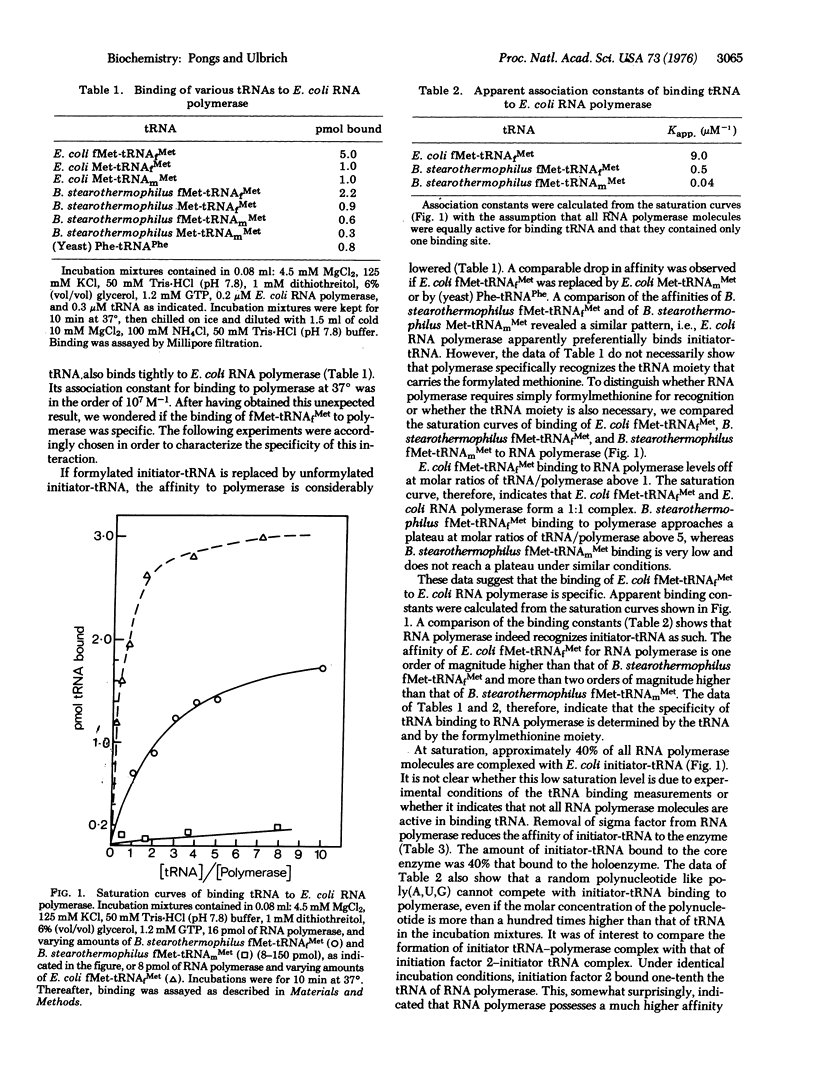
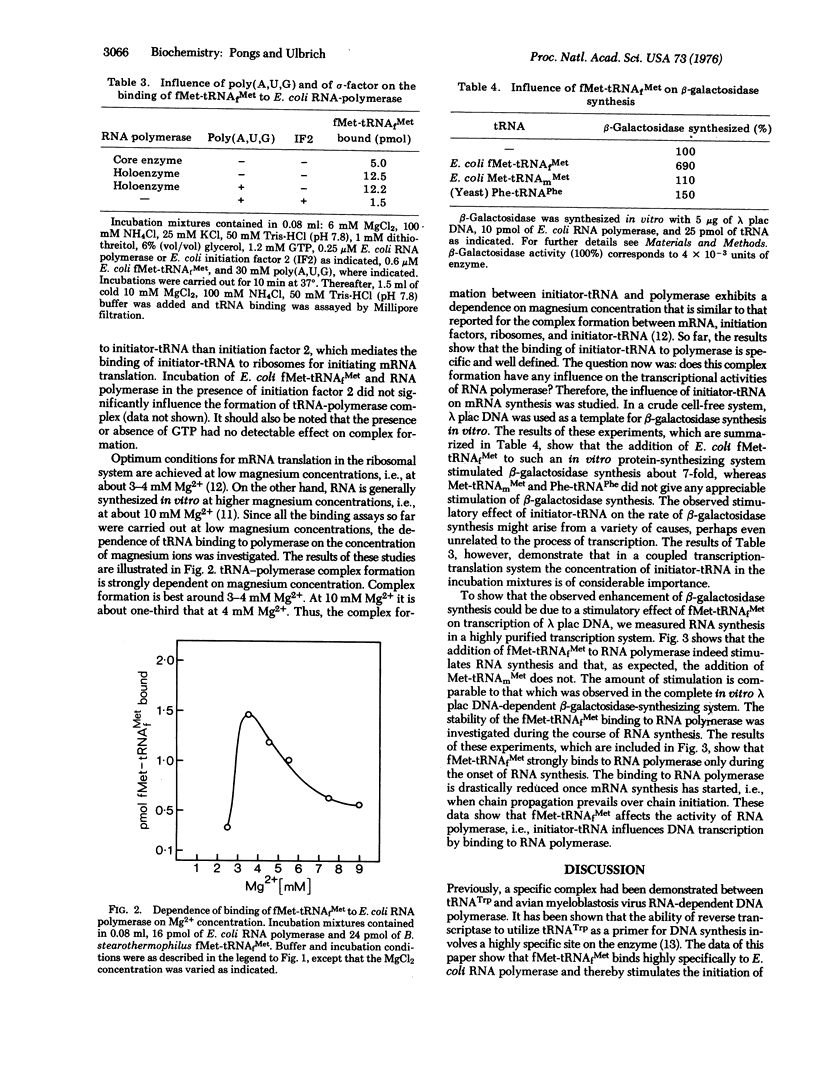
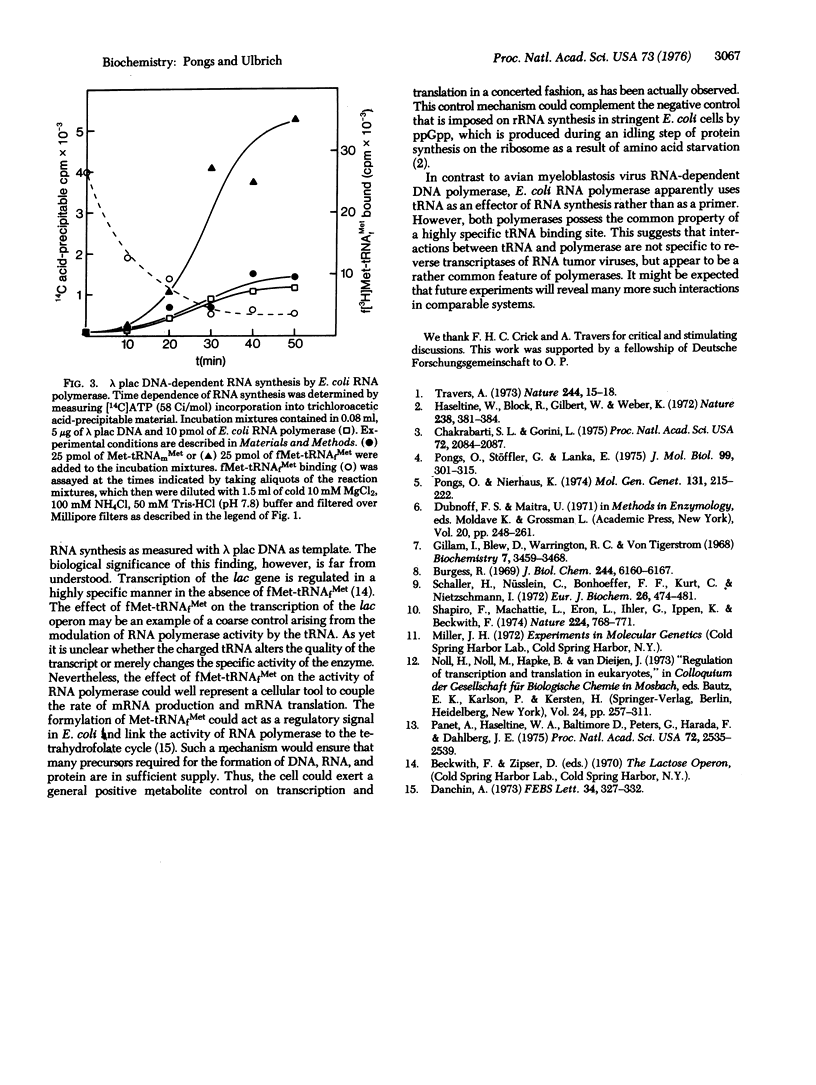
Abstract
E. coli fMet-tRNAfMEet and E. coli RNA plymerase (RNA nucleotidyltransferase; EC 2.7.7.6; nucleoside-triphosphate:RNA nucleotidyltransferase) form a 1:1 complex with an apparent association constant of 9.0 X 10(6)M-1 at 37 degrees. The affinity of polymerase to tRNA depends on the tRNA as well as the formyl methionine moiety. Core polymerase has a greatly reduced affinity for initiator tRNA. Optimal binding conditions are similar to those that are also optimal for binding initiator tRNA to ribosomes. Binding of initiator tRNA to polymerase stimulates the transcription of lambda plac DNA, as determined in a crude cell-free system for beta-galactosidase (EC 3.2.1.23; beta-D-galactoside galactohydrolase) synthesis as well as in a highly purified transcription system.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A. Does formylation of initiator tRNA act as a regulatory signal in E. coli? FEBS Lett. 1973 Aug 15;34(2):327–332. doi: 10.1016/0014-5793(73)80823-3. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Nierhaus K. H. Recognition of normal and modified tRNA by streptomycin sensitive and resistant ribosomes of Escherichia coli. Mol Gen Genet. 1974;131(3):215–222. doi: 10.1007/BF00267961. [DOI] [PubMed] [Google Scholar]
- Pongs O., Stöffler G., Lanka E. The codon binding site of the Escherichia coli ribosome as studied with a chemically reactive A-U-G analog. J Mol Biol. 1975 Dec 5;99(2):301–315. doi: 10.1016/s0022-2836(75)80148-3. [DOI] [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Shapiro J., Machattie L., Eron L., Ihler G., Ippen K., Beckwith J. Isolation of pure lac operon DNA. Nature. 1969 Nov 22;224(5221):768–774. doi: 10.1038/224768a0. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]


