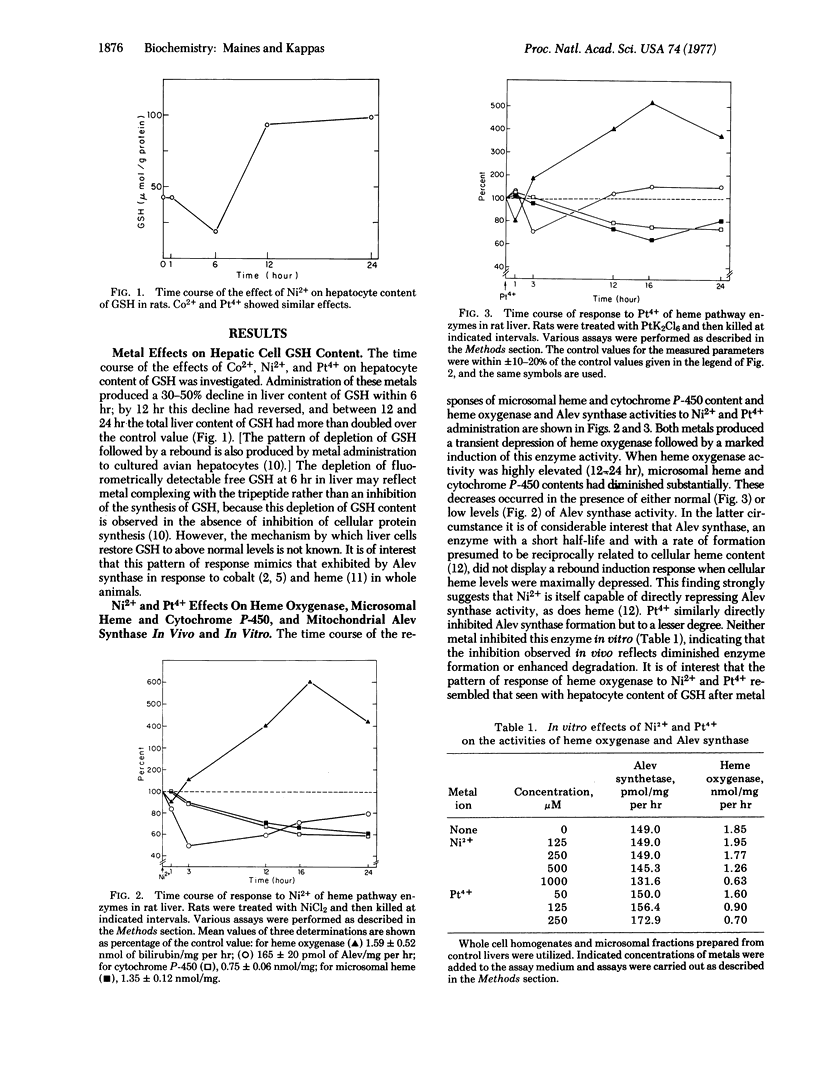
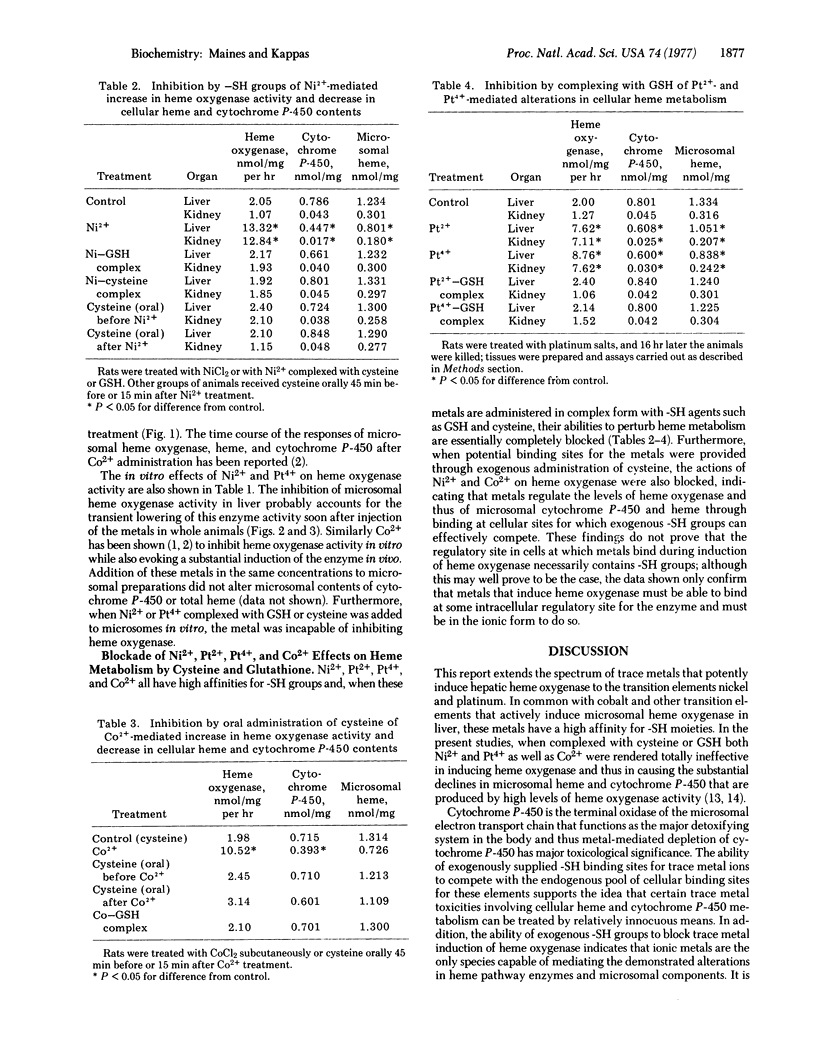
Abstract
The trace metals nickel and platinum, which are not substrates for ferrochelatase and thus do not form heme in biological systems, were found to act similaryl to cobalt, and heme itself, in regulating heme metabolism in liver and kidney. These metals induced heme oxygenase activity in both organs with the peak of induced enzyme activity reached approximately 16 hr after single injections in rats. Both metals caused transient depression of cellular glutathione content followed by increases above normal after 12 hr in liver. Nickel and platinum were more potent inducers of heme oxygenase in kidney than in liver (10-13 times normal versus 5-6 times normal). At high concentrations, they inhibited heme oxygenase [heme, hydrogen-donor:oxygen oxidoreductase (alpha-methene-oxidizing, hydroxylating), EC 1.14.99.3] in vitro. Both were active in regulating heme metabolism only when administered in the ionic form. Complexing of the metals with sulfhydryl agents completely blocked their actions on heme metabolism. Administration of cysteine orally prior to or shortly after administration of the metals had a similar blocking effect. Nickel and platinum produced depression of delta-aminolevulinate synthase [succinyl-CoA:glycine c-succinyltransferase (decarboxylating), EC 2.3.1.37] activity in liver, but neigther inhibited this rate-limiting ennzyme for heme synthesis in vitro. Furthermore, despite the substantial decreases in cellular heme and hemoprotein contents mediated by the metal, production of delta-amimolevulinate synthase did not undergo the compensatory increase that would be expected if there were a direct reciprocal feedback relationship between cellular heme level and synthesis of this enzyme. These findings indicate that it is not necessary for metal ions to be chelated in the porphyrin ring in order to regulate the enzymes of heme synthesis and heme oxidation. Accordingly, it is suggested that the iron atom of heme is the proximately active regulator of delta-aminolevulinate synthase and heme oxygenase--actions generally ascribed to the iron-tetrapyrrole complex itself--and that the tetrapyrrole moiety of the complex functions primarily as a means of transport of the metal to regulatory sites in cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn V. H., Lyle J. A fluorometric assay for glutathione. Anal Biochem. 1966 Mar;14(3):434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Kappas A., Maines M. D. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976 Apr 2;192(4234):60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Janousek V., Tomio J. M., Kappas A. Cobalt inhibition of synthesis and induction of delta-aminolevulinate synthase in liver. Proc Natl Acad Sci U S A. 1976 May;73(5):1499–1503. doi: 10.1073/pnas.73.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res. 1976;8 (Suppl 17):39–46. [PubMed] [Google Scholar]
- Maines M. D., Sinclair P. Cobalt regulation of heme synthesis and degradation in avian embryo liver cell culture. J Biol Chem. 1977 Jan 10;252(1):219–223. [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Delta-aminolevulinic acid synthetase. I. Studies in liver homogenates. J Biol Chem. 1966 Jun 25;241(12):2803–2809. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Waxman A. D., Collins A., Tschudy D. P. Oscillations of hepatic delta-aminolevulinic acid synthetase produced in vivo by heme. Biochem Biophys Res Commun. 1966 Sep 8;24(5):675–683. doi: 10.1016/0006-291x(66)90377-9. [DOI] [PubMed] [Google Scholar]


