Abstract
Borrelia burgdorferi, the Lyme disease agent, causes joint inflammation in an experimental murine model. Inflammation occurs, in part, due to the ability of B. burgdorferi to induce the production of proinflammatory cytokines and a strong CD4+ T helper type 1 response. The mechanisms by which spirochetes induce these responses are not completely known, although transcription factors, such as NF-κB in phagocytic cells, initiate the proinflammatory cytokine burst. We show here that the mitogen-activated protein (MAP) kinase of 38 kDa (p38 MAP kinase) is involved in the proinflammatory cytokine production elicited by B. burgdorferi Ags in phagocytic cells and the development of murine Lyme arthritis. B. burgdorferi Ags activated p38 MAP kinase in vitro, and the use of a specific inhibitor repressed the spirochete-induced production of TNF-α. The infection of mice that are deficient for a specific upstream activator of the kinase, MAP kinase kinase 3, resulted in diminished proinflammatory cytokine production and the development of arthritis, without compromising the ability of CD4+ T cells to respond to borrelial Ags or the production of specific Abs. Overall, these data indicated that the p38 MAP kinase pathway plays an important role in B. burgdorferi-elicited inflammation and point to potential new therapeutic approaches to the treatment of inflammation induced by the spirochete.
Lyme disease usually begins with a skin rash (erythema migrans) and may progress to involve the musculoskeletal, cardiovascular, or neurologic systems (1). The pathogenesis of Lyme disease is related to the number of Borrelia burgdorferi in the affected organs (2), spirochetal virulence (3–5), and the humoral and cellular responses arising during infection (6–11). Experimental infections of mice suggest a strong correlation between the production of proinflammatory cytokines, including IL-12 and IFN-γ, and the development of acute murine Lyme arthritis and spirochetal adaptation (6, 7, 10, 12–15). The modulation of the CD4+ T cell helper phenotype arising during experimental infection of mice affects the development of joint inflammation. Blockade of IL-12 and IFN-γ reduced arthritis severity at the peak of infection in immunocompetent mice (6, 7, 10). Furthermore, the infection of mice deficient for IL-6 production, a strong inducer of Th2 responses (16) and inhibitor of CD4+ Th1 differentiation (17), results in decreased Th2 responses and increased incidence of inflammation (18). The role of IFN-γ on the development of acute murine Lyme arthritis is controversial. Although the treatment of infected mice with blocking mAbs reduced the degree of arthritis (7, 10), the infection in the footpad of mice deficient for this cytokine (19) or its receptor (20) revealed a degree of inflammation similar to that of their wild-type counterparts.
B. burgdorferi induces the production of proinflammatory cytokines in different cell types (21–23). B. burgdorferi lipoproteins up-regulate chemokines and adhesion molecules in endothelial cells (24, 25) and fibroblasts (24), which is mediated by the activation of the transcription factor NF-κB (24–26). Moreover, B. burgdorferi is able to induce the production of proinflammatory cytokines in monocytes, mast cells, and other cell types, such as TNF-α, IL-12, and IFN-γ (22, 27, 28). The LPS receptor CD14 has been shown to facilitate the activation of human monocytic cells by B. burgdorferi (23, 29–31). CD14 engagement activates and induces transcriptional activity of NF-κB, probably through its interaction with Toll-like receptors (TLRs)3 (32). Indeed, outer surface protein A recently has been shown to induce the activation of TLR-2 with nuclear translocation of NF-κB (33) and induction of IL-12 p40 gene transcription (34).
Signal transduction through mitogen-activated protein (MAP) kinases plays a key role in several cellular responses, including growth factor-induced proliferation, differentiation, and cell death. Several parallel MAP kinase signal transduction pathways that are functionally independent have been defined in mammalian cells (35, 36), including the extracellular signal-regulated kinase (37, 38), c-Jun amino-terminal kinases (also known as stress-activated protein kinases) (39, 40), and p38 MAP kinase (41–43). These MAP kinases are activated by phosphorylation on Thr and Tyr by dual-specificity MAP kinase kinases (MKKs) (39, 44).
In mammalian cells, p38 MAP kinase can be activated by multiple stimuli, such as proinflammatory cytokines (e.g., IL-1β and TNF-α), LPS, and environmental stress (41–45). Upstream activators of the p38 MAP kinase include MKK3, MKK4, and MKK6 (46–49), which phosphorylate p38 MAP kinase on Thr and Tyr within the tripeptide motif TGY in kinase subdomain VIII, increasing enzymatic activity (44). The p38 MAP kinase is implicated in the expression of cytokines and the regulation of cell proliferation and death (45, 50). In vitro studies demonstrate that activating transcription factor 2 is phosphorylated and activated by p38 MAP kinase (44, 46, 49). In addition, p38 MAP kinase activates p62 ternary complex factor, C/EBP homologous protein, myocyte enhancer factor 2C, and serum-response factor accessory protein 1 transcription factors (46, 49, 51–53). p38 MAP kinase also phosphorylates and activates the eukaryotic initiation factor 4E protein kinases MAP kinase interacting kinase 1 and 2 (54, 55) and the small heat shock protein 27 protein kinase MAP kinase-activated protein kinase (43, 45, 56).
The p38 MAP kinase is a selective target for pyridinyl imidazole drugs (42). These drugs appear to act by inhibiting p38 MAP kinase activity through competition with ATP at the ATP binding pocket (57–59). These compounds are candidate drugs for the treatment of arthritis, bone resorption, and endotoxic shock (60–62). The SB 203580 compound possesses therapeutic activity in collagen-induced arthritis in DBA/LACJ mice, resulting in significant inhibition of paw inflammation and serum amyloid protein levels (61). This antiarthritic activity is associated with reduced production of proinflammatory cytokines, such as IL-1β and TNF-α, by activated macrophages (61).
We have now addressed the role of p38 MAP kinase activation in the development of acute Lyme arthritis in a murine model of infection.
Materials and Methods
Mice
MKK3-deficient mice (129/SvJ × C57BL/6 (129 × B6)) that had been previously generated (63) were used throughout the studies and were obtained by breeding second-generation deficient mice, as described (64). Control mice were derived from the same generation littermates. The mice were maintained in filter-framed cages and euthanized with CO2. All procedures that involve animals were approved by institutional guidelines for animal care.
B. burgdorferi and infections
The mice were infected with low-passaged B. burgdorferi N40 by intradermal injection of 104 spirochetes in the back, as previously described (6). The mice were sacrificed at 2 wk (peak of inflammation) and 8 wk (resolution of disease) of infection. The infectious status of the animals was determined by culture of different specimens (bladder, spleen, and skin at the inoculation site) in modified Barbour Stoenner Kelly II medium for 2 wk at 34°C (6).
B. burgdorferi lysates were obtained from late-log phase cultures of the spirochetes in Barbour Stoenner Kelly II medium by three freeze-thaw cycles in endotoxin-free water, as assessed by the Limulus amebocyte assay.
Histology
At sacrifice, hearts and joints were fixed in formalin and embedded in paraffin to assess signs of inflammation. Arthritis was assessed in both the knee and tibiotarsal joints and is characterized by neutrophilic infiltration, which may be accompanied by edema and hyperproliferation of the syno-vial membrane. Carditis is most evident at the base of the heart with periaortic infiltration of macrophages and is not graded. All determinations were made in a blinded fashion.
In vitro stimulations
The macrophage cell line RAW 264.7 was used to determine the ability of B. burgdorferi to induce the activation of p38 MAP kinase. A total of 2 × 106 cells/ml were incubated with 10 μg/ml of a B. burgdorferi extract for up to 60 min. The cells were then lysed and the extracts were subjected to PAGE and transferred to a nitrocellulose membrane. The membrane was then tested by Western blot for the presence of the phosphorylated form of p38 MAP kinase, using a specific Ab (Cell Signaling, Beverly, MA). Once developed, the membrane was reprobed with an Ab specific for actin (Santa Cruz Biotechnology, Santa Cruz, CA).
For the inhibition of p38 MAP kinase in vitro, the cells were stimulated with 10 μg/ml of a B. burgdorferi extract in the presence of increasing concentrations of SB 203580. The supernatants were collected after 16 h and analyzed for the presence of TNF-α by capture ELISA. All in vitro assays were performed in the presence of 10 μg/ml polymixin B.
CD4+ T cell restimulation
CD4+ T cells were isolated from spleens by negative selection as described (6), using biotinylated Abs against CD8a, MHC class II, panNK, Ly6G, CD11b, and B220 (BD PharMingen, San Diego, CA), followed by incubation with avidin bound to magnetic microbeads and passed through a magnetic column (Miltenyi Biotec, Auburn, CA). A total of 106 purified CD4+ T cells per milliliter were incubated with 106/ml syngeneic mytomycin C-treated APCs in the presence of 10 μg/ml of a B. burgdorferi extract. The supernatants were collected at 40 h of incubation and analyzed for the presence of IFN-γ by ELISA.
Cytokine ELISA
The levels of IFN-γ, TNF-α, and IL-12 in restimulation supernatants and murine sera were determined by capture ELISA, as described (6). Purified anti-IFN-γ, anti-TNF-α, and anti-IL-12 (2 μg/ml; BD PharMingen) as capture Abs, the corresponding biotinylated Abs (1 μg/ml; BD PharMingen), horseradish-conjugated streptavidin (1/1000 dilution; BD PharMingen), and the tetramethylbenzidine microwell peroxidase substrate and stop solution (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were used, according to the recommended protocol (BD PharMingen). As standards, recombinant IFN-γ, TNF-α, and IL-12 (BD PharMingen) were used.
Ab isotype ELISA
B. burgdorferi-specific IgM and IgG subclass levels in the sera from the infected animals were determined by ELISA using biotinylated rat monoclonal anti-mouse Abs specific to mouse IgM, IgG1, IgG2b, and IgG3 (BD PharMingen), as described (6).
Flow cytometry
Whole splenocytes depleted of red cells were stained for activation markers in different cell populations. The analysis of memory (CD44high CD45RBlow) and naive (CD44lowCD45RBhigh) populations of CD4+ T cells was performed by triple staining with anti-CD4CyChrome, CD44PE, and CD45RBFITC. CD4+ T cells were also analyzed for the levels of surface expression of IL-2Rα and L-selectin with anti-C25PE and CD62LFITC, respectively. Phagocytic cell activation was analyzed by staining with either anti-CD11bFITC or anti-Ly6GPE and biotinylated anti-IFN-γRα, followed by incubation with PE- or FITC-labeled streptavidin, respectively. The analysis was performed in a FACSCalibur apparatus (BD Biosciences, Mountain View, CA) and the data were analyzed using the CellQuest software package (BD Biosciences).
Results
B. burgdorferi induces the phosphorylation of p38 MAP kinase in RAW 264.7 cells
The MAP kinase p38 has been associated with the production of proinflammatory cytokines such as IL-12, TNF-α, and IL-1β (42, 65). B. burgdorferi is able to induce the release of proinflammatory cytokines in vitro through a mechanism that involves, at least partially, its interaction with CD14 (23, 33) and TLRs (33, 34, 66). This suggested to us a potential activation of the p38 MAP kinase that in turn could be important for proinflammatory cytokine production and the development of inflammation during Lyme borreliosis.
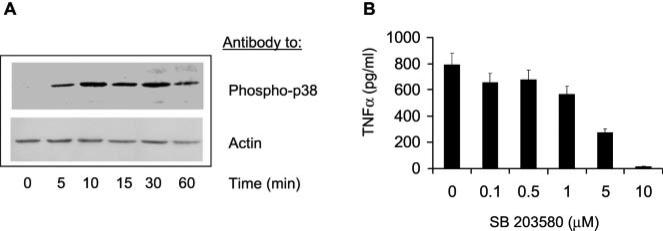
To assess the involvement of B. burgdorferi Ags in p38 MAP kinase activation, we analyzed the ability of whole B. burgdorferi lysates to induce the phosphorylation of the kinase in the murine macrophage cell line RAW 264.7 by Western blot analysis. B. burgdorferi extracts induced the phosphorylation of p38 MAP kinase in this cell line. The p38 MAP kinase phosphorylation was evident as soon as 5 min after exposure to B. burgdorferi lysates (Fig. 1A).
FIGURE 1.
B. burgdorferi induction of p38 MAP kinase phosphorylation results in proinflammatory cytokine production. A, The murine macrophage cell line RAW 264.7 (2 × 106 cells/ml) was incubated with 10 μg/ml of a B. burgdorferi extract for different time points in the presence of 10 μg/ml polymixin B, and the phosphorylation status of p38 was analyzed by Western blot. Equal loads were assured by reprobing the membranes with an anti-actin Ab (control). B, The p38 MAP kinase specific inhibitor SB 203580 inhibits B. burgdorferi-induced TNF-α production in RAW 264.7 cells. A total of 2 × 106 cells/ml was activated with 10 μg/ml of a B. burgdorferi lysate in the presence of different molar concentrations of SB 203580 and 10 μg/ml polymixin B for 16 h. The supernatants were analyzed for TNF-α by capture ELISA. The results are representative of at least three independent experiments.
The involvement of the p38 MAP kinase pathway in cytokine production by the macrophage cell line RAW 264.7 in response to B. burgdorferi Ags was then evaluated. The compound SB 203580 is a specific inhibitor of the p38 MAP kinase pathway. We studied the effect of SB 203580 on the production of B. burgdorferi-induced TNF-α production by the macrophage cell line RAW 264.7. The cells were incubated with 10 μg/ml of a B. burgdorferi lysate in the presence of increasing concentrations of the inhibitor, and 16 h later the supernatants were analyzed for the presence of TNF-α. The presence of increasing concentrations of SB 203580 inhibited the production of TNF-α (Fig. 1B), indicating that this pathway is implicated in the production of proinflammatory cytokines in response to spirochetal Ags in vitro and suggesting that it may be important for the production of inflammation as a result of infection. The observed effects of the inhibitor on the macrophage cell line were not due to toxicity, as assessed by visual evaluation of their morphology and trypan blue exclusion after 72 h of incubation with 10 μM SB203580 (data not shown).
B. burgdorferi-infected MKK3-deficient mice develop a lower inflammatory response than do wild-type controls
MKK3-deficient mice have fundamental defects in the inflamma-tory response and in Th1 CD4+ T cell responses (63). LPS-activated p38 MAP kinase was reduced, but not absent, in MKK3-deficient macrophages, resulting in an almost complete blockade of the induction of IL-12 production and IL-12 p40 mRNA expression. This defect, plus intrinsic defects in T cells, led to reduced IFN-γ production by differentiated CD4+ T cells (63). To clarify the contribution of MKK3 in the activation of p38 MAP kinase and subsequent development of Lyme arthritis and carditis, we infected MKK3-deficient mice (63) and wild-type controls with 104 spirochetes in the midline of the back (6). Two weeks later, the mice were analyzed for disease appearance by histological evaluation of the joints in the rear limbs and the hearts (67). Compared with wild-type controls, MKK3-deficient mice developed arthritis with a significantly lower incidence (eight of eight infected control mice developed arthritis vs one of five MKK3-deficient mice; Fisher's exact test, p = 0.007; Table I), indicating that the lack of activation of p38 MAP kinase by MKK3 affected the development of inflammation. The difference in arthritis was also evident at 8 wk of infection, the period of disease resolution (Table I). Carditis prevalence was not affected by the lack of this gene. At the peak of disease (2 wk) and at the time at which inflammation is regressing (2 mo), both groups of mice had similar cardiologic involvement (Table I).
Table I.
Arthritis and carditis prevalence in MKK3-deficient mice at 2 and 8 wk of infectiona
| Mice | Day of Sacrifice | Infection | Arthritis | Carditis |
|---|---|---|---|---|
| Control | 14 | 8/8 | 8/8 | 3/8 |
| MKK3–/– | 14 | 5/5 | 1/5b | 2/5 |
| Control | 60 | 7/7 | 4/7 | 3/7 |
| MKK3–/– | 60 | 5/5 | 0/5b | 3/5 |
At sacrifice, rear limbs and hearts were fixed in formalin and embedded in paraffin. For arthritis prevalence, both knees and tibiotarsi were analyzed.
Significantly different from control-infected mice. Value of p < 0.05 (Student's t test).
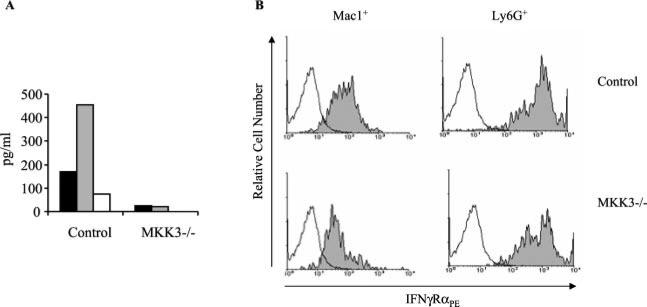
We then analyzed the levels of proinflammatory cytokines in the sera of the infected mice. As expected, the sera of the MKK3-deficient infected mice contained lower levels of IL-12, IFN-γ, and TNF-α than did control infected animals (Fig. 2A). These data indicated that infection with B. burgdorferi of MKK3-deficient mice results in lower proinflammatory cytokine production and arthritis development.
FIGURE 2.
B. burgdorferi-infected MKK3-deficient mice develop a lower inflammatory response. A, Two-week-infected mice were analyzed for IL-12 (filled bars), IFN-γ (shaded bars), and TNF-α (open bars) in the serum. B, The activation status of Mac-1+ and Ly6G+ splenocytes was assessed by fluorescent staining of surface IFN-γRα. Splenocytes were obtained from 2-wk-infected MKK3-deficient and wild-type controls with B. burgdorferi, double stained with anti-CD11b or anti-Ly6G plus anti-IFN-γRα, and analyzed by flow cytometry. The results shown are representative of five to six mice in each group in two independent experiments.
Proinflammatory cells express lowered levels of IFN-γRα in MKK3-deficient mice infected with B. burgdorferi
Inflammation as a result of infection with B. burgdorferi is at least partially dependent on the recruitment and activation of phagocytic cells (67). These cells are activated in response to T cell signals (i.e., cytokines and CD40 ligand cross-linking) as well as by a direct response to bacterial components (33). To establish the activation status of macrophages and neutrophils, which could explain the lower development of inflammation in MKK3-deficient mice, we analyzed the surface expression of the α subunit of the receptor for IFN-γ. In agreement with our previous results, macrophages (Fig. 2B, left panels) and, to a lesser extent, neutrophils (Fig. 2B, right panels) expressed lower levels of IFN-γRα, suggesting a diminished activation status and a lowered ability to respond to this proinflammatory cytokine.
CD4+ T cells in MKK3-deficient mice infected with B. burgdorferi produce less IFN-γ in response to spirochetal Ags
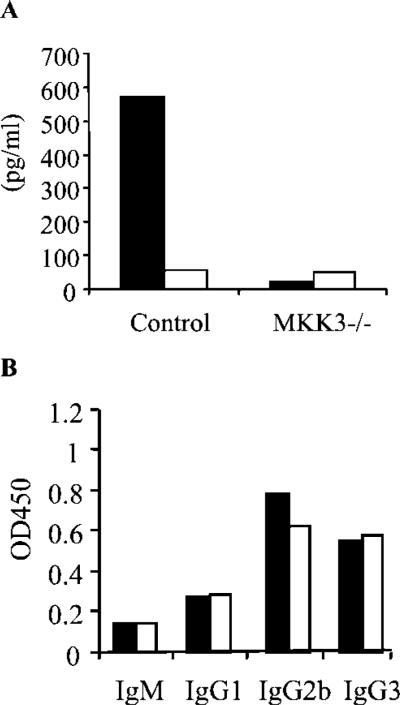
The differentiation phenotype of CD4+ T cells during Lyme borreliosis also affects the development of inflammation. A predominant Th1 phenotype has been observed in mice experimentally infected with B. burgdorferi (7, 10, 68) and in humans (69). Therefore, we assessed the cytokine production pattern of CD4+ T cells from the infected mice in restimulation assays in vitro (6). CD4+ T cells from infected MKK3-deficient mice produced lower levels of IFN-γ in response to B. burgdorferi Ags than did those from wild-type controls, indicating a lower Th1 phenotype in response to B. burgdorferi infection (Fig. 3A). However, this reduction in the production of IFN-γ by CD4+ T cells was not accompanied by an increase in the production of IL-4 (Fig. 3A), suggesting that the lack of the mkk3 gene inhibited Th1 differentiation without affecting Th2 cells.
FIGURE 3.
A, IFN-γ and IL-4 production by CD4+ T cells in response to B. burgdorferi Ags in vitro. CD4+ T cells were purified from MKK3-deficient and wild-type controls at 2 wk of infection and restimulated in vitro with 10 μg/ml of a B. burgdorferi lysate in the presence of syngeneic APCs. The supernatants were analyzed by capture ELISA for IFN-γ (filled bars) and IL-4 (open bars) at 40 h of stimulation. Results are the average of four mice and are representative of three independent experiments. B, B. burgdorferi-specific Ab isotype titers at 2 wk of infection in MKK3-deficient mice (open bars) and wild-type controls (filled bars). The results are representative of at least five experiments with similar results.
We also tested whether the lack of the mkk3 gene had affected the ability of CD4+ T cells to become activated during infection with B. burgdorferi. The activation phenotype of CD4+ T cells was equivalent in both control and MKK3-deficient mice. Naive (CD44lowCD45RBhigh) and activated (CD44highCD45RBlow) CD4+ T cell populations were identical in both groups, as was the level of surface expression of activation markers such as CD25 and L-selectin (CD62L) (data not shown). These results suggest that the lack of the mkk3 gene reduces the ability of CD4+ T cells to produce IFN-γ in response to B. burgdorferi Ags but does not affect the ability of CD4+ T cells to become activated upon infection with the spirochete.
In correlation with the similar level of activation of CD4+ T cells, the levels of B. burgdorferi-specific Ab isotypes were equivalent in MKK3-deficient mice compared with controls (Fig. 3B), indicating that the lack of the gene encoding MKK3 had not affected the development of a spirochete-specific Ab response.
Discussion
Inflammation elicited by B. burgdorferi is controlled by several factors. Both the intrinsic ability of specific isolates to activate different cell types and factors in the host that influence the capacity to respond to the invading microorganism can influence the degree of proinflammatory responses. The mechanisms by which B. burgdorferi Ags induce the production of proinflammatory cytokines have been partly elucidated. TLR-2 appears to have a role in the provision of the signals that lead to the up-regulation of proinflammatory genes in response to lipoproteins of B. burgdorferi, like outer surface protein A (33, 66). This is accomplished at least in part by the activation of the transcription factor NF-κB (24–26).
In this work we show that B. burgdorferi Ags induce the phosphorylation of the p38 MAP kinase in a macrophage cell line in vitro and ex vivo in isolated macrophages (data not shown). The relevance of the activation of this MAP kinase in vivo is underscored by the diminished incidence of arthritis in mice that lack the gene that encodes MKK3, a specific activator of the p38 MAP kinase. Not surprisingly, the lowered inflammatory symptoms appearing on MKK3-deficient mice correlate with diminished levels of systemic proinflammatory cytokines, including IFN-γ, TNF-α, and IL-12.
Overall, our data show that the interaction of B. burgdorferi with innate immune cells results in the activation of the p38 MAP kinase pathway that may be acting in coordination with NF-κB to result in the activation of these cells. These results also suggest that the p38 MAP kinase pathway may have an indirect effect on the activation of phagocytic cells by inducing the expression of surface receptors for other proinflammatory factors like IFN-γ. The lack of MKK3-mediated p38 MAP kinase activity results in lower levels of the α subunit of the IFN-γ receptor, which in turn may preclude these cells from responding to the same extent to this proinflammatory cytokine.
The role of IFN-γ in the development of acute murine Lyme arthritis is controversial. Reports have concluded that the lack of the gene that encodes the cytokine or its receptor does not affect the development of inflammation upon infection (19, 20). However, we have demonstrated that IFN-γ-mediated signals are important for the adaptation of B. burgdorferi to the murine host (15), and a correlation between IFN-γ production upon infection and inflammation has been extensively established (7, 10, 15). The discrepancy may result from the inoculation route used in the different experimental approaches. Indeed, although strain-specific disease resistance and susceptibility has been determined in mice intradermally infected with spirochetes (2), hind foot inoculation results in the same degree of arthritis in disease-resistant (BALB/c) and susceptible (C3H) strains of mice (70). The discrepancy was related to the route of spirochetal dissemination and accessibility of anatomical sites in which spirochetes may preferentially replicate and cause disease (70). Cultured spirochetes show a distinct pattern of expression compared with “adapted” spirochetes after several days of infection (71). We have demonstrated that indeed the lack of pathogenicity of high-passaged derivatives of the N40 isolate is associated with impaired gene expression (71) and adaptation to the murine host (15), which may result, at least in part, because of the inability of this nonpathogenic derivative to induce strong proinflammatory cytokine production, including production of IFN-γ (15). Overall, our results show that MKK3 deficiency resulted in lower production of all proinflammatory cytokines tested (IFN-γ, TNF-α, and IL-12), underscoring the importance of this pathway in B. burgdorferi-induced inflammatory responses.
Our results also show that the differentiation of CD4+ T cells to a Th1 phenotype is dependent on the p38 MAP kinase pathway. We have previously shown that mice that lack the mkk3 gene are defective in innate cell LPS-dependent production of IL-12. This defect is partially responsible for a lowered Th1 phenotype when CD4+ T cells are activated in vitro (63). An intrinsic defect in CD4+ T cells in these mice also prevents them from a complete Th1 differentiation. Indeed, p38 MAP kinase activity is involved in IFN-γ production by effector Th1 cells (72). However, this effect on CD4+ T cells does not impair the ability of the mice to develop strong Ab responses against the spirochete, including isotypes that are borreliacidal and can fix complement.
Carditis prevalence was not affected by the lack of the specific activator of p38 MAP kinase, MKK3, suggesting as previously reported (64, 73) that the mechanisms that trigger both inflamma-tory processes (arthritis and carditis) are different. The lack of modulation of carditis prevalence could also be due to differences in activity regulation of the kinase in the joints and the heart. Indeed, p38 MAP kinase isoform distribution in different tissues is not homogeneous (74), and p38 MAP kinase can also be activated by the upstream kinase MKK6 (49). Our results suggest that MKK3 activity is more relevant for joint than cardiac inflammation during Lyme borreliosis. Further work is required to understand the differences between both inflammatory phenomena.
In summary, we show in this work that the p38 MAP kinase cascade may have a profound impact on the overall immune response to the infection with B. burgdorferi, in activating innate immune cells with subsequent production of proinflammatory cytokines and the development of Th1 responses. Because pyridinyl imidazole drugs that specifically target this pathway are currently under evaluation under clinical trials, the knowledge of specific mechanisms used by the spirochete to induce inflammation will bring new therapeutic approaches to treat this highly prevalent infection, without compromising the Ab response that is necessary for the bacterial clearance.
Acknowledgments
We thank Debby Beck and Manchuan Chen for technical assistance.
Footnotes
This work was supported by grants from the National Institutes of Health (to J.A., E.F., S.W.B., R.J.D., and R.A.F.), the American Heart Association (to J.A. and R.A.F.), and the Arthritis Foundation (to R.A.F.). E.F. is the recipient of a Clinical-Scientist Award in Translational Research from the Burroughs Wellcome Fund. R.A.F. and R.J.D. are investigators of the Howard Hughes Medical Institute.
Abbreviations used in this paper: TLR, Toll-like receptor; MAP, mitogen-activated protein; MKK, MAP kinase kinase.
References
- 1.Steere AC. Lyme disease. N. Engl. J. Med. 2001;345:115. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Weis JH, Eichwald E, Kolbert CP, Persing DH, Weis JJ. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 1994;62:492. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll JA, Dorward DW, Gherardini FC. Identification of a transferrin-binding protein from Borrelia burgdorferi. Infect. Immun. 1996;64:2911. doi: 10.1128/iai.64.8.2911-2916.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes CA, Engstrom SM, Coleman LA, Kodner CB, Johnson RC. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect. Immun. 1993;61:5115. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris SJ, Howell JK, Garza SA, Ferdows MS, Barbour AG. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 1995;63:2206. doi: 10.1128/iai.63.6.2206-2212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anguita J, Persing DH, Rincón M, Barthold SW, Fikrig E. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J. Clin. Invest. 1996;97:1028. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keane-Myers A, Nickell SP. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 1995;155:2020. [PubMed] [Google Scholar]
- 8.Keane-Myers A, Nickell SP. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 1995;154:1770. [PubMed] [Google Scholar]
- 9.Lengl-Janssen B, Strauss AF, Steere AC, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A in patients with treatment-resistant or treatment-responsive Lyme arthritis. J. Exp. Med. 1994;180:2069. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matyniak JE, Reiner SL. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 1995;181:1251. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Ma Y, Schoenfeld R, Griffiths M, Eichwald E, Araneo B, Weis JJ. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect. Immun. 1992;60:3033. doi: 10.1128/iai.60.8.3033-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher Doyle M, Telford SR, III, Criscione L, Lin SR, Spielman A, Gravallese EM. Cytokines in murine Lyme carditis: Th1 cytokine expression follows expression of proinflammatory cytokines in a susceptible mouse strain. J. Infect. Dis. 1998;177:242. doi: 10.1086/517364. [DOI] [PubMed] [Google Scholar]
- 14.Keane-Myers A, Maliszewski CR, Finkelman FD, Nickell SP. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J. Immunol. 1996;156:2488. [PubMed] [Google Scholar]
- 15.Anguita J, Thomas V, Samanta S, Persinski R, Hernanz C, Barthold SW, Fikrig E. Borrelia burgdorferi-induced inflammation facilitates spirochete adaptation and variable major protein-like sequence locus recombination. J. Immunol. 2001;167:3383. doi: 10.4049/jimmunol.167.6.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Inter-leukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 1997;185:461. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincón M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS-1. Immunity. 2000;13:805. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 18.Anguita J, Rincón M, Samanta S, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased Lyme arthritis. J. Infect. Dis. 1998;178:1512. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 19.Brown CR, Reiner SL. Experimental Lyme arthritis in the absence of interleukin-4 or γ interferon. Infect. Immun. 1999;67:3329. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glickstein L, Edelstein M, Dong JZ. γ-Interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 2001;69:3737. doi: 10.1128/IAI.69.6.3737-3743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatro JB, Romero LI, Beasley D, Steere AC, Reichlin S. Borrelia burgdorferi and Escherichia coli lipopolysaccharides induce nitric oxide and interleukin-6 production in cultured rat brain cells. J. Infect. Dis. 1994;169:1014. doi: 10.1093/infdis/169.5.1014. [DOI] [PubMed] [Google Scholar]
- 22.Radolf JD, Arndt LL, Akins DR, Curetty LL, Levi ME, Shen Y, Davis LS, Norgard MV. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 1995;154:2866. [PubMed] [Google Scholar]
- 23.Sellati TJ, Bouis DA, Caimano MJ, Feulner JA, Ayers C, Lien E, Radolf JD. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 1999;163:2049. [PubMed] [Google Scholar]
- 24.Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S. Borrelia burgdorferi activates nuclear factor-κB and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibro-blasts. J. Immunol. 1997;158:3285. [PubMed] [Google Scholar]
- 25.Wooten RM, Modur VR, McIntyre TM, Weis JJ. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-κB and inflammatory activation in human endothelial cells. J. Immunol. 1996;157:4584. [PubMed] [Google Scholar]
- 26.Norgard MV, Arndt LL, Akins DR, Curetty LL, Harrich DA, Radolf JD. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κB. Infect. Immun. 1996;64:3845. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Weis JJ. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect. Immun. 1993;61:3843. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talkington J, Nickell SP. Borrelia burgdorferi spirochetes induce mast cell activation and cytokine release. Infect. Immun. 1999;67:1107. doi: 10.1128/iai.67.3.1107-1115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 1999;67:140. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellati TJ, Bouis DA, Kitchens RL, Darveau RP, Pugin J, Ulevitch RJ, Gangloff SC, Goyert SM, Norgard MV, Radolf JD. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 1998;160:5455. [PubMed] [Google Scholar]
- 31.Wooten RM, Morrison TB, Weis JH, Wright SD, Thieringer R, Weis JJ. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 1998;160:5485. [PubMed] [Google Scholar]
- 32.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 1999;163:2382. [PubMed] [Google Scholar]
- 34.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 35.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK): from inflammation to development. Curr. Opin. Cell Biol. 1998;10:205. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 36.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 37.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 38.Boulton TG, Yancopoulos GD, Gregory JS, Slaughter C, Moomaw C, Hsu J, Cobb MH. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 39.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 40.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase sub-family of c-Jun kinases. Nature. 1994;369:156. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 41.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 42.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 43.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphor-ylation of the small heat shock proteins. Cell. 1994;78:1027. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 44.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 45.Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 46.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 1996;271:2886. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 48.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, et al. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J. Biol. Chem. 1996;271:13675. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 49.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 1996;16:1247. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 51.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 52.Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272:1347. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 53.Whitmarsh AJ, Yang SH, Su MS, Sharrocks AD, Davis RJ. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol. 1997;17:2360. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLaughlin MM, Kumar S, McDonnell PC, Van Horn S, Lee JC, Livi GP, Young PR. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J. Biol. Chem. 1996;271:8488. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 57.Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat. Struct. Biol. 1997;4:311. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- 58.Wilson KP, McCaffrey PG, Hsiao K, Pazhanisamy S, Galullo V, Bemis GW, Fitzgibbon MJ, Caron PR, Murcko MA, Su MS. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem. Biol. 1997;4:423. doi: 10.1016/s1074-5521(97)90194-0. [DOI] [PubMed] [Google Scholar]
- 59.Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr SA, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 1997;272:12116. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 60.Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 2000;47:185. doi: 10.1016/s0162-3109(00)00206-x. [DOI] [PubMed] [Google Scholar]
- 61.Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J. Pharmacol. Exp. Ther. 1996;279:1453. [PubMed] [Google Scholar]
- 62.Jackson JR, Bolognese B, Hillegass L, Kassis S, Adams J, Griswold DE, Winkler JD. Pharmacological effects of SB 220025, a selective inhibitor of P38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J. Pharmacol. Exp. Ther. 1998;284:687. [PubMed] [Google Scholar]
- 63.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fikrig E, Barthold SW, Chen M, Grewal IS, Craft J, Flavell RA. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J. Immunol. 1996;157:1. [PubMed] [Google Scholar]
- 65.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914. [PMC free article] [PubMed] [Google Scholar]
- 66.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 2002;168:348. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 67.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 1990;162:133. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 68.Schaible UE, Kramer MD, Wallich R, Tran T, Simon MM. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur. J. Immunol. 1991;21:2397. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 69.Yssel H, Shanafelt MC, Soderberg C, Schneider PV, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 1991;174:593. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect. Immun. 1997;65:3107. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anguita J, Samanta S, Revilla B, Suk K, Das S, Barthold SW, Fikrig E. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 2000;68:1222. doi: 10.1128/iai.68.3.1222-1230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rincón M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, Penix LA, Davis RJ, Flavell RA. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fikrig E, Barthold SW, Chen M, Chang CH, Flavell RA. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J. Immunol. 1997;159:5682. [PubMed] [Google Scholar]
- 74.Nick JA, Avdi NJ, Young SK, Lehman LA, McDonald PP, Frasch SC, Billstrom MA, Henson PM, Johnson GL, Worthen GS. Selective activation and functional significance of p38α mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J. Clin. Invest. 1999;103:851. doi: 10.1172/JCI5257. [DOI] [PMC free article] [PubMed] [Google Scholar]