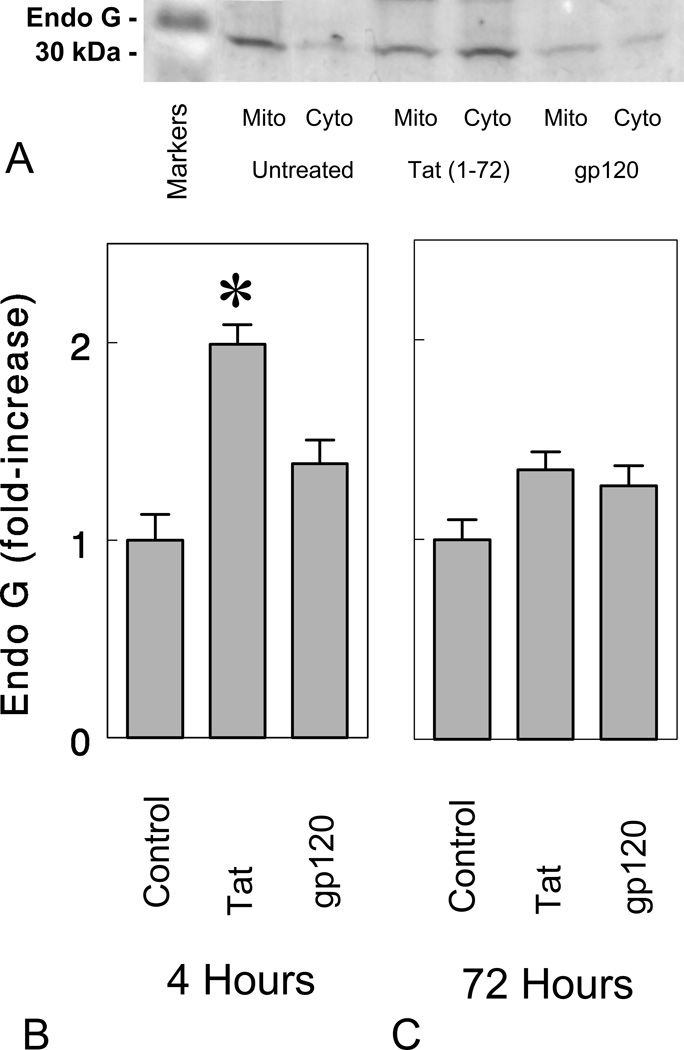
We additionally investigated the role of endonuclease-G (endo G), a mitochondrion-specific nuclease that translocates into the nucleus during apoptosis (Parrish et al, 2001; Li et al, 2001). To test whether endo G is released following HIV-1 protein neurotoxicity, we exposed neuronal cultures to Tat1-72 (100 nM) or gp120 (500 pM) for 4 h and 72 h (Fig. 7A–C). Tat1-72, but not gp120, significantly increased the translocation of endo G from mitochondria into the cytoplasm at 4 h following exposure (*P < 0.05 versus medium-treated control cultures). The results suggested that Tat can induce apoptosis by activating endo G in addition to caspase activation, while gp120 failed to activate the endo G apoptotic pathway. This difference may explain why Z-DEVD-FMK was able to significantly attenuate gp120 neurotoxicity but unable to allay Tat1-72 toxicity in the present study.
Figure 7.
Effects of Tat and gp120 on endonuclease G (endo G) in the cytoplasm at 4 and 72 h following continuous treatment. HIV-1 Tat1-72 (100 nM) increased cytoplasmic levels of in striatal neurons at 4 hours in vitro (*P < 0.05 versus vehicle-treated control cultures), while gp120 (500 pM) failed to increase endo G levels in the cytoplasm (Cyto) at 4 h or 72 h. Although cytoplasmic changes in endo G are shown (B,C), mitochondrial (Mito) levels were also assessed and varied inversely to cytoplasmic levels in control and Tat-treated neurons as expected (A). The results show the mean ± SEM of n = 4 experiments.
DISCUSSION
The work presented here indicates that HIV-1 Tat1-72 and gp120 are toxic to striatal neurons, and suggests they trigger neurodegeneration by activating caspase-3 and/or endonuclease G apoptotic pathways. Although the mechanisms underlying HIV-induced cell death in the nervous system are not fully understood, results presented herein suggest that underlying neurotoxic pathways are highly complex and likely to differ for each viral protein. HIV-1 viral products can be released by infected microglia and astroglia (Ensoli et al, 1990; Munis et al, 1992; Robert-Guroff et al, 1990; Chang et al, 1997; Shutt and Soil, 1999; Jones et al, 1998; Jones et al, 2000), and can also induce the production and release of proinflammatory cytokines and cellular toxins in neural cells (Yeung et al, 1995; Adamson et al, 1996; Bagetta et al, 1996a; Kaul and Lipton, 1999; Nath et al, 1999). Numerous lines of evidence suggest that extracellular proinflammatory cytokines and cellular toxins can induce neuronal apoptosis (Gabuzda et al, 1986; Gendelman et al, 1994; Shi et al, 1998; New et al, 1998; Bagetta et al, 1999; Holden et al, 1999; Zheng et al, 1999; Park et al, 2001; Takahashi et al, 1996). Furthermore, several studies suggest that HIV-1 Tat and gp120 interact with neuronally-expressed receptors to activate multiple pro-apoptotic signaling cascades in neurons (Ma and Nath, 1997; Moore et al, 1997; New et al, 1997; Shi et al, 1998; Piller et al, 1999; Bonavia et al, 2001; Corasaniti et al, 2001; Haughey et al, 2001).
The present study shows that both Tat1-72 and gp120 activated the apoptotic effector caspase-3. This is consistent with reports that HIV proteins, including Tat, Vpr, gp41 and Nef, can initiate apoptotic cascades in other neural cell types (Adamson et al, 1996; Yang et al, 1997; Kruman et al, 1998; Bartz and Emerman, 1999; Liu et al, 2000; Trillo-Pazos et al, 2000; Parrish et al, 2001; Li et al, 2001). The protective role of caspase inhibitors in experimental models of HIVE supports the involvement of caspases in the pathogenesis of neuroAIDS (Garden et al, 2002). The pan caspase inhibitor Z-DEVD-FMK (Z-VAD-FMK) is an important tool to assess the role of caspases in cell death, while the substrate used to detect activity (Ac-DEVD-AMC) is selective for caspase-3 (Lei et al, 2002). Interestingly, although both Tat1-72 and gp120 caused increases in caspase-3 activity followed by neuronal cell death, only gp120 neurotoxicity was significantly blocked by caspase inhibition suggesting that Tat1-72 can induce neuronal death independent of caspase activation.
The release of cytochrome c from mitochondria into the cytosol can be a key event in the apoptotic process (Springer et al, 1999). Our results indicate that exposure of mouse striatal neurons with Tat alone significantly increased cytochrome c levels in the cytoplasm following 4 h or 72 h exposure, while gp120 failed to induce significant increases in cytochrome c in the cytoplasm.
Endonuclease G is primarily synthesized in a propeptide form in mammalian cells and its import into mitochondria is mediated by an amino-terminal mitochondrion-targeting sequence (Ruiz-Carillo and Renaud, 1987; Cote and Ruiz-Carillo, 1993). Upon entry into the mitochondria, the signal peptide is cleaved-off and the mature endo G can be released from mitochondria following an appropriate apoptotic signal (Li et al, 2001). We report here that exposure of mouse striatal neurons with HIV-1 Tat1-72 significantly increased the translocation of endo G from mitochondria into the cytoplasm and this may be sufficient to induce death despite the presence of caspase inhibition. Thus, endo G levels in brains of HIV infected patients may be an indirect indicator of the presence of Tat. In contrast, gp120 did not induce endo G translocation from mitochondria at 4 h or 72 h suggesting an alternative apoptotic pathway different from Tat. Studies in progress are more directly addressing the role of endo G in Tat neurotoxicity.
Collectively, these findings suggest the involvement of at least two independent pathways, which execute neuronal apoptosis in neuro-AIDS. The existence of multiple apoptotic pathways may underlie fundamental differences in Tat versus gp120 mediated neurotoxicity in the striatum. Understanding the differences in HIV protein-mediated neurotoxicity is critical to the development of new therapeutic approaches for HIV dementia.
ACKNOWLEDGEMENTS
We thank Ms. Susan Goebel and Mr. Kenneth M. Martin for expert technical assistance. Anti-endonuclease G antibodies were the kind gift of Dr. Xiaodong Wang (University of Texas, Dallas Southwestern Medical Center). This work was funded by NIH DA13559 and is part of ongoing studies exploring the mechanisms by which drug abuse exacerbates HIV-induced neurotoxicity.
REFERENCES
- Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F, Noonan D. HIV-1 Tat protein is a heparin-binding angiogenic growth factor. Oncogene. 1996a;12:289–297. [PubMed] [Google Scholar]
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Famussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 Tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. NatMedl: 1996b:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Aloe L, Berliocchi L, Costa N, Finazzi-Agro A, Nistico G. Intracerebral injection of HIV-1 coat protein gp120 differentially affects the expression of NGF and nitric oxide synthase in the hippocampus of rat. Proc Natl Acad Sci USA. 1996a;93:928–933. doi: 10.1073/pnas.93.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Malorni W, Rainaldi G, Costa N, Berliocchi A, Finazzi-Agro A, Nistico G. The HIV-1 gp120 causes ultrastructural changes typical of apoptosis in the rat cerebral cortex. NeuroReport. 1996b;7:1722–1724. doi: 10.1097/00001756-199607290-00005. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Berliocchi L, Nistico R, Giammarioli AM, Malorni W, Aloe L, Finazzi-Agro A. Involvement of interleukin-ip in the mechanism of human immunodeficiency virus type 1 (HIV-1) recombinant protein gp120-induced apoptosis in the neocortex of rat. Neuroscience. 1999;89:1051–1066. doi: 10.1016/s0306-4522(98)00363-7. [DOI] [PubMed] [Google Scholar]
- Barillari G, Gendelman R, Gallo RC, Ensoli B. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz SR, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycan through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Piccirilli S, Paoletti A, Nistico R, Stringaro A, Malorni W, Finazzi-Agro A, Bagetta G. Evidence that HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci Lett. 2001;31:267–270. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- Cote J, Ruiz-Carilloo A. Primers for mitochondrial DNA replication generated by endonuclease G. Science. 1993;261:765–769. doi: 10.1126/science.7688144. [DOI] [PubMed] [Google Scholar]
- Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem. Soc.Trans. 2001;29:696–702. doi: 10.1042/0300-5127:0290696. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of ADDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Gabuzda DH, Ho DD, de la Monte SM, Hirsch MS, Rota TR, Sobel RA. Immunohistochemical identification of HTLV-III antigen in brains of patients with ADDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LC. Apoptotic neurons in brain from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS. The neuropathogenesis of HIV-1 infection. J Leukocyte Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Martin KM, Goebel SM, Hauser KF. Dynorphin A toxicity in striatal neurons via an a-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA)/kainate receptor mechanism. Neuroscience. 2003;116:807–816. doi: 10.1016/s0306-4522(02)00563-8. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. Journal of Acquired Immune Deficiency Syndr. 2002;31:S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Foldes JK, Turbek CS. Dynorphin A (1–13) neurotoxicity in vitro: Opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp. Neurol. 1999;160:361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Holden CP, Nath A, Haughey NJ, Geiger JD. Involvement of Na+/H+ exchangers, Ca2+ channels, and excitatory amino acid receptors in intracellular Ca2+ responses to HIV-1 gp120 in cultured human fetal brain cells. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Huang MB, Bond VC. Involvement of protein kinase C in HIV-1 gp120-induced apoptosis in primary endothelium. J Acquir Immune Defic Syndr. 2000;25:375–89. doi: 10.1097/00042560-200012150-00001. [DOI] [PubMed] [Google Scholar]
- James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, Gelbard HA. Expression of caspase-3 in brains from pediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–386. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) Tat protein causes inflammation, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14:2709–2713. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Kaaya EE, Castanos-Velex E, Amir H, Lema L, Luande J, Kitinya J, Patarroyo M, Biberfeld P. Expression of adhesion molecules in endemic and epidemic Kaposi’s sarcoma. Histopathology. 1996;29:337–346. doi: 10.1111/j.1365-2559.1996.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lee JW, Gersuk GM, Kiener PA, Beckham C, Ledbetter JA, Deeg HJ. HLA-DR-triggered inhibition of hemopoiesis involves Fas/Fas ligand interactions and is prevented by c-Kit ligand. J Immunol. 1997;159:3211–3219. [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Neuropathogenesis of acquired immunodeficiency syndrome dementia. Curr Opin Neurol. 1997;10:247–253. doi: 10.1097/00019052-199706000-00014. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Dementia associated with the acquired immunodeficiency syndrome. New Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 Tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Seines OA, Jacobson LP. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- Munis JR, Kornbluth RS, Guatelli JC, Richman DD. Ordered appearance of human immunodeficiency virus type 1 nucleic acids following high multiplicity infection of macrophages. J Gen Virol. 1992;73:1899–1906. doi: 10.1099/0022-1317-73-8-1899. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neuroviroll: 2001:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes: A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. JNeurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor a and activation of non-N-methyl-D-aspartate receptors by a NFκB-independent mechanism. J Biol Chem. 1998;273:17582–17588. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- Park IW, Ullrich CK, Schoenberger K, Ganju RK, Groopman JE. HIV-1 Tat induces microvascular endothelial apoptosis through caspase activation. J Immunol. 2001;167:2766–2771. doi: 10.4049/jimmunol.167.5.2766. [DOI] [PubMed] [Google Scholar]
- Parrish J, Li L, Klotz K, Ledwich D, Wang X, Xue D. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 2001;412:90–94. doi: 10.1038/35083608. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Piller SC, Ewart GD, Jans DA, Gage PW, Cox GB. The amino-terminal region of Vpr from human immunodeficiency virus type 1 forms ion channels and kills neurons. J Virol. 1999;73:4230–4238. doi: 10.1128/jvi.73.5.4230-4238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Sanchez JF, Dimitri CA, Gelbard HA, Dewhurst S, Maggirwar SB. Neurotrophins prevent HIV Tat-induced apoptosis via a nuclear factor-kappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001;78:874–889. doi: 10.1046/j.1471-4159.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Rigamonti D, Bauer JH, De-Fraja C, Contio L, Sipione S, Sciorati C, Clementi E, Hackman A, Hay den MR, Li Y, Cooper JK, Ross CA, Govoni S, Vincentz C, Cattaneo E. Wild-type Huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 2000;20:3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M, Popovic M, Gartner S, Markham P, Gallo RC, Reitz MS. Structure and expression of tat-, rev-, and nef- specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. J Virol. 1990;64:3391–3398. doi: 10.1128/jvi.64.7.3391-3398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carillo A, Renaud J. Endonuclease G: a (dG)n X (dC)n-specific DNase from higher eukaryotes. EMBO J. 1987;6:401–407. doi: 10.1002/j.1460-2075.1987.tb04769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer AD, Hedley DW, Penn LZ, Minden MD. Receptor- and mitochondrial-mediated apoptosis in acute leukemia: a translational view. Blood. 2001;98:3541–3553. doi: 10.1182/blood.v98.13.3541. [DOI] [PubMed] [Google Scholar]
- Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996;98:1979–90. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998;4:281–90. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- Shutt DC, Soll DR. HIV-induced T-cell syncytia release a two component T-helper cell chemoattractant composed of Nef and Tat. J Cell Sci. 1999;112:3931–3941. doi: 10.1242/jcs.112.22.3931. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Knapp PE, Nath A, Hauser KF. HIV-1 Tat and gp120 induce apoptotic cell death in mouse striatal neurons through partly overlapping apoptotic cascades. J. Neurochem. 2003;85(Suppl. 1):35. [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1411:299–306. doi: 10.1016/s0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- Su JH, Nichol KE, Sitch T, Sheu P, Chubb C, Miller BL, Tomaselli KJ, Kim RC, Cotman CW. DNA damage and activated caspase-3 expression in neurons and astrocytes: evidence for apoptosis in frontotemporal dementia. Exp Neurol. 2000;163:9–19. doi: 10.1006/exnr.2000.7340. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridizatiuon and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rail GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP. Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res. 2000;864:315–326. doi: 10.1016/s0006-8993(00)02213-7. [DOI] [PubMed] [Google Scholar]
- Vogel BE, Lee SJ, Hilderbrand A, Craig W, Pierschbacher MD, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the αvβs integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993;121:461. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yeung MC, Pulliam I, Lau AS. The HIV envelope protein gp120 is toxic to human brain-cell cultures through the induction of interleukin-6 and tumor necrosis factor-alpha. AIDS. 1995;9:137–143. [PubMed] [Google Scholar]
- Yeung MC, Geertsma F, Liu J, Lau AS. Inhibition of HIV-1 gp120-induced apoptosis in neuroblastoma SK-N-SH cells by an antisense oligodeoxynucleotide against p53. AIDS. 1998;12:349–54. doi: 10.1097/00002030-199804000-00002. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]