Abstract
Background and Aims
Amino acid (AA) availability is critical to maintain protein homeostasis and reduced protein intake causes a decline in protein synthesis. Citrulline, an amino acid metabolite, has been reported to stimulate muscle protein synthesis in malnourished rats.
Methods
To determine whether citrulline stimulates muscle protein synthesis in healthy adults while on a low-protein diet, we studied 8 healthy participants twice in a cross-over study design. Following a 3-days of low-protein intake, either citrulline or a non-essential AA mixture (NEAA) was given orally as small boluses over the course of 8 hours. [ring-13C6] phenylalanine and [15N] tyrosine were administered as tracers to assess protein metabolism. Fractional synthesis rates (FSR) of muscle proteins were measured using phenylalanine enrichment in muscle tissue fluid as the precursor pool.
Results
FSR of mixed muscle protein was higher during the administration of citrulline than during NEAA (NEAA: 0.049 ± 0.005; citrulline: 0.060 ± 0.006; p=0.03), while muscle mitochondrial protein FSR and whole-body protein turnover were not different between the studies.
Citrulline administration increased arginine and ornithine plasma concentrations without any effect on glucose, insulin, C-peptide, and IGF-1 levels. Citrulline administration did not promote mitochondria protein synthesis, transcripts, or citrate synthesis.
Conclusions
Citrulline ingestion enhances mixed muscle protein synthesis in healthy participants on 3-day low-protein intake. This anabolic action of citrulline appears to be independent of insulin action and may offer potential clinical application in conditions involving low amino acid intake.
Keywords: citrulline, protein synthesis, phenylalanine, muscle
INTRODUCTION
Citrulline is an amino acid metabolite which is not a component of proteins but a metabolic product of the urea cycle and nitric oxide (NO) formation1. Until recently, no specific function has been identified for citrulline. Circulating citrulline is synthesized in the gut from arginine and glutamine2 but unlike other amino acids, citrulline is neither taken up nor released by the liver1, in normal physiology (S1-S2). Citrulline is however, taken up by the kidney where arginine is resynthesized and released for whole-body requirements1. For the above reason, there is a substantial decline in plasma citrulline and arginine in short bowel syndrome (SBS) (S3) and in other situations where the gut function is compromised (S4). This prompted Osowska et al3 to test the effects of citrulline supplementation on net protein balance. In a rat model with short bowel syndrome, it has been shown that citrulline supplementation normalizes plasma and tissue arginine and improves nitrogen balance (S4). In a rat model of endotoxemia, citrulline absorption is decreased but remains higher than the absorption of arginine4. Furthermore, Osowska et al (S5), demonstrated that, in malnourished old rats on a low protein diet for 12 weeks, a citrulline-enriched diet administered for one week during the refeeding period enhanced muscle protein accretion by increasing absolute protein synthesis with no effect on fractional synthesis rate (FSR). Moreover, a recent in vitro study by Moinard et al (S6) showed that in isolated muscle from adult malnourished rats incubated with citrulline, protein synthesis was increased by 27% compared to the control experiment without citrulline. Taken together, the results of these different studies3 (S5-S6) strongly suggest that citrulline, an amino acid metabolite, may play an important role in protein anabolism when protein intake is low. There are potential explanations for the anabolic effect of citrulline.
Provision of all amino acids is critical for synthesis of proteins. The systemic AA availability is determined not only by intestinal absorption of AAs (95-99%) from ingested protein but also by selective removal of AAs by the liver (50%), mainly via ureagenesis (S7). Following intestinal absorption, portal flux of arginine stimulates ureagenesis not only as a substrate of ureagenesis but also by allosteric activation of ureagenesis key enzyme N-acetyl glutamate synthetase (S8). Thus, dietary arginine favors not only its own catabolism but also of those of other AA via ureagenesis. In situations where protein intake is low and ureagenesis is consecutively slowed-down, intestinal arginase and ornithine carbamoyl transferase are activated resulting in an increase in the conversion of arginine to citrulline1. The newly formed citrulline is released into the portal vein, and since citrulline is not taken up by the liver5 (S8), it is released into systemic circulation, unlike arginine which is partially taken up in the liver. Therefore it is likely that by limiting ureagenesis all AAs are spared and are available in systemic circulation (i.e. for muscle protein synthesis).
Secondly, low protein intake results in a reduction in AA supply. It has been well established that AAs are major stimulant of protein synthesis and amino acids availability is critical for insulin’s stimulatory effect on protein synthesis6(S9). Both insulin and certain AAs (especially leucine)7 (S10) have direct or indirect effects on mTOR phosphorylation and downstream signaling pathway, which is considered as a nitrogen sensing pathway regulating protein anabolic processes8. However, insulin activation of mTOR pathway is through upstream activation of AKT, while AA activation of protein synthesis is independent from AKT activation and considered as independent from insulin action. It has also been shown that citrulline like leucine may stimulate protein synthesis via the mTOR signaling pathway9. We therefore hypothesized that in situations of low protein intake, citrulline could play a critical role in stimulating muscle protein synthesis and that this action is independent of insulin secretion and action in humans. To test this hypothesis we have studied the effect of citrulline intake on whole-body and mixed and mitochondrial muscle protein synthesis in healthy volunteers following 3 days of relatively low-protein diet.
MATERIALS AND METHODS
Subjects
Healthy adults (n=8; 4 women and 4 men: 25.9 ± 2.0 years) were recruited for this study and informed written consent has been obtained after detailed review of the protocol, which has been approved by the Institutional Review Board of the Mayo Clinic and Foundation. All participants underwent physical examination including detailed history, hematological and biochemical profile. Exclusion criteria included patients with BMI > 25, altered liver functions, fasting blood glucose values above 6.2 mmol/l, serum creatinine > 1.5 mg/l, and any evidence of alterations in any organ functions and any active diseases. Volunteers taking medications, such as steroids that may affect the outcome measures were also excluded. Subject characteristics are given in Table 1.
Table 1. Participant characteristics (n=8).
| Parameters | ||
|---|---|---|
| Weight (kg) | 73.3±4.4 | |
| BMI (kg/m2) | 24.1±0.4 | |
| Fat free mass (kg) | 48.5±5.5 | |
| Fat percentage (%) | 32.0±3.7 | |
| Usual | Low Protein Diet | |
| Protein intake (g/kg/day) | 1.26 | 0.69 |
| Carbohydrate intake (g/kg/day) | 3.99 | 4.77 |
| Fat intake (g/kg/day) | 1.21 | 1.29 |
| Energy distribution (%): prot/carbs/fat | 15:49:33 | 8:56:35 |
Data are given as means ± SE
Study design
In this randomized cross over design, each participant underwent two separate studies at the Mayo Clinic CTSA Research Unit (CRU). Participants received citrulline or NEAA (alanine, glycine, serine, proline, aspartate in equimolar amounts) mixture on the first study day and vice and versa for the second study day. There was a washout period of at least 3 weeks between the two study days during which the participants were allowed to consume their normal diet. Body composition (fat and fat free mass or FFM) was measured using dual-energy X-ray absorptiometry (DPX-L, Lunar, Madison, WI). Subjects were on a weight-maintaining low-protein diet (protein/carbohydrate/fat 8:60:32% by calories) provided by CRU for 3 consecutive days before each inpatient study period. They were put on a low-protein diet based on our working hypothesis and on the fact that citrulline supplementation has been shown to enhance protein anabolism in animals in a protein deficient state (S5). During the screening visit, a member of the CRU-SMH dietetics staff performed a diet assessment, based on usual US daily intake of normal weight people (BMI<25) from NHANES in order to determine dietary needs for the 3 days of weight maintenance low-protein meals. Table 1 provides the dietary composition of the usual and low protein diet. All meals were prepared in the CRU kitchen. The volunteers were asked to eat two meals per day in the CRU kitchen and take home the other one meal prepared by the CRU kitchen. The volunteers were asked to completely consume these meals.
On the 3rd day, the participants were admitted to the CRU at 5 PM and ingested a low-protein meal at 6 PM. Thereafter, they fasted, except for water, until the end of the inpatient period the following day.
The following morning, a retrograde IV catheter was inserted in one hand to withdraw blood samples. The hand was kept in a “hot box” (60°C) prior to collections to obtain arterialized venous blood samples (S11). A second intravenous catheter was placed in the contralateral forearm for infusion of a primed, continuous infusion of [ring-13C6] phenylalanine (1.5 mg/kg FFM prime with a 1.5 mg/kg FFM/hr continuous infusion), and [15N] tyrosine (prime 0.6 mg/kg FFM with a 0.6 mg/kg FFM/hr continuous infusion). The non-radioactive tracers (Cambridge Isotope Laboratories, Andover, MA) infusions started at 6 AM after obtaining a baseline blood sample.
Oral administration of either the citrulline (Kyowa Hakko, Tokyo, Japan) or NEAA mixture (Sigma-Aldrich, St. Louis, MO) containing a sugar-free flavor, started following baseline blood samples. The NEAA mix contained alanine, serine, proline, and glycine. The two mixtures were isonitrogenous (nitrogen content: 72 mg/kg FFM). After a first bolus of 60 ml providing 9 mg of N/kg FFM, the mixtures were given as small boluses of 30 ml (4.5 mg of N/kg FFM) every 30 min for 8 hours. The total amount of citrulline administered was 11 – 24 g depending on the fat free mass of the participants. A previous dose-effect study (S6) in healthy people demonstrated that all doses up to 15 g of citrulline given as a single dose were well tolerated and no adverse effects (such as diarrhea or hypoglycaemia) were observed. Two percutaneous needle biopsies (200-300 mg) were taken under local anesthesia (lidocaine 2% buffered with 8.4% NaBicarb) from the vastus lateralis muscle as previously described10 following 180 and 480 min of tracer infusion. A portion of the muscle obtained at 480 min was kept on ice for the mitochondrial enzyme assay. The remaining muscle samples were quickly frozen in liquid nitrogen and stored, along with plasma samples, at −80 °C until analysis.
Blood samples were taken at baseline and every hour to measure isotopic enrichment and plasma AA concentrations. Additional samples were collected every two hours to measure hormones (insulin, C-peptide, growth hormone and insulin like growth factor 1 (IGF-1)) and substrates (glucose, non-esterified fatty acid (NEFA)). Urine samples were also collected.
Analysis
Hormones and Substrates
Insulin and growth hormone were measured with two-site immunoenzymatic assays (Access system, Beckman Instruments, Chaska, MN). C-peptide was measured by direct radioimmunoassays (Linco Research, St. Louis, MO). After separation from its binding proteins with a simple organic solvent, total insulin-like growth factor-I (IGF-I) was measured with two-site immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX). AA levels were measured by ion-exchange chromatography with ninhydrin detection on an AA analyser (Jéol, Croissy-sur-Seine, France)11. Glucose was measured with a Beckman Glucose Analyzer (Beckman Instruments, Porterville, CA). Non-esterified fatty acids (NEFA) were measured using an enzymatic colorimetric assay (NEFA C, Wako Chemicals USA, Richmond, VA).
Urinary nitrogen
Nitrogen levels in timed urine samples were measured using a Beckman GM7 Analox Microstat.
Indirect Calorimetry
Respiratory gas exchanges were measured using the DeltaTrac system (Sensormedics, Yorba Linda, CA) for 45 minutes at 11AM.
Citrate synthase activity
citrate synthase activity was measured in fresh mitochondria preparation and in muscle samples at 480 min using spectrometric assay as previously described12.
Whole-body protein synthesis
plasma concentrations and enrichment levels of [13C6] phenylalanine, [13C6] tyrosine and [15N] tyrosine were determined using gas chromatography/mass spectrometry (GC/MS)13 (S12). Whole-body phenylalanine and tyrosine kinetics were calculated utilizing equations described previously14 (S13).
Briefly, the rate of fluxes of both phenylalanine (Raph) and tyrosine were calculated using the tracer dilatation approach as previously described using [13C6] phenylalanine and [15N] tyrosine as tracer14 (S13). Phenylalanine conversion to tyrosine (Phe-Tyr) was calculated as follows:
[13C6] Tyr and [13C6] Phe represents plasma enrichment of tyrosine and phenylalanine isotopic plateau.
I [13C6] Phe represents the infusion rate of [13C6] phenylalanine. Phenylalanine incorporation into protein or protein synthesis (Phe→Prot) is calculated by subtracting Phe→Tyr from RaPhe. The details of the derivation of these equations are described elsewhere14 (S13).
Fractional synthesis rate of mixed muscle proteins
a piece of tissue was used to prepare total mixed muscle proteins and to isolate free tissue fluid AAs10 (S14). Isotopic enrichment of [13C6] Phenylalanine was measured using modified gas chromatography (GC)/on-line combustion/isotope ratio mass spectrometry (IRMS) as previously described15. Muscle tissue fluid phenylalanine enrichment was also measured using GC/MS15.
The fractional synthesis rate for mixed muscle proteins was calculated as previously described (S13) using the following equation:16
where Ef and Ei represent the enrichments as atom percent excess of13C6 derived from the combustion of muscle fraction phenylalanine obtained from the 480 and 180 min muscle biopsies, respectively. Ep is the precursor pool (tissue fluid phenylalanine) enrichment, and t represents the time between biopsies in hours.
Quantification of mRNA
The transcript levels of selected genes were examined by real-time quantitative PCR (Applied Biosystems 7900)10. RNA was extracted from frozen muscle samples collected from individual subjects using RNeasy Fibrous Tissue Kit (Qiagen) following the manufacturer’s instruction. Total RNA was reverse transcribed using Taqman Reverse Transcription kit (Applied Biosystems). The primers for nuclear-encoded genes were designed to cover the boundaries of two adjacent exons, and the primers for mitochondrial-encoded genes were designed to expand the coding region and poly-A tail, thereby eliminating the possibility of amplifying DNA. Sequences for the primers and probes that have been previously published are: COX317, COX4, PGC-1, NRF1, TFAM18, 28S rRNA10. The abundance of each target gene was normalized to 28S ribosomal RNA which was coamplified in the same well.
Quantification of phosphorylated and total forms of protein kinase B (AKT)
muscle tissues were homogenized in ice-cold cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with protease inhibitors (Mini Complete, Roche, Indianapolis, IN) and incubated on ice for 30 min. After high-speed centrifugation, supernatant from each sample were used for Western blot analysis. The same amount of protein was loaded (30 μg) onto each lane. The proteins were separated by polyacrylamide gel electrophoresis and transferred to PVDF membranes (Biorad, Hercules, CA). Following blocking in non-fatted milk, membranes were incubated overnight at 4C with primary antibodies directed against the total or phosphorylated (Ser 473) forms of protein kinase B (AKT, Cell Signaling). After incubation with horseradish peroxidase-conjugated secondary antibodies and the ECL-Plus detection system (Amersham Biosciences, Piscataway, NJ), images were captured on Biomax XAR film (Kodak Scientific, New Haven, CT) and analyzed using Kodak Molecular Imaging software. Data were expressed as the ratio of phosphorylated: total protein signal for each subject.
Statistical analysis
The cross-over designe was analyzed using a random effects model (S15). For all variables except the FSR, the change from baseline over the course of the 8 hour study was used as the primary endpoint/dependent variable to account for baseline differences in AA concentrations at the start of each study. The FSR, through its calculation, only yielded one value and this value accordingly was used as the dependent variable in the cross over analysis., Due to the length of the washout period in between two study days (i.e. 3 weeks) and the recommendation by Senn19, no carry over effect of treatments (citrulline or NEAA) were hypothesized. Given the limited sample size and the need for parsimony in the statistical model, the period effect was tested at the alpha=0.05 level of significance and removed from the final model on account of it not being statistically significant. In this manner, and since values were normally distributed, the analysis simplifies conceptually to a one sample (paired) t-test on the delta score of the response under citrulline ingestion relative to NEAA ingestion; however, a key advantage of the mixed model approach is that participants that were missing one or more of the assessments were still included in the estimation of the variance component (S15). Changes from baseline measurements are summarized as the mean and standard error of the mean (m ± SEM), mean and standard deviation was used to describe sample parameters and baseline values. SAS Version 9.1.3 software package was used. The level of significance was set at P<0.05. No correction factor for multiple comparisons has been applied to reported p-values.
RESULTS
Hormones and substrates
Concentrations for hormones were not significantly different in the baseline samples between the two studies.
Several AA were found to be significantly different at baseline between the two studies, so the change from baseline was used to minimize the heterogeneity of values between the two studies (Table 2).
Table 2.
Plasma hormones and substrates concentrations at baseline and at completion of the study periods
| NEAA treatment at Baseline |
Citrulline treatment at Baseline |
Change in value following NEAA Ingestion |
Change value following Citrulline Ingestion |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std Dev | N | Mean | Std Dev | p-value (a) | Mean | SE | Mean | SE | p-value (b) | |
| Insulin | 7 | 35.82 | 8.79 | 7 | 33.34 | 13.76 | 0.67 | −12.40 | 3.21 | −12.40 | 3.21 | 1.00 |
| C-peptide (nmol/L) | 8 | 0.51 | 0.14 | 8 | 0.50 | 0.13 | 0.82 | −0.19 | 0.04 | −0.20 | 0.04 | 0.77 |
| HGH | 7 | 0.22 | 0.44 | 7 | 0.22 | 0.34 | 0.97 | 0.47 | 0.17 | 0.10 | 0.17 | 0.23 |
| IGF-1 (nmol/L) | 8 | 39.02 | 2.26 | 8 | 39.20 | 3.21 | 0.87 | 0.41 | 1.29 | −1.85 | 1.29 | 0.17 |
| Glucose (mmol/L) | 5 | 5.11 | 0.21 | 5 | 5.06 | 0.38 | 0.78 | −0.06 | 0.08 | 0.15 | 0.08 | 0.11 |
| NEFA (mEq/L) | 8 | 0.02 | 0.01 | 8 | 0.02 | 0.01 | 0.66 | 0.0148 | 0.0051 | 0.0092 | 0.0051 | 0.19 |
| Non-Essential Amino Acids mix |
||||||||||||
| Alanine (μmol/L) | 7 | 313.27 | 54.88 | 8 | 362.79 | 92.37 | 0.08 | 71.4036 | 45.3336 | −112.02 | 42.56 | 0.006 |
| Aspartate (μmol/L) | 6 | 6.05 | 1.46 | 8 | 6.58 | 2.26 | 0.29 | 1.31 | 0.74 | −1.28 | 0.67 | 0.041 |
| Glycine (μmol/L) | 7 | 275.69 | 54.50 | 8 | 305.59 | 71.00 | 0.015 | 175.90 | 30.05 | −48.89 | 27.61 | 0.001 |
| Serine (μmol/L) | 7 | 120.98 | 24.63 | 8 | 128.85 | 29.41 | 0.024 | 135.64 | 21.05 | −13.99 | 19.36 | <0.001 |
| Proline (μmol/L) | 7 | 158.66 | 20.38 | 8 | 166.00 | 28.91 | 0.017 | 302.72 | 48.74 | −20.68 | 6.02 | <0.001 |
| Citrulline (μmol/L) | 7 | 34.49 | 9.95 | 8 | 35.59 | 8.40 | 0.20 | −8.99 | 71.24 | 741.29 | 65.47 | <0.001 |
| Other Amino Acids | ||||||||||||
| Asparagine (μmol/L) | 7 | 44.05 | 8.62 | 8 | 52.64 | 13.30 | 0.021 | −3.67 | 2.13 | −8.21 | 2.06 | 0.028 |
| Arginine (μmol/L) | 7 | 77.32 | 15.20 | 8 | 88.20 | 13.34 | 0.010 | −9.95 | 14.48 | 188.61 | 13.27 | <0.001 |
| Cysteine (μmol/L) | 7 | 52.78 | 8.47 | 8 | 53.80 | 11.96 | 0.12 | −3.07 | 2.03 | −2.09 | 1.99 | 0.41 |
| Glutamate (μmol/L) | 7 | 58.01 | 14.60 | 8 | 57.62 | 18.86 | 0.92 | −4.58 | 4.71 | −10.28 | 4.38 | 0.32 |
| Glutamine (μmol/L) | 7 | 533.98 | 65.95 | 8 | 582.10 | 90.75 | 0.037 | 55.22 | 23.62 | −42.72 | 22.04 | 0.007 |
| Histidine (μmol/L) | 7 | 62.78 | 9.58 | 8 | 72.59 | 12.86 | 0.003 | −0.38 | 4.51 | −8.44 | 4.35 | 0.050 |
| Isoleucine (μmol/L) | 7 | 58.80 | 10.82 | 8 | 61.46 | 8.39 | 0.13 | −10.50 | 2.93 | −18.53 | 2.70 | 0.064 |
| Leucine (μmol/L) | 7 | 126.50 | 25.81 | 8 | 128.03 | 22.70 | 0.15 | −11.86 | 5.78 | −23.82 | 5.46 | 0.072 |
| Lysine (μmol/L) | 7 | 167.69 | 34.69 | 8 | 175.86 | 49.83 | 0.034 | −16.88 | 9.72 | −15.58 | 9.56 | 0.80 |
| Methionine (μmol/L) | 7 | 22.68 | 3.58 | 8 | 24.49 | 4.43 | 0.021 | −2.76 | 0.99 | −3.46 | 0.98 | 0.12 |
| Ornithine (μmol/L) | 7 | 48.29 | 14.52 | 8 | 49.35 | 14.50 | 0.19 | 8.26 | 8.14 | 84.91 | 7.42 | <0.001 |
| Phenylalanine (μmol/L) | 7 | 55.03 | 4.14 | 8 | 58.69 | 5.99 | 0.032 | 12.13 | 3.02 | 13.07 | 2.94 | 0.63 |
| Taurine (μmol/L) | 7 | 64.32 | 11.73 | 8 | 58.54 | 17.56 | 0.76 | −10.98 | 4.34 | −8.43 | 4.06 | 0.60 |
| Threonine (μmol/L) | 7 | 115.50 | 33.05 | 8 | 140.75 | 45.19 | 0.031 | −8.38 | 5.88 | −15.39 | 5.66 | 0.17 |
| Tyrosine (μmol/L) | 7 | 60.22 | 12.65 | 8 | 62.40 | 13.53 | 0.020 | −0.25 | 3.65 | 2.71 | 3.48 | 0.38 |
| Valine (μmol/L) | 7 | 211.64 | 33.46 | 8 | 215.91 | 35.16 | 0.038 | −15.45 | 7.47 | −28.55 | 7.21 | 0.05 |
Std Dev=standard deviation, SE=standard error of means;
=differences in baseline values;
=differences in Δ or changes over NEAA vs citrulline ingestion
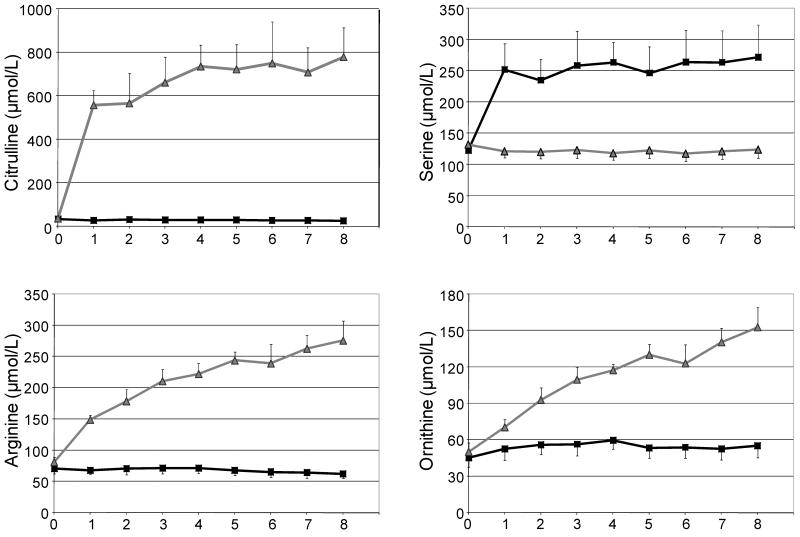
As expected, the plasma levels of AA given in the NEAA mixture (alanine, aspartate, glycine, proline and serine) increased from 60 to 100% in the first hour and then reached a plateau. Serine levels are shown in Figure 1 as an example of the kinetic behavior of NEAA administred. The NEAA mixture produced a statistically significant increase in each of these five AA relative to the citrulline ingestion (Table 2). Change in glutamine plasma levels following 8 hours of treatment with NEAA were significantly (p=0.007) higher than following citrulline administration (Table 2)
Figure 1.
Effect of small bolus intakes of citrulline (grey line with triangle) or a non-essential amino acid (NEAA) mixture (black line with square) on citrulline, serine, arginine and ornithine plasma concentrations over the course of 8 hours. Data are means ± SE. The differences in the change in levels over the course of treatment are reported in Table 2.
Following the citrulline ingestion, plasma levels of citrulline increased 20 fold during the first hour and then reached a plateau. Citrulline intake also resulted in an increase in plasma concentration of arginine and ornithine (Figure 1). There were no differences for other AAs between the two trials (Table 2).
Indirect calorimetry
VO2, VCO2, the respiratory quotient and resting energy expenditure were similar during citrulline and NEAA adminitration (Table 3).
Table 3. Indirect calorimetry.
| NEAA treatment means ± SE |
Citrulline treatment means ± SE |
p-value(a) | |
|---|---|---|---|
| VO2 (ml/min) | 215±15 | 203±15 | 0.14 |
| VCO2 (ml/min) | 251±14 | 253±18 | 0.89 |
| Respiratory quotient | 0.85±0.02 | 0.81±0.02 | 0.12 |
| Energy expenditure (kcal/day) | 1748±103 | 1738±127 | 0.90 |
Number of participants = 8
Measurement over 45 min. during the experimental periods (see material and methods for details).
Data are means ± SE.
Student’s t test (paired) was used to compare groups.
Whole-body protein metabolism and muscle protein synthesis
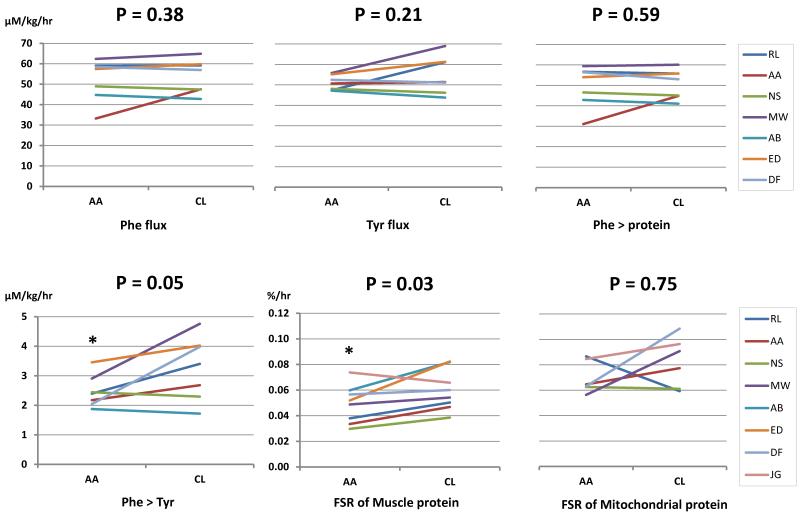
Ingestion of citrulline had no significant effect on phenylalanine and tyrosine flux when compared with NEAA group; indicating that citrulline did not alter whole body protein turnover (Figure 2). However, phenylalanine conversion to tyrosine was modestly higher (p=0.049) in the citrulline group than in the NEAA group, without any significant differences observed on phenylalanine incorporation into protein between groups indicating that citrulline had no significant effect on whole body protein synthesis. As noted changes in phenylalanine levels were not statistically different between the two studies (Table 2). Citrulline administration was associated with higher fractional synthesis rate (FSR) of mixed muscle proteins (Fig.2) than the one observed following NEAA ingestion although no significant treatment effect was observed for FSR of muscle mitochondrial protein.
Figure 2.
Effect of citrulline on whole body (A) and mixed muscle (B) protein synthesis. No significant differences for phenylalanine (Phe flux) and tyrosine flux (Tyr flux) and phenylalanine incorporation into a protein (Phe protein) was noted between NEAA (AA) and citrulline (CL) days. Phenylalanine conversion to tyrosine (Phe Tyr) was modestly (p=0.05) higher during citrulline and NEAA days. A higher (p=0.03) fractional synthesis rate (FSR) of mixed muscle protein was noted on citrulline day. Data are means ± SE. Cross over analysis (see methods) was used to compare groups. *P<0.05 citrulline vs. NEAA.
Citrate synthase activity and average mRNA levels of mitochondrial genes in muscle
Citrulline ingestion had no effect on citrate synthase activity or mRNA levels of mitochondrial related genes when compared with NEAA group (PGC1, COX3, COX4, TFAM) (Table 4).
Table 4.
Citrate synthase activity average mRNA levels of mitochondrial genes Akt activation in muscle (n=8)
| NEAA treatment | Citrulline treatment |
p-value | |
|---|---|---|---|
|
Citrate synthase activity
(μmol/min/g protein) |
46.6±4.3 | 49.4±3.2 | 0.62 |
| Average mRNA levels (AU) | |||
| PGC1/28S | 1.31±0.11 | 1.41±0.14 | 0.61 |
| COX4/28S | 1.27±0.09 | 1.41±0.19 | 0.56 |
| COX3/28S | 4.29±0.73 | 4.81±1.24 | 0.51 |
| TFAM/28S | 0.99±0.15 | 1.28±0.28 | 0.46 |
|
Akt activation (phosphorylated
to the total) (AU) at 180 min |
1.01±0.21 | 0.9±0.22 | 0.42 |
| at 480 min | 0.92±0.08 | 0.85±0.12 | 0.44 |
Data (means ± SE) were obtained after 8 hours of tracers infusion and ingestion of citrulline or a mixture of NEAA. Student’s t test (paired) was used to compare groups.
AKT activation
There was no effect of citrulline ingestion on AKT activation when compared with NEAA group (Table 4).
DISCUSSION
The current study demonstrates that oral ingestion of citrulline stimulates mixed muscle protein synthesis in the postabsorptive state without altering whole body protein turnover in healthy lean people while on low protein diet. To our best knowledge, this study is the first human study demonstrating a stimulatory effect of citrulline on muscle proteins. This effect occurred without altering insulin, growth hormone (GH) or IGF-1 levels. The comparison of citrulline effect is with iso-nitrogenous non-essential amino acids indicating that the increased muscle protein synthesis is not a non-specific effect of increased nitrogen intake. The current study was performed while the participants were on a relatively low protein diet. The usual daily intake of proteins in these participants represented approximately 15% of their total daily calories and they received only 8% of their caloric intake as proteins for three days prior to the study. This protein restriction was implemented as previous studies performed in rodents have shown that the anabolic effect of citrulline occurred while the animals were recovering from a restricted protein and caloric intake (S5). Of interest, in the current study we only modestly restricted protein intake in the human participants but demonstrated an enhancement (approximately 20%) of muscle protein synthesis in the citrulline group compared to the NEAA group. It cannot be excluded that more pronounced increase in protein synthesis may have occurred if the subjects had been on a low-protein diet for longer period as shown in rodents. Also, it would have been interesting to measure protein FSR and mitochondrial enzyme activities prior to the 3-day diet to have a pre-to post-comparison.
Citrulline administration had no effect on whole-body protein turnover. Muscle protein turnover is recognized to be slow compared to other organs such as liver and gut20. In humans, muscle protein synthesis contributes to only about 25% of whole body protein synthesis21 and a 20% increase in muscle protein synthesis would contribute to less than 7% to whole body protein synthesis. Of note, Osowska et al. (S5) showed that when malnourished old rats were re-fed with a citrulline-enriched diet, muscle protein synthesis was higher while hepatic protein synthesis was lower than in controls fed NEAA. These data may explain the lack of citrulline effect at the whole-body level.
In the current study we did not measure muscle protein breakdown. There are no methods to directly measure muscle protein breakdown, although the rate of protein breakdown across leg (mostly muscle) can be measured using arterio-venous approaches22 (S16-S17). It is possible that muscle protein degradation may be reduced or increased by citrulline. An increase in muscle protein degradation despite an increase in muscle protein synthesis may not result in a net muscle proteins accretion. However, animal studies (S5) demonstrated that citrulline not only increased absolute muscle protein synthesis but also muscle protein content, indicating that muscle protein breakdown was either decreased or not altered. We also noted a modest increase in whole-body phenylalanine conversion to tyrosine but the importance of this observation is uncertain. This observation indicates that tyrosine flux is a combination of tyrosine appearing from protein degradation and a small fraction appearing from phenylalanine conversion to tyrosine. The change in phenylalanine conversion to tyrosine was taken into account in estimating whole body protein synthesis.
In the current study we compared citrulline effect to that of a mixture of non-essential AA. Previous studies have shown that a mixture of essential and non-essential AA given intravenously resulted in an enhanced muscle protein synthesis and inhibition of muscle protein degradation6. This effect of AA was independent from insulin levels. In addition, studies have also shown that orally administered proteins such as whey protein results in greater muscle protein accrual than its constituent essential AA23 although it has been reported that essential AA are primarily responsible for the AA-induced stimulation of muscle protein synthesis (S17). To our best knowledge it remains to be shown that dietary non-essential AA have any effect on muscle protein synthesis. Muscle anabolic effect of citrulline could potentially be mediated through the stimulation of anabolic hormone secretions via citrulline-induced increase in arginine level. Indeed, repeated citrulline ingestion was responsible for a progressive increase in arginine and ornithine plasma levels which are recognized for their ability to stimulate insulin (S18-S20) and GH (S21-S22) secretion. However, we did not observe any effect of citrulline administration on insulin, C-peptide, or IGF-1 levels. Previous studies (S19-20) having shown an insulin secretagogue effect of arginine occur when pharmacological doses of arginine is administered intravenously whereas, in the present work, the increase in arginine plasma levels remained within the physiological range (equivalent to what is observed at the postprandial state). It is therefore unlikely that citrulline-induced increase in muscle protein synthesis is mediated by hormonal changes.
The mTOR phosphorylation and downstream signaling pathway is considered as a nitrogen sensing pathway regulating skeletal muscle protein synthesis8. Experimental and clinical studies24 (S21,S23-S24) have shown that essential AAs and particularly leucine can activate the mTOR pathway and consequently stimulate muscle protein synthesis. It was recently demonstrated in animal experiments that as citrulline also stimulates muscle protein synthesis through activation of the mTORC1 pathway25. In agreement, Moinard and9 showed an increased activation of proteins downstream of mTORC1, P70S6K and S6R (S6 ribosomal protein) in chronically malnourished old rats re-fed with a citrulline-enriched diet. Therefore, it is likely that in the present study, the observed citrulline-related stimulation of muscle protein synthesis might be via an activation of mTOR signaling pathway. Insulin stimulates muscle protein synthesis through an activation of AKT upstream of mTORC1. Liu et al.26 showed that AA could stimulate muscle protein synthesis independently from AKT activation. Therefore, not surprisingly and in accordance with low plasma level of insulin, we did not observe a citrulline-related activation of AKT. Taken together, these data show that the anabolic action of citrulline on muscle is independent from insulin action and AKT activation. Of note, there is no study comparing the effects of citrulline with those of leucine or of a mixture of EAA. It is therefore not possible to conclude whether citrulline’s impact on muscle protein synthesis is similar in magnitude as that of leucine or BCAA.
Considering that citrulline action is specific to muscle, it may affectall muscle proteins fraction (mitochondrial, sarcoplasmic and myofibrillar) equally. We sought to determine whether citrulline enhanced muscle mitochondrial biogenesis since previous studies have shown that AAs when administered in combination with insulin increased muscle mitochondrial biogenesis as noted by an increase mRNA levels of genes encoding mitochondrial proteins17. However we did not observe any higher mRNA levels of genes encoding mitochondrial proteins or fractional synthesis rate of muscle mitochondrial proteins following citrulline administration in comparison with NEAA administration. The enhancement of mitochondrial biogenesis might only occur when both insulin and AA are increased or it may be a specific effect of branched-chain AA which levels did not differ between the two groups. It remains to be studied if the positive effect of citrulline on mixed mucle protein synthesis is similar for sarcoplasmic and myofibrillar proteins or specific to one of these protein fractions.
Potential clinical applications of citrulline have been demonstrated in several experimental studies1,2(S22). The importance of the current study is that it shows in humans, that citrulline stimulates muscle protein synthesis following 3 days of low protein intake. This finding could potentially be of clinical importance in situations where muscle protein synthesis is decreased due to improper protein intake, such as aging and/or malnutrition. Indeed, Osowska et al. (S5) demonstrated that in malnourished old rats that a citrulline-enriched diet administered during the refeeding period enhanced muscle protein accretion by increasing protein synthesis.
We must also take into account the potential adverse effects in a clinical situation. Indeed, recent research suggests that in mice there is a close association between obesity state and plasma citrulline level28.Moreover, findings in human studies are far less consistent. Increased plasma citrulline levels were reported in obese subjects with hyperglycemia in one study29 although the effect of insulin resistance as a factor has not been investigated. To our knowledge there is no publication describing negative consequences of long term citrulline intakes or a direct causal link between citrulline intake and development of metabolic syndrome. Next steps to this pilot study are two ongoing randomized controlled clinical trials studying long term effect of citrulline intake. The first trial evaluates the effectiveness of 12-week citrulline supplementation on muscle mass and strength in combination with physical exercise in the elderly. This study lasting 12 weeks, it will also assess the safety of long term use of citrulline. Another study evaluates whole-body protein metabolism of malnourished hospitalized elderly patients treated for 2 weeks with citrulline.
In conclusion, the current pilot study, the first performed in humans, demonstrates that oral ingestion of citrulline stimulated muscle protein synthesis in healthy participants while on a short-term low protein diet. This anabolic action of citrulline is independent of insulin action and is specific of muscle. Overall, this novel finding opens the potential opportunity for clinical application of citrulline in situations where muscle anabolism is diminished.
ACKNOWLEDGEMENTS
We are grateful to Mr. Bushra Ali for technical assistance, the nursing staff of the CTSA Clinical Research Unit, the Immunochemistry Core Laboratory and the Metabolomics Core Laboratory of Mayo Clinic CTSA for support.
SOURCES OF SUPPORT
MJ was supported by fellowships from IFN and SFNEP. The study was supported by grants from NIH grant R01 DK41973 and UL1 TR000135 and from the MERT (contrat quadriennal EA 2498)
Footnotes
AUTHOR CONTRIBUTIONS
MJ: design, performed analysis and preparation of manuscript; KSN: design, performance, supervision of analysis, and preparation of manuscript; REC: statistical analysis and preparation of manuscript; JS: analysis and preparation of manuscript; GCF: analysis and preparation of manuscript; JM: analysis and preparation of manuscript; CA: analysis and preparation of manuscript; LC: design and manuscript preparation.
CONFLICT OF INTEREST
Conflict of interest: L. Cynober is share holder of Citrage company. No other authors have any conflict to disclose.
Reference List
- 1.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 2.Crenn P, Cynober L. Effect of intestinal resections on arginine metabolism practical; implication for nutrition support. Curr Opin Clin Nutr Metab Care. 2009;13:65–69. doi: 10.1097/MCO.0b013e328333c1a8. [DOI] [PubMed] [Google Scholar]
- 3.Osowska S, Moinard C, Neveux N, Loi C, Cynober L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut. 2004;53(12) doi: 10.1136/gut.2004.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elwafi F, Curis E, Zerrouk N, Neveux N, Chaumeil JC, Arnaud P, Cynober L, Moinard C. Endotoxemia affects citrulline, arginine and glutamine bioavailability. Eur J Clin Invest. 2012;42:282–9. doi: 10.1111/j.1365-2362.2011.02581.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Morris SM. Arginine metabolism: nitrice oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 7.Anthony J, Anthony T, Kimball SR, Vary T, Jefferson LS. Orally-administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–45. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 8.Gulati P, Thomas G. Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem Soc Trans. 2007;35:236–8. doi: 10.1042/BST0350236. [DOI] [PubMed] [Google Scholar]
- 9.Moinard C, Cynober L. Citrulline: a new player in the control of nitrogen homeostasis. J Nutr. 2007;137:1621S–1625S. doi: 10.1093/jn/137.6.1621S. [DOI] [PubMed] [Google Scholar]
- 10.Balagopal P, Schimke JC, Ades PA, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- 11.Neveux N, David P, Cynober L. Measurements of amino acids concentrations in biological fluids and tissues using ion exchange chromatography. In: Cynober L, editor. Metabolic and therapeutic aspects of amino acids in clinical nutrition. CRC Press; Boca Raton: 2004. [Google Scholar]
- 12.Short KR, Bigelow ML, Kahl JC, Singh R, Coenen-Schimke JM, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichement (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spec. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 14.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splachnic and leg tissues in type I diabetic patients. J Clin Invest. 1995;95:2926–2937. doi: 10.1172/JCI118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balagopal P, Ford GC, Ebenstein DB, Nadeau DA, Nair KS. Mass spectrometric methods for determination of [13C]leucine enrichment in human muscle protein. Anal Biochem. 1996;239:77–85. doi: 10.1006/abio.1996.0293. [DOI] [PubMed] [Google Scholar]
- 16.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stump CS, Short KR, Bigelow ML, Schimke JC, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short KR, Vittone J, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 19.Senn S. Cross-over trials in clinical research. John Wiley & Sons, Ltd; 2002. [Google Scholar]
- 20.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50:2652–2658. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 21.Nair KS, Schwartz RG, Welle SL. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263:E928–E934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- 22.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab. 2006;291:E729–36. doi: 10.1152/ajpendo.00003.2006. [DOI] [PubMed] [Google Scholar]
- 23.Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008;28:651, 8. doi: 10.1016/j.nutres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–23. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Plenier S, Walrand S, Noirt R, Cynober L, Moinard C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: A common activation pathway? Amino Acids. 2012;43:1171–8. doi: 10.1007/s00726-011-1172-z. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Jahn LA, Wei L, Long W, Barrett EJ. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab. 2002;87:5553–8. doi: 10.1210/jc.2002-020424. [DOI] [PubMed] [Google Scholar]
- 27.Moinard C, Nicolis I, Neveux N, Darquy S, Bénazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr. 2008;99:855–62. doi: 10.1017/S0007114507841110. [DOI] [PubMed] [Google Scholar]
- 28.Sailer M, Dahlhoff C, Giesbertz P, Eidens MK, de Wit N, Rubio-Aliaga I, Boekschoten MV, Müller M, Daniel H. Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS One. 2013;8:e63950. doi: 10.1371/journal.pone.0063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdam FJ, Greve JW, Roosta S, van Eijk H, Bouvy N, Buurman WA, Rensen SS. Small intestinal alterations in severely obese hyperglycemic subjects. J Clin Endocrinol Metab. 2011;96:E379–83. doi: 10.1210/jc.2010-1333. [DOI] [PubMed] [Google Scholar]