Abstract
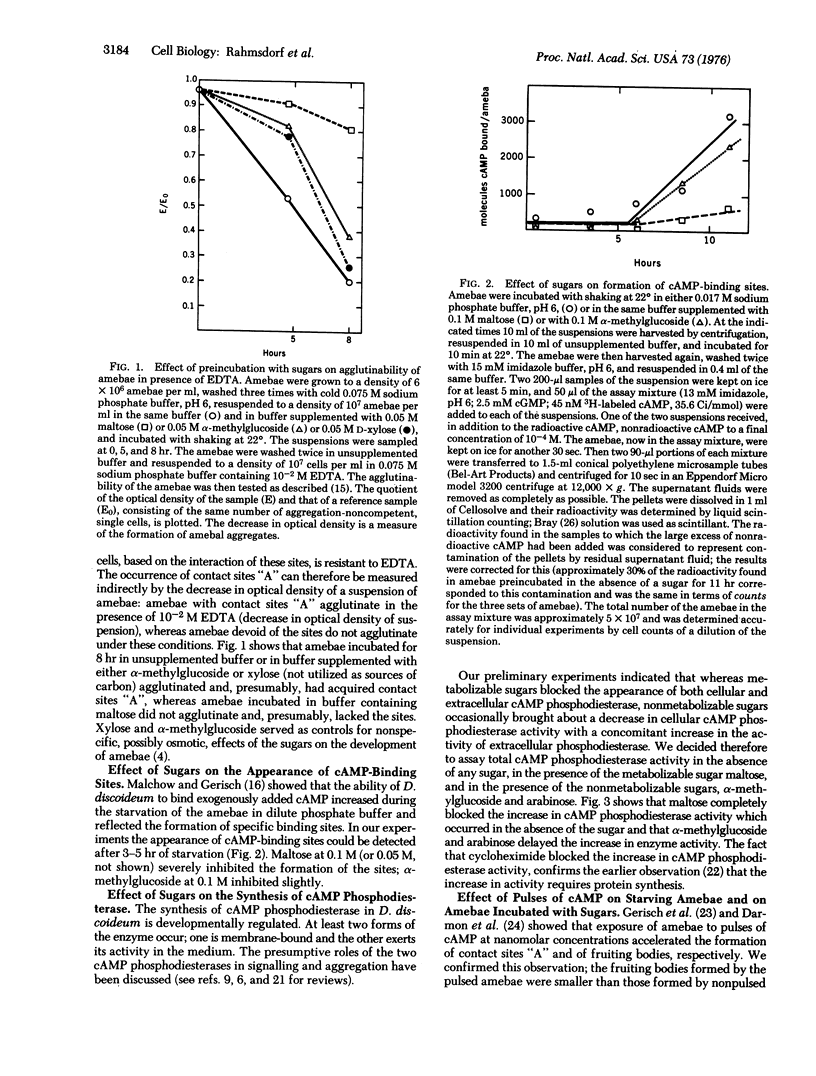
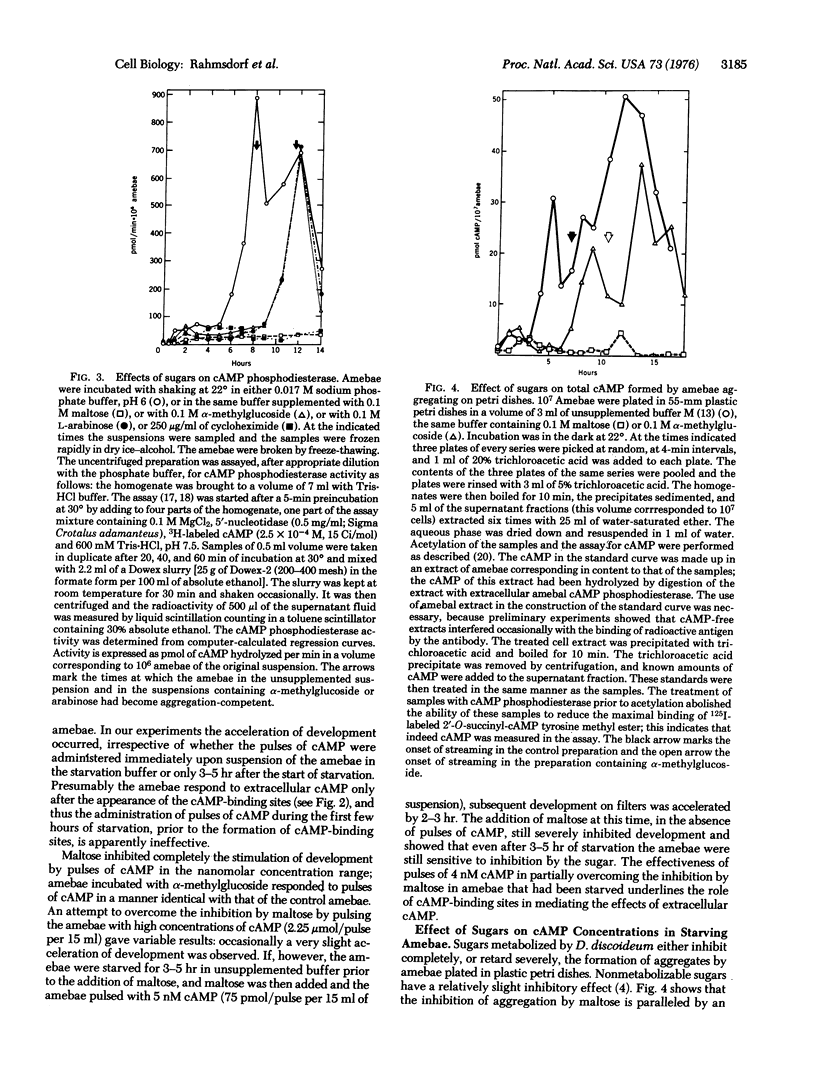
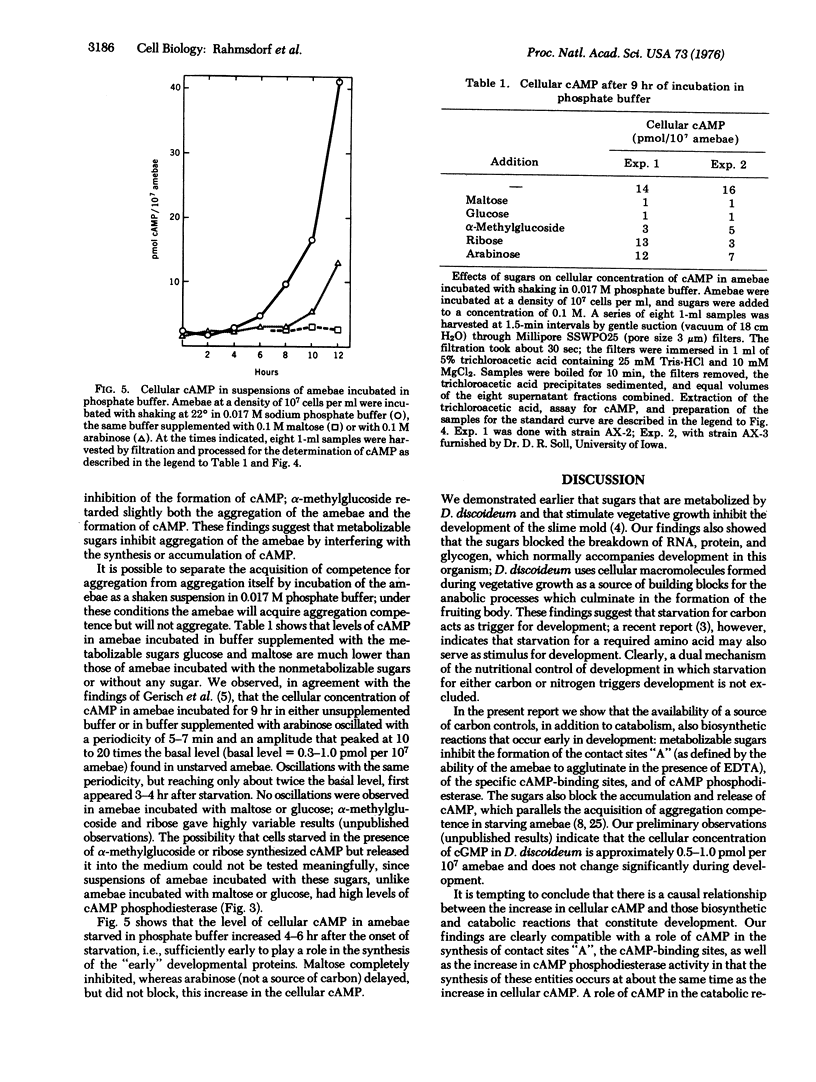
Metabolizable sugars blocked development of the slime mold Dictyostelium discoideum; the same sugars also inhibited the formation of contact sites "A", of membranal 3':5'-cyclic adenosine monophosphate (cAMP)-binding sites, and of total cAMP phosphodoesterase (3':5'-cyclic-nucleotide phosphodiesterase; EC 3.1.4.17; 3':5'-cyclic-nucleotide 5'-nucleotidohydrolase). These inhibitory effects of the sugars on the synthesis of cellular components, required for the aggregation of developing amebae, were paralleled by an inhibition of the accumulation of cAMP which normally accompanies development. The inhibition by sugars could be overcome partially by pulsing the amebae with nanomolar concentrations of cAMP only after the amebae had acquired cAMP-binding sites. The findings suggest that metabolizable sugars inhibit development by blocking the formation of cAMP and, conversely, that development in D. discoideum may be related to the energetic state of the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Gerisch G. A micromethod for routine measurement of cell agglutination and dissociation. J Immunol Methods. 1972 Nov;2(1):49–57. doi: 10.1016/0022-1759(72)90017-8. [DOI] [PubMed] [Google Scholar]
- Beug H., Katz F. E., Gerisch G. Dynamics of antigenic membrane sites relating to cell aggregation in Dictyostelium discoideum. J Cell Biol. 1973 Mar;56(3):647–658. doi: 10.1083/jcb.56.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. T., Barkley D. S., Hall E. M., Konijn T. M., Mason J. W., O'Keefe G., 3rd, Wolfe P. B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev Biol. 1969 Jul;20(1):72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- Cailla H. L., Racine-Weisbuch M. S., Delaage M. A. Adenosine 3',5' cyclic monophosphate assay at 10-15 mole level. Anal Biochem. 1973 Dec;56(2):394–407. doi: 10.1016/0003-2697(73)90205-4. [DOI] [PubMed] [Google Scholar]
- Darmon M., Brachet P., Da Silva L. H. Chemotactic signals induce cell differentiation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3163–3166. doi: 10.1073/pnas.72.8.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Fromm H., Huesgen A., Wick U. Control of cell-contact sites by cyclic AMP pulses in differentiating Dictyostelium cells. Nature. 1975 Jun 12;255(5509):547–549. doi: 10.1038/255547a0. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Wick U. Intracellular oscillations and release of cyclic AMP from Dictyostelium cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):364–370. doi: 10.1016/s0006-291x(75)80102-1. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Klein C. Induction of phosphodiesterase by cyclic adenosine 3':5'-monophosphate in differentiating Dictyostelium discoideum amoebae. J Biol Chem. 1975 Sep 25;250(18):7134–7138. [PubMed] [Google Scholar]
- Lee K. C. Permeability of Dictyostelium discoideum towards amino acids and inulin: a possible relationship between initiation of differentiation and loss of "pool" metabolites. J Gen Microbiol. 1972 Oct;72(3):457–471. doi: 10.1099/00221287-72-3-457. [DOI] [PubMed] [Google Scholar]
- Malchow D., Fuchila J., Nanjundiah V. A plausible role for a membrane-bound cyclic AMP phosphodiesterase in cellular slime mold chemotaxis. Biochim Biophys Acta. 1975 Apr 7;385(2):421–428. doi: 10.1016/0304-4165(75)90371-2. [DOI] [PubMed] [Google Scholar]
- Malchow D., Gerisch G. Short-term binding and hydrolysis of cyclic 3':5'-adenosine monophosphate by aggregating Dictyostelium cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2423–2427. doi: 10.1073/pnas.71.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkinson A. M., Ashworth J. M. Adenosine 3':5'-cyclic monophosphate concentrations and phosphodiesterase activities during axenic growth and differentiation of cells of the cellular slime mould Dictyostelium discoideum. Biochem J. 1973 May;134(1):311–319. doi: 10.1042/bj1340311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin F. T. Regulation of development in Dictyostelium discoideum: I. Initiation of the growth to development transition by amino acid starvation. Dev Biol. 1976 Jan;48(1):110–117. doi: 10.1016/0012-1606(76)90050-6. [DOI] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V., Rahmsdorf H. J., Campbell A., North M. J., Kwasniak J., Ashworth J. M. Inhibition of development in Dictyostelium discoideum by sugars. J Bacteriol. 1975 Oct;124(1):212–219. doi: 10.1128/jb.124.1.212-219.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A., Drage D. J., Cohen M. H. Control of Aggregation in Dictyostelium discoideum by an External Periodic Pulse of Cyclic Adenosine Monophosphate. Science. 1972 Jan 21;175(4019):333–335. doi: 10.1126/science.175.4019.333. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]


