Abstract
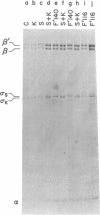
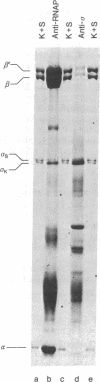
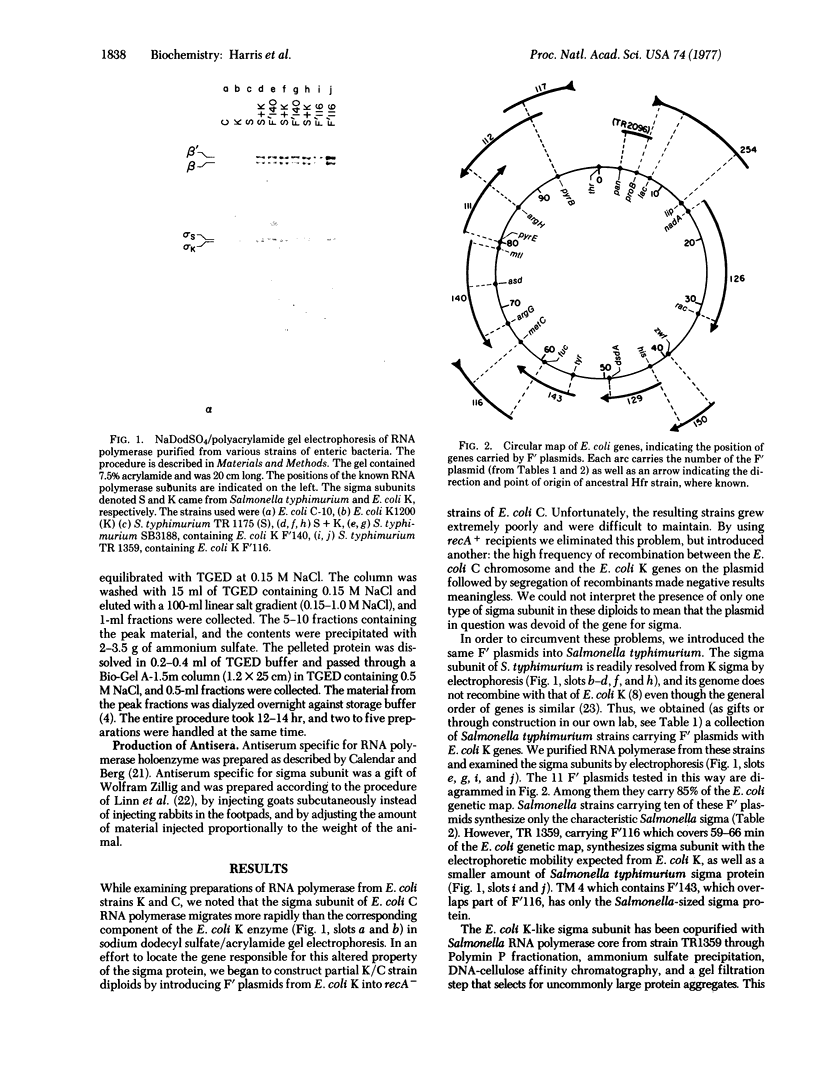
The RNA polymerase sigma subunits of Escherichia coli K, E. coli C, and Salmonella typhimurium can be resolved by electrophoresis. Using this technique, we have analyzed Salmonella strains carrying F' plasmids from E. coli K in order to map the gene for the sigma factor. Partial diploid analyses show the location of the sigma gene at 62-66 min on the E. coli genetic map. This gene is cotransducible with toIC and dnaG, at 66 min.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Barrand P., Fritsch A., Goldthwait D. A., Jacob F. Cohesive sites on the deoxyribonucleic acids from several temperate coliphages. J Mol Biol. 1966 Jun;17(2):343–357. doi: 10.1016/s0022-2836(66)80146-8. [DOI] [PubMed] [Google Scholar]
- Bowden D. W., Twersky R. S., Calendar R. Escherichia coli deoxyribonucleic acid synthesis mutants: their effect upon bacteriophage P2 and satellite bacteriophage P4 deoxyribonucleic acid synthesis. J Bacteriol. 1975 Oct;124(1):167–175. doi: 10.1128/jb.124.1.167-175.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindahl G. Attachment of prophage P2: gene order at different host chromosomal sites. Virology. 1969 Dec;39(4):867–881. doi: 10.1016/0042-6822(69)90023-3. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Roth J. R. Histidine regulatory mutants in Salmonella typhiumium. VI. Dominance studies. J Mol Biol. 1968 May 14;33(3):547–557. doi: 10.1016/0022-2836(68)90305-7. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G., Banerjee S. A mutation increasing the amount of a constitutive enzyme in Escherichia coli, glucose 6-phosphate dehydrogenase. J Mol Biol. 1971 Feb 28;56(1):183–194. doi: 10.1016/0022-2836(71)90093-3. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Parker J., Watson R. J., Bendiak D., Reeh S. V., Pedersen S., Fiil N. P. A transducing bacteriophage lambda carrying the structural gene for elongation factor Ts. Mol Gen Genet. 1976 Oct 18;148(1):93–98. doi: 10.1007/BF00268549. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Sunshine M. G., Calendar R. Bacteriophage P2 head morphogenesis: cleavage of the major capsid protein. Virology. 1973 May;53(1):1–23. doi: 10.1016/0042-6822(73)90461-3. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Cluster of genes in Escherichia coli for ribosomal proteins, ribosomal RNA, and RNA polymerase subunits. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2743–2747. doi: 10.1073/pnas.72.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Structural interactions between amino acid residues at positions 22 and 211 in the tryptophan synthetase alpha chain of Escherichia coli. J Bacteriol. 1974 Feb;117(2):444–448. doi: 10.1128/jb.117.2.444-448.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Control of template specificity of E. coli RNA polymerase by a phage-coded protein. Nature. 1969 Sep 13;223(5211):1111–1113. doi: 10.1038/2231111a0. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. A bacteriophage specific for F- Salmonella strains. Science. 1961 Jun 30;133(3470):2069–2070. doi: 10.1126/science.133.3470.2069. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Infective heredity in bacteria. Cold Spring Harb Symp Quant Biol. 1953;18:261–269. doi: 10.1101/sqb.1953.018.01.037. [DOI] [PubMed] [Google Scholar]
- Zillig W., Fujiki H., Blum W., Janeković D., Schweiger M., Rahmsdorf H., Ponta H., Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]