Abstract
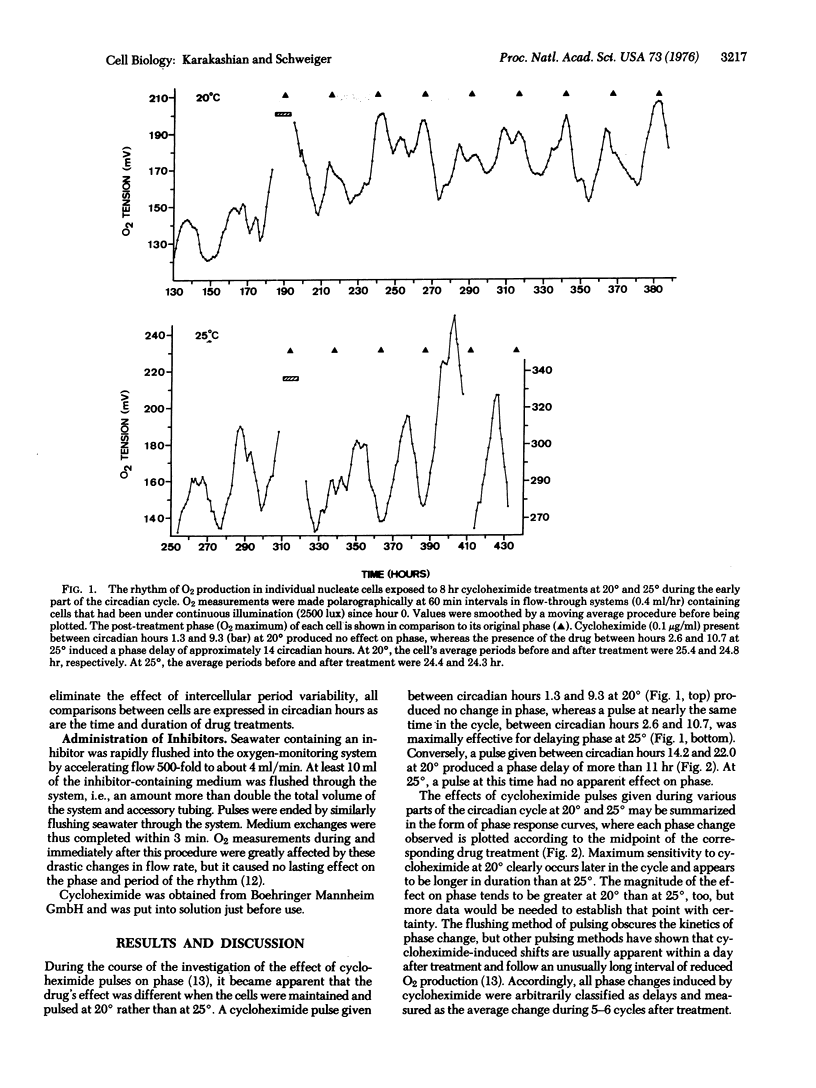
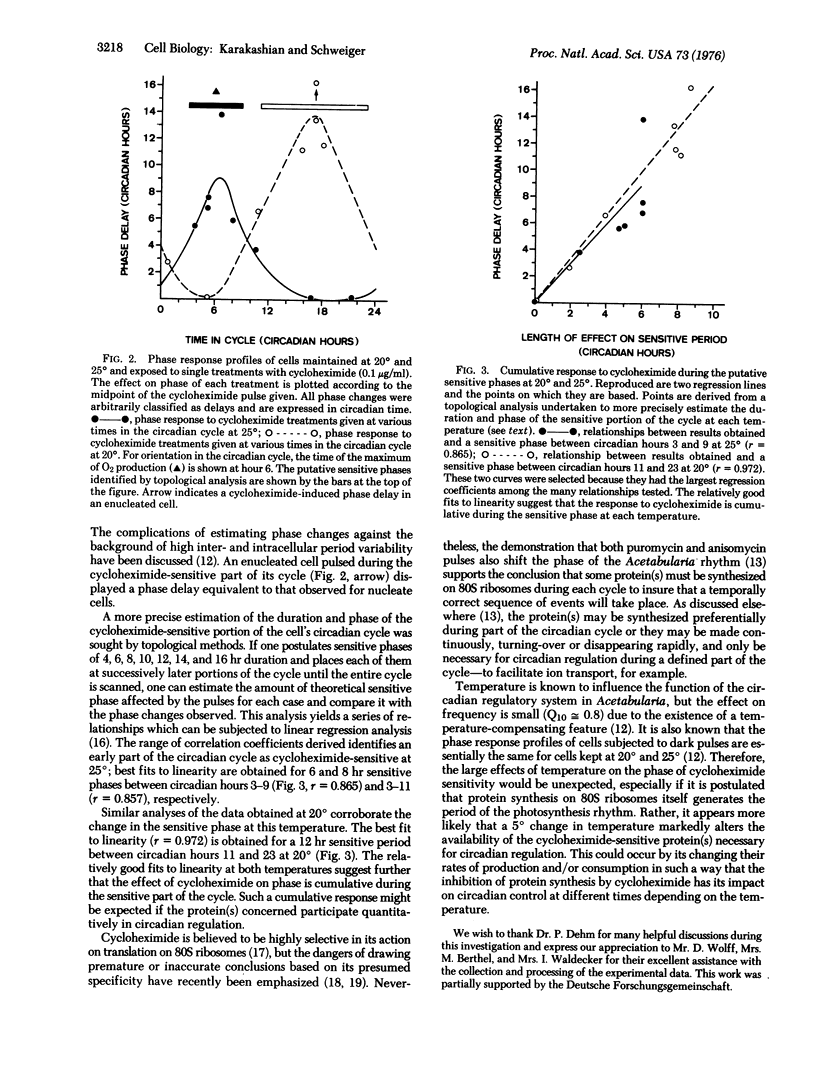
The biochemical nature of the circadian regulatory system that controls many cellular activities is still unclear. Recent results obtained from the application of protein synthesis inhibitors to individual Acetabularia cells expressing circadian rhythms of photosynthesis indicate that some protein(s) must be synthesized on 80S ribosomes during a discrete part of each cycle to insure correct time-keeping. A comparative study of the effects of brief cycloheximide treatments on cells investigated at different temperature has revealed that the phase of cycloheximide sensitivity is 4-6 hr longer and occurs about 8 hr later in the cycle when cells are kept at 20 degrees rather than 25 degrees. Temperature is known to influence the function of the circadian regulatory system in Acetabularia, but the effect on frequency is small (Q10 approximately equal to 0.8) due to the existence of a temperature-compensating feature. The large effects of temperature observed here thus favor the interpretation that protein synthesis on 80S ribosomes, while providing an essential component of the circadian timing mechanism, does not itself generate the period of the photosynthesis rhythm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce V. G. Mutants of the biological clock in Chlamydomonas reinhardi. Genetics. 1972 Apr;70(4):537–548. doi: 10.1093/genetics/70.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F. Lengthening the period of a biological clock in Euglena by cycloheximide, an inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1080–1087. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W. Biochemical aspects of rhythms: phase shifting by chemicals. Cold Spring Harb Symp Quant Biol. 1960;25:131–143. doi: 10.1101/sqb.1960.025.01.012. [DOI] [PubMed] [Google Scholar]
- KARAKASHIAN M. W., HASTINGS J. W. THE EFFECTS OF INHIBITORS OF MACROMOLECULAR BIOSYNTHESIS UPON THE PERSISTENT RHYTHM OF LUMINESCENCE IN GONYAULAX. J Gen Physiol. 1963 Sep;47:1–12. doi: 10.1085/jgp.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Circadian properties of the rhythmic system in individual nucleated and enucleated cells of Acetabularia mediterranea. Exp Cell Res. 1976 Feb;97(2):366–377. doi: 10.1016/0014-4827(76)90628-5. [DOI] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Evidence for a cycloheximide-sensitive component in the biological clock of Acetabularia. Exp Cell Res. 1976 Mar 15;98(2):303–312. doi: 10.1016/0014-4827(76)90442-0. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenhagen D., Schweiger H. G. Recording the oxygen production of a single Acetabularia cell for a prolonged period. Exp Cell Res. 1973 Oct;81(2):360–364. doi: 10.1016/0014-4827(73)90526-0. [DOI] [PubMed] [Google Scholar]
- Mergenhagen D., Schweiger H. G. The effect of different inhibitors of transcription and translation on the expression and control of circadian rhythm in individual cells of Acetabularia. Exp Cell Res. 1975 Sep;94(2):321–326. doi: 10.1016/0014-4827(75)90499-1. [DOI] [PubMed] [Google Scholar]
- Schweiger H. G. Cell biology of acetabularia. Curr Top Microbiol Immunol. 1969;50:1–36. doi: 10.1007/978-3-642-46169-9_1. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M., Tuffli C. F., Jr, Rubin R. H. The circadian rhythm in photosynthesis in Acetabularia in the presence of actinomycin D, puromycin, and chloramphenicol. J Gen Physiol. 1967 Jan;50(3):647–659. doi: 10.1085/jgp.50.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]


