Abstract
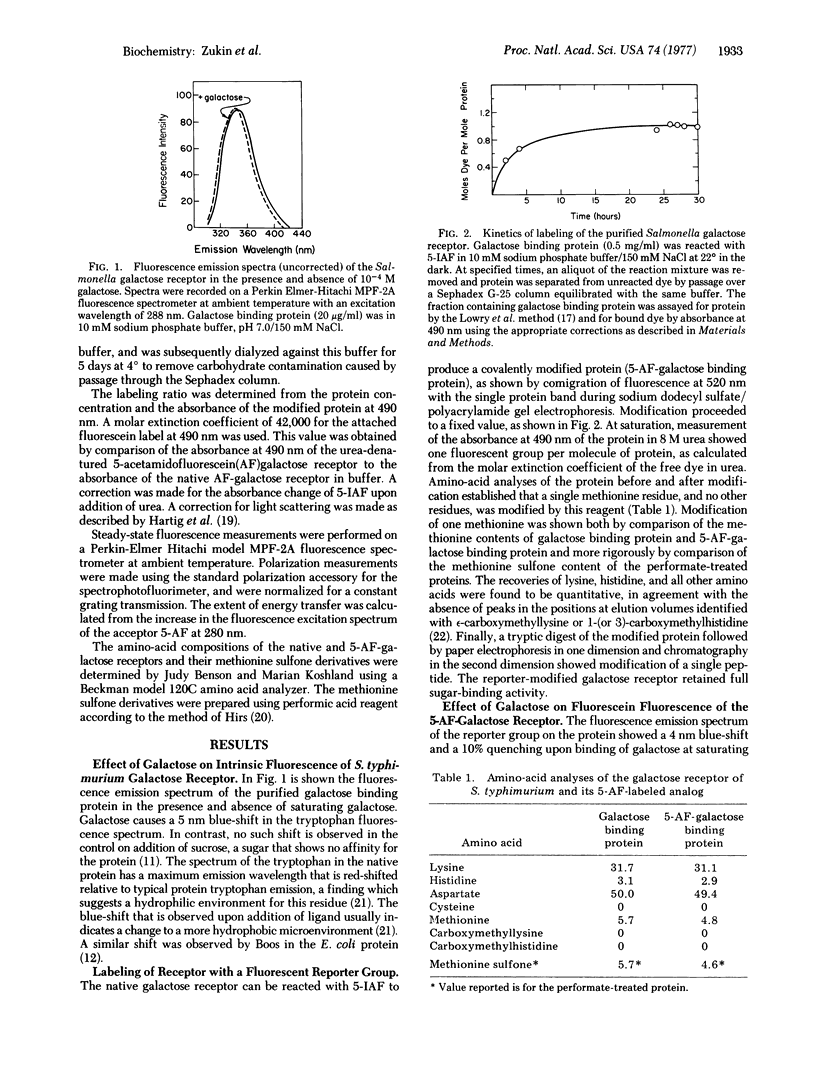
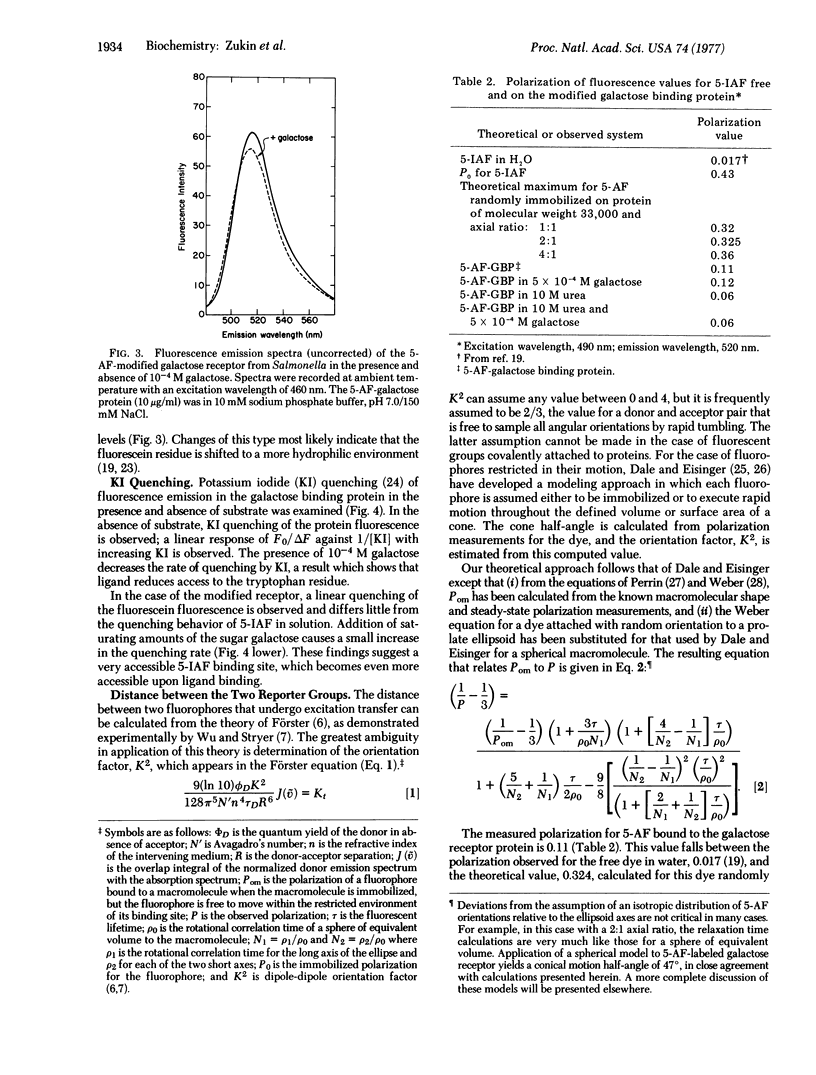
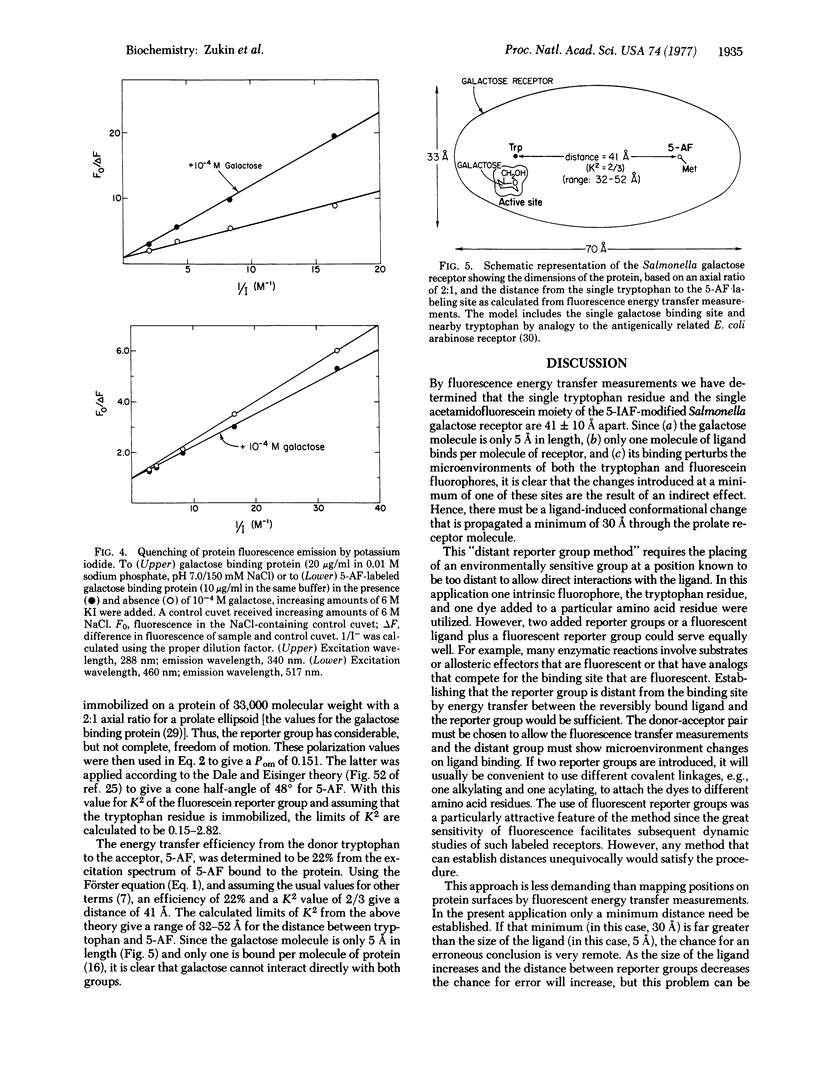
A highly sensitive method for demonstrating ligand-induced conformational changes in protein molecules in solution is described. The method utilizes an environmentally sensitive reporter group that is known to be distant from the active site. In the present application a conformational change is demonstrated in the galactose receptor of Salmonella typhimurium, involved in bacterial sensing and transport, by means of an extrinsic fluorophore, 5-iodoacetamidofluorescein, attached at a single methionine residue, and the intrinsic tryptophan fluorophore. Binding of the ligand galactose perturbs the microenvironment of both the fluorescein and tryptophan, as shown by both spectral and potassium iodide quenching changes. The distance between the two dyes is established by fluorescence energy transfer methods to be 41 +/- 10A. Since only one molecule of galactose binds per molecule of receptor and since the galactose molecule is only about 5 A in length, changes at one of these sites reflect the result of an indirect effect. Hence, there must be a ligand-induced conformational change that is propagated a minimum of 30 A through the receptor molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., McPherson A., Jr, Rossmann M. G., Schevitz R. W., Wonacott A. J. The structure of the nicotinamide-adenine dinucleotide coenzyme when bound to lactate dehydrogenase. J Mol Biol. 1970 Jul 14;51(1):31–38. doi: 10.1016/0022-2836(70)90267-6. [DOI] [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. II. Properties of galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3123–3127. [PubMed] [Google Scholar]
- BURR M., KOSHLAND D. E., Jr USE OF "REPORTER GROUPS" IN STRUCTURE-FUNCTION STUDIES OF PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:1017–1024. doi: 10.1073/pnas.52.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Gordon A. S., Hall R. E., Price H. D. Transport properties of the galactose-binding protein of Escherichia coli. Substrate-induced conformational change. J Biol Chem. 1972 Feb 10;247(3):917–924. [PubMed] [Google Scholar]
- Boos W., Gordon A. S. Transport properties of the galactose-binding protein of Escherichia coli. Occurrence of two conformational states. J Biol Chem. 1971 Feb 10;246(3):621–628. [PubMed] [Google Scholar]
- Boos W. Structurally defective galactose-binding protein isolated from a mutant negative in the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5414–5424. [PubMed] [Google Scholar]
- Dale R. E., Eisinger J. Intramolecular energy transfer and molecular conformation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):271–273. doi: 10.1073/pnas.73.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDLACH H. G., STEIN W. H., MOORE S. The nature of the amino acid residues involved in the inactivation of ribonuclease by iodoacetate. J Biol Chem. 1959 Jul;234(7):1754–1760. [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lever J. E. Quantitative assay of the binding of small molecules to protein: comparison of dialysis and membrane filter assays. Anal Biochem. 1972 Nov;50(1):73–83. doi: 10.1016/0003-2697(72)90487-3. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- McGowan E. B., Silhavy T. J., Boos W. Involvement of a tryptophan residue in the binding site of Escherichia coli galactose-binding protein. Biochemistry. 1974 Feb 26;13(5):993–999. doi: 10.1021/bi00702a025. [DOI] [PubMed] [Google Scholar]
- Parsons R. G., Hogg R. W. A comparison of the L-arabinose- and D-galactose-binding proteins of Escherichia coli B-r. J Biol Chem. 1974 Jun 10;249(11):3608–3614. [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Mahajan V. K., Siu A. K., Quiocho F. A. Structure of L-arabinose-binding protein from Escherichia coli at 5 A resolution and preliminary results at 3.5 A. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2186–2190. doi: 10.1073/pnas.73.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange P. G., Koshland D. E., Jr Receptor interactions in a signalling system: competition between ribose receptor and galactose receptor in the chemotaxis response. Proc Natl Acad Sci U S A. 1976 Mar;73(3):762–766. doi: 10.1073/pnas.73.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Stryer L. Proximity relationships in rhodopsin. Proc Natl Acad Sci U S A. 1972 May;69(5):1104–1108. doi: 10.1073/pnas.69.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKEELOV J. A., Jr, KOSHLAND D. E., Jr EVIDENCE FOR CONFORMATION CHANGES INDUCED BY SUBSTRATES OF PHOSPHOGLUCOMUTASE. J Biol Chem. 1965 Apr;240:1593–1602. [PubMed] [Google Scholar]
- Zukin R. S., Strange P. G., Heavey R., Koshland D. E. Properties of the galactose binding protein of Salmonella typhimurium and Escherichia coli. Biochemistry. 1977 Feb 8;16(3):381–386. doi: 10.1021/bi00622a007. [DOI] [PubMed] [Google Scholar]


