Abstract
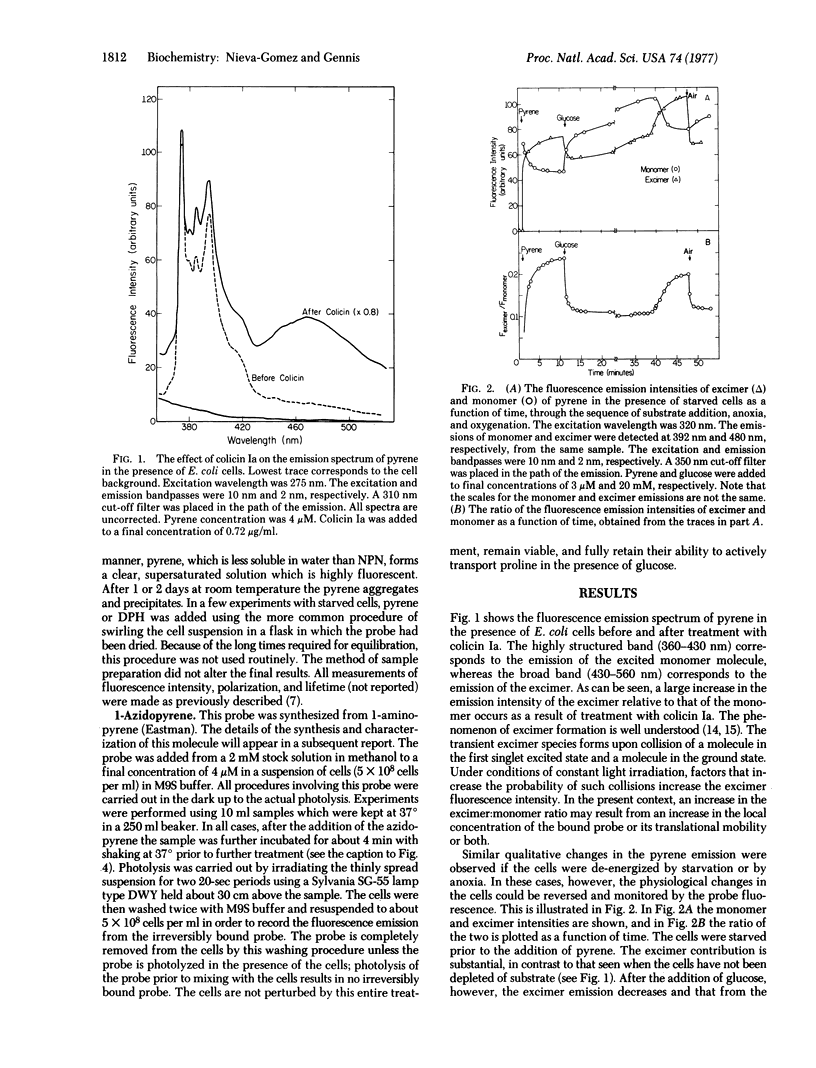
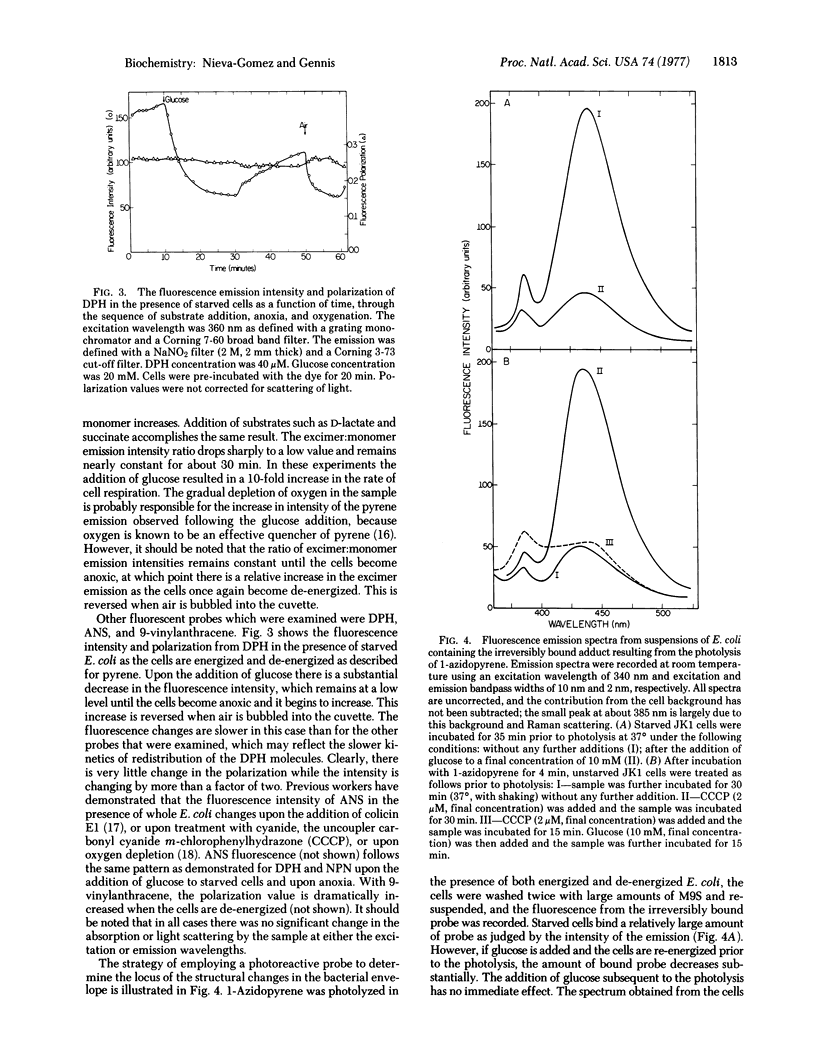
The fluorescence parameters of several common membrane probes in the presence of whole E. coli have been examined. The probes included electrically neutral lipophilic molecules N-phenyl-1-naphthylamine, pyrene, and 1,6-diphenyl-1,3,5-hexatriene as well as the negatively charged molecule 8-anilino-1-naphthalene sulfonate. It is demonstrated in each case that certain fluorescence parameters are a function of the state of energization of the cells. All the probes appear to monitor structural changes in the E. coli envelope which accompany the energization and de-energization of the cells. tthe phenomenon is completely reversible as demonstrated by re-energizing anoxic cells by the addition of oxygen, or starved cells by the addition of substrate. All the results are qualitatively consistent with an increased binding of probe by de-energized cells and a subsequent expulsion of probe when the cells are re-energized. A pyrene substituted with a photosensitive group, 1-azidopyrene, has been synthesized. Photolysis in the presence of a suspension of energized E. coli reveals a relatively small amount of probe irreversibly bound to the cells. However, in the presence of cells that have been de-energized the amount of irreversibly bound probe is dramatically increased. This molecule should be useful for localizing the regions of the bacterial envelope that are involved in the structural changes being monitored in these experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abendano J. J., Kepes A. Sensitization of D-glucuronic acid transport system of E. coli to protein group reagents in presence of substrate or absence of energy source. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1342–1346. doi: 10.1016/0006-291x(73)91134-0. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J. The state of energization of the membrane of Escherichia coli as affected by physiological conditions and colicin K. Biochemistry. 1976 Apr 6;15(7):1387–1392. doi: 10.1021/bi00652a006. [DOI] [PubMed] [Google Scholar]
- Brocklehurst J. R., Freedman R. B., Hancock D. J., Radda G. K. Membrane studies with polarity-dependent and excimer-forming fluorescent probes. Biochem J. 1970 Feb;116(4):721–731. doi: 10.1042/bj1160721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P., Khorana G. A new approach to the study of phospholipid-protein interactions in biological membranes. Synthesis of fatty acids and phospholipids containing photosensitive groups. Biochemistry. 1975 Nov 18;14(23):5021–5033. doi: 10.1021/bi00694a001. [DOI] [PubMed] [Google Scholar]
- Chance B. Fluorescent probe environment and the structural and charge changes in energy coupling of mitochondrial membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):560–571. doi: 10.1073/pnas.67.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Phillips S. K. Response of an Escherichia coli-bound fluorescent probe to colicin E1. J Bacteriol. 1970 Nov;104(2):819–825. doi: 10.1128/jb.104.2.819-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Postma P. W., Helgerson S. L. An evaluation of N-phenyl-1-naphthylamine as a probe of membrane energy state in Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):401–411. doi: 10.1016/0005-2728(76)90151-1. [DOI] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Gilchrist M. J., Konisky J. Effects of colicin Ia on transport and respiration in Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2457–2462. [PubMed] [Google Scholar]
- Griniuviene B., Dzheia P., Grinius L. Anilinonaphthalenesulfonate as a fluorescent probe of the energized membrane state in Escherichia coli cells and sonicated membrane particles. Biochem Biophys Res Commun. 1975 May 19;64(2):790–796. doi: 10.1016/0006-291x(75)90390-3. [DOI] [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Kepes A. Changes in accessibility of the membrane bound transport enzyme glucose phosphotransferase of E. coli to protein group reagents in presence of substrate or absence of energy source. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1335–1341. doi: 10.1016/0006-291x(73)91133-9. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Harris J. M., Lytle F. E. Evidence for a microviscosity increase in the Escherichia coli cell envelope caused by colicin E1. Biochemistry. 1974 Jul 16;13(15):3057–3061. doi: 10.1021/bi00712a010. [DOI] [PubMed] [Google Scholar]
- Klip A., Gitler C. Photoactive covalent labeling of membrane components from within the lipid core. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1155–1162. doi: 10.1016/0006-291x(74)90433-1. [DOI] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S. Interaction of colicin Ia with bacterial cells. Direct measurement of Ia-receptor interaction. J Biol Chem. 1972 Oct 25;247(20):6524–6529. [PubMed] [Google Scholar]
- Lanyi J. K. Influence of electron transport on the interaction between membrane lipids and Triton X-100 in Halobacterium cutirubrum. Biochemistry. 1973 Mar 27;12(7):1433–1438. doi: 10.1021/bi00731a025. [DOI] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A. Estimations of membrane potentials in Streptococcus faecalis by means of a fluorescent probe. Biochem Biophys Res Commun. 1974 Apr 8;57(3):620–626. doi: 10.1016/0006-291x(74)90591-9. [DOI] [PubMed] [Google Scholar]
- Luzikov V. N., Romashina L. V. Studies on stabilization of the oxidative phosphorylation system. II. Electron transfer-dependent resistance of succinate oxidase and NADH oxidase systems of submitochondrial particles to proteinases and cobra venom phospholipase. Biochim Biophys Acta. 1972 Apr 20;267(1):37–47. doi: 10.1016/0005-2728(72)90136-3. [DOI] [PubMed] [Google Scholar]
- Nieva-Gomez D., Konisky J. Membrane changes in Escherichia coli induced by colicin Ia and agents known to disrupt energy transduction. Biochemistry. 1976 Jun 29;15(13):2747–2753. doi: 10.1021/bi00658a006. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nordenbrand K., Ernster L. Studies of the energy-transfer system of submitochondrial particles. Fluorochrome response as a measure of the energized state. Eur J Biochem. 1971 Jan;18(2):258–273. doi: 10.1111/j.1432-1033.1971.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Okada M., Hirose S., Tamaura Y., Yamazaki S., Ushiwata A. Changes in reactivity of mitochondria with 1-dimethylaminonaphthalene-5-sulfonyl chloride (dansyl chloride) transforming from deenergized to energized state. Arch Biochem Biophys. 1974 May;162(1):316–319. doi: 10.1016/0003-9861(74)90132-5. [DOI] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Weil R., Kaback H. R. Energy-dependent binding of dansylgalactoside to the lac carrier protein: direct binding measurements. Proc Natl Acad Sci U S A. 1976 Jan;73(1):109–112. doi: 10.1073/pnas.73.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Staros J. V., Richards F. M. Photochemical labeling of the surface proteins of human erythrocytes. Biochemistry. 1974 Jun 18;13(13):2720–2726. doi: 10.1021/bi00710a010. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Callis J. B. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry. 1974 Sep 10;13(19):4000–4006. doi: 10.1021/bi00716a028. [DOI] [PubMed] [Google Scholar]
- Wong M., Kulpa C. F., Thomas J. K. Kinetic processes in Escherichia coli membranes and cells. A laser photolysis study using derivatives of pyrene. Biochim Biophys Acta. 1976 Apr 5;426(4):711–722. doi: 10.1016/0005-2736(76)90136-x. [DOI] [PubMed] [Google Scholar]


