Abstract
Purpose
To compare the rates of retinal nerve fiber layer (RNFL) loss in patients suspect of having glaucoma who developed visual field damage (VFD) to those who did not develop VFD, and to determine whether the rate of RNFL loss can be used to predict who will develop VFD..
Design
Prospective observational cohort study
Participants
Glaucoma suspects, defined as having glaucomatous optic neuropathy or ocular hypertension (Intraocular pressure (IOP)>21 mmHg) without repeatable VFD at baseline from the Diagnostic Innovations in Glaucoma Study, and the African Descent and Glaucoma Evaluation Study.
Methods
Global and quadrant RNFL thickness (RNFLT) were measured with the Spectralis spectral-domain optical coherence tomography (SD-OCT). VFD was defined as having 3 consecutive abnormal visual fields. The rate of RNFL loss in eyes developing VFD was compared with eyes not developing VFD using multivariable linear mixed-effects models. A joint longitudinal survival model utilized the estimated RNFLT slope to predict the risk of developing VFD, while adjusting for potential confounding variables.
Main Outcome Measures
The rate of RNFL thinning and the probability of developing VFD.
Results
Four hundred and fifty-four eyes of 294 glaucoma suspects were included. The average number of SD-OCT examinations was 4.6 (range, 2–9) with median follow-up time of 2.2 (0.4–4.1) years. Forty eyes (8.8%) developed VFD. The estimated mean rate of global RNFL loss was significantly faster in eyes developing VFD compared with eyes that did not (−2.02μm/year vs. −0.82μm/year, P<0.001). The joint longitudinal survival model showed that each 1μm/year faster rate of global RNFL loss corresponded to a 2.05 times higher risk of developing VFD (Hazards Ratio (HR)=2.05, 95% Confidence Interval (CI): 1.14–3.71; p=0.017).
Conclusions
The rate of global RNFL loss was more than twice as fast in eyes developing VFD compared with eyes that did not develop them. Joint longitudinal survival model showed that a 1μm/year faster rate of RNFLT loss corresponded to a 2.05 times higher risk of developing VFD. These results suggest that measuring the rate of SD-OCT RNFL loss may be a useful tool to help identify patients who are at a high risk of developing visual field loss.
Introduction
Glaucoma is a progressive optic neuropathy characterized by structural changes in the optic nerve head (ONH), retinal nerve fiber layer (RNFL) defect, and accompanying visual field damage (VFD).1 In glaucoma management, detecting progression is essential in both early and late stages of the disease. In patients with an established diagnosis of glaucoma, evidence of progression will influence a clinician’s decision whether to modify glaucoma therapy. In patients who are suspected of having the disease, progression detection can confirm the diagnosis, and help decide how to manage the patient.
Although standard automated perimetry (SAP) has been the most commonly used test to monitor glaucomatous progression, evidence suggests that in some eyes substantial structural damage can be detected before the development of clinically detectable VFD.2,3 Visual field test results tend to be affected by various factors including subjects’ alertness, technicians’ experience, and short- or long-term fluctuations.4–6 These limitations suggest the necessity of more objective and sensitive methods to detect glaucoma progression.
Structural assessment of ONH and RNFL with imaging devices is a promising alternative. Many studies have reported the usefulness of event-based analyses,7–14 trend-based analyses,15–23 and other statistical analyses24–29 of change in ONH or RNFL over time, using either confocal scanning laser ophthalmoscope,7–10,12,15,16,19,22,24–29 scanning laser polarimetry,18,19 or optical coherence tomography (OCT).11,13,14,17,20–23 Of these, trend-based analysis of RNFL thickness (RNFLT) is advantageous in that it provides estimates of the rates of change (slope) in RNFLT so clinicians can better assess whether the patient is progressing quickly and therefore is at a higher risk of visual impairment.
Spectral-domain OCT (SD-OCT) with its improved resolution, scan speed, and reproducibility compared with its predecessor, time-domain OCT, potentially leads to earlier and more accurate detection of glaucoma progression.23,30 SD-OCT has been used to evaluate the patterns of progressive RNFL defect,14 and to estimate the number of retinal ganglion cells.31 However, to our knowledge, there have been no reports evaluating the rate of SD-OCT based RNFL change and its usefulness in detecting the development of glaucomatous VFD in patients suspected of having glaucoma.
The purpose of this study was to compare the rates of change in RNFLT as measured with the Spectralis SD-OCT (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany) in glaucoma suspect eyes that developed VFD to those who did not, and to use the rate of RNFL loss to predict who will develop VFD. The effects of other factors such as age, race, intraocular pressure (IOP), corneal thickness, and visual field pattern standard deviation (PSD) on the development of VFD were also analyzed.
Methods
Study design
Subjects were enrolled from the Diagnostic Innovations in Glaucoma Study, and the African Descent and Glaucoma Evaluation Study. The study design has been described in detail previously.32 In brief, participants in these studies were evaluated every 6 months in which patients underwent extensive predetermined clinical examinations. Inclusion criteria for these studies were open angles, a best corrected acuity of 20/40 or better, a spherical refraction within ± 5.0 Diopter (D), and cylinder correction within ± 3.0 D. Subjects were excluded if they had a history of intraocular surgery (except for uncomplicated glaucoma and cataract surgery). Subjects with secondary causes of elevated IOP, other intraocular eye disease, other diseases affecting visual field, or with medications known to affect visual field sensitivity were also excluded. All participants with suspected glaucoma who had at least two Spectralis examinations of sufficient quality were analyzed in the current study. Glaucoma suspect was defined as having glaucomatous optic neuropathy or suspicious appearing optic discs based on stereophotograph review by two experienced graders or ocular hypertension (OHT; IOP >21 mm Hg) at baseline without evidence of repeatable glaucomatous VFD at baseline. Other information such as race, age, visual field pattern standard deviation (PSD), central corneal thickness, and mean IOP during follow up were also collected.
The 3-site collaboration includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California, San Diego (UCSD), the New York Eye and Ear Infirmary, and the Department of Ophthalmology, University of Alabama, Birmingham. Informed consent was obtained from all participants and all methods were approved by the institutional review boards at all 3 sites. All methods adhered to the declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act. This study was registered at http://clinicaltrials.gov (NCT00221923) on September 14, 2005..
Follow-up
At the baseline visit and at each annual follow-up visit, subjects underwent complete ophthalmologic examination including slitlamp biomicroscopy, intraocular pressure measurement, dilated stereoscopic fundus examination, and stereophotography of the optic nerve head. Standard automated perimetry and SD-OCT imaging were completed at baseline and every 6 months during follow-up.
Standard Automated Perimetry
Standard automated perimetry visual field tests were completed using SITA (Humphrey Field Analyzer; Carl Zeiss Meditec) strategies during follow-up. Only reliable tests (≤33% fixation losses and false negatives, and ≤15% false positives) were included. SAP result was considered to be abnormal if a glaucoma hemifield test was outside of normal limits or a pattern standard deviation fell outside of the 95% normal confidence limits. Eyes were divided into two groups; eyes developing VFD (VFD group) and eyes that did not develop VFD (non-VFD group). Development of VFD was defined as having 3 or more consecutive abnormal SAP results during follow-up.
Stereophotography
Optic disc damage was evaluated by masked assessment of optic disc stereophotographs. Simultaneous stereoscopic optic disc photographs were reviewed by 2 experienced graders with a stereoscopic viewer (Screen-Vu stereoscope, PS Mfg, Portland, OR) and classified as glaucomatous or normal. Glaucomatous optic disc appearance was defined based on the presence of neuroretinal rim thinning, excavation, notching, or characteristic RNFL defects. Each grader was masked to the subject’s identity and other test results. Discrepancies between the two graders were resolved by adjudication of the two graders. Only photographs of adequate quality were included.
Spectralis SD-OCT
RNFLT was measured with the Spectralis SD-OCT parapapillary circle scan (software version 5.4.7.0). Basic principles of the SD-OCT technique have been described in the literature.30 Spectralis incorporates a real-time eye tracking system that couples confocal laser scanning ophthalmoscope and SD-OCT scanners to adjust for eye movements and to ensure that the same location of the retina is scanned over time. In addition, automatic real time function (ART) mode resamples multiple frames (B-scans) for noise reduction. The examiner is required to manually place the scan around the optic disc at the baseline examination. After the reference image is manually identified by the operator, the system recognizes the reference image scanning area and automatically positions the retest scan on the same location in follow-up examinations. The scan circle contains 1536 A-scan points from a 12 degree circle which equates to a retinal diameter of 3.5mm in eyes with standard corneal curvature. The acquisition rate is 40000 a-scans per second at an axial resolution of 3.9 μm and a lateral resolution of 6 μm. The software also provides the quality score that indicates the signal strength. The quality scores range from 0 dB (poor) to 40 dB (excellent).
Software module version 5.4.6.7 was used to process the scans and measure RNFLT. The temporal (316–45 degrees), temporal-superior (46–90 degrees), nasal-superior (91–135 degrees), nasal (136–225 degrees), nasal-inferior (226–270 degrees), temporal-inferior (271–315 degrees) and global mean RNFLT were provided by the software. All images were processed and reviewed by the Imaging Data Evaluation and Assessment (IDEA) Center in the UCSD. Images with noncentered scans, inaccurate segmentation of the RNFL, or quality scores of ≤15 dB were excluded from the analysis. Overall, approximately 5% of Spectralis RNFL circle scan visits reviewed by the IDEA center are excluded because of poor image quality.
Statistical Analysis
Descriptive statistics including mean and standard deviation for normally distributed variables and median, first quartile, and third quartile for non-normally distributed variables were computed to describe the study population.
We used mixed effects modeling to estimate the rates of change of global RNFLT in each group (Visual Field Damage (VFD) vs. Non-Visual Field Damage). We have previously used mixed effects modeling with random intercepts and random slopes to account for repeated measurements over time.18,19 A set of mixed effects models were fit with VFD, time, VFD*time interaction as the fixed effects and random intercept and slope for each eye nested within subject. The “VFD*time” interaction term indicates the difference between eyes developing VFD and eyes not developing VFD in estimated rate of change of RNFLT over time, and the P value shows the significance of the difference. Other possible predictors such as age, central corneal thickness (CCT), race, IOP and baseline PSD were also included in the model.
A joint longitudinal survival model was used to investigate the relationship between longitudinal RNFLT and risk of developing VFD. Potential influence of inter-eye correlation was taken into account by using a bootstrap resampling procedure (n=500), where the subject was considered as the unit of resampling for estimating the standard errors. Details about these models have been discussed previously in the literature.33,34 This model was used to evaluate the predictive ability of rates of structural loss on the risk of glaucomatous progression as previously described. 35,36
In brief, they are composed of a longitudinal submodel and a survival submodel which are tied together by sharing random effects. The longitudinal submodel was composed of a linear mixed model, which specifically accounts for measurement error of the marker by postulating that the observed level of the outcome (the RNFLT measurements) equals the unobserved true value plus a random error term. Covariates can be included in the estimation process by means of the design matrices for the fixed-effects regression coefficients and random-effects coefficients. In this study, we evaluated the effect of the baseline covariates such as age, race, visual field PSD, central corneal thickness, and the mean IOP during follow-up, on the intercept and slopes of RNFLT.
To quantify the strength of the association between the slope value and the risk for developing VFD, a survival submodel was used to determine the hazard function. This model was estimated jointly with the longitudinal submodel and allowed an evaluation of the relationship between the marker value (RNFLT measurements) and the risk for the event (development of the visual field damage). In this study, we were particularly interested in the relationship between the slopes of RNFLT loss is and risk of the event. Therefore, the coefficient for the first derivative of the marker profile, which demonstrates how strongly the slope of the longitudinal marker was associated with the risk for an event, was estimated.
To evaluate whether baseline and longitudinal measurements were predictive of the study endpoints, only tests acquired before the event date were analyzed in the study. For eyes that developed VFD, follow-up time was defined as the time between the first OCT visit and the date of the third abnormal visual field result (the study endpoint). For eyes without VFD, follow-up time was defined as the time between the first OCT visit and the date of last available follow-up. Eyes that did not develop the study endpoint were considered censored at the last follow-up visit. All tests up to the last available follow-up date were analyzed for these eyes.
Statistical analyses were performed using statistical software R 2.14.1 (R foundation, Vienna, Austria) and STATA version 12.0 (STATA corp, College Station, TX).P-values less than 0.05 were considered statistically significant.
Results
Study Population
454 eyes of 294 glaucoma suspects were included. One hundred and eighty-three patients (62.2%) were women. Mean age ± SD at baseline was 64.5 ± 11.3 years. 92 subjects (31.3%) were of African Descent (AD) and 202 subjects (68.7%) were of European Descent (ED). 239 eyes (52.6%) were categorized as OHT at the baseline examination. The average number of OCT examinations per eye was 4.6 (range, 2–9) with median follow-up time of 2.2 years.
Baseline Factors
Forty eyes (8.8%) developed repeatable VFD and 414 eyes did not develop repeatable VFD (non-VFD group) during the follow-up period. Baseline demographics and RNFLT of the VFD group and the non-VFD groups are summarized in tables 1 and 2, respectively. Participants that developed repeatable VFD had higher visual field PSD compared with participants that did not. Other factors such as gender, race, diagnosis, image quality, and mean IOP during follow-up were not significantly different between the group that developed VFD and the group that did not.
Table 1.
Baseline demographics of the group the visual field damage group and the non-visual field damage group
| By Subject | Visual Field Damage (n=38) | Non-Visual Field Damage (n=256) | Difference (P-value) |
|---|---|---|---|
| Gender (% Female) | 63.2% | 62.1% | 1.000 |
|
| |||
| Age (years) | 67.8 ± 10.7 | 64.0 ± 11.3 | 0.111 |
|
| |||
| Race | |||
| African descent, % | 36.8 | 30.5 | 0.456 |
| European descent, % | 63.2 | 69.5 | |
| By Eye | Visual Field Damage (n=40) | Non-Visual Field Damage (n=414) | Difference (P-value) |
|---|---|---|---|
| Diagnosis | |||
| GON, % | 60.0 | 46.1 | 0.100 |
| OHT, % | 40.0 | 53.9 | |
|
| |||
| Mean IOP during follow-up (mmHg) | 18.6 ± 4.7 | 18.1 ± 3.8 | 0.613 |
|
| |||
| MD (dB) | −0.8 ± 1.4 | −0.3 ± 1.3 | 0.026* |
|
| |||
| CCT (μm) | 547.7 ± 41.7 | 554.5 ± 40.7 | 0.431 |
|
| |||
| PSD | 1. 7 ± 0.3 | 1.5 ± 0.3 | <0.001* |
GON: glaucomatous optic neuropathy, OHT: ocular hypertension, IOP: intraocular pressure, MD: mean deviation, CCT: central corneal thickness, PSD: Pattern Standard Deviation
Table 2.
Baseline retinal nerve fiber layer thickness (μm) of the visual field damage group and the non-visual field damage group
| Visual Field Damage (n=40) | Standard deviation | Non-Visual Field damage (n=414) | Standard deviation | Total (n=454) | Standard deviation | Difference between groups (P-value) | |
|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | |||||
| Global | 83.1 | 13.9 | 89.8 | 11.7 | 89.2 | 12.0 | 0.003* |
| Temporal | 60.2 | 12.2 | 67.6 | 12.5 | 67.0 | 12.6 | 0.001* |
| Nasal | 66.0 | 15.3 | 67.9 | 13.6 | 67.7 | 13.8 | 0.384 |
| Superior | 100.0 | 18.7 | 106.9 | 17.5 | 106.3 | 17.7 | 0.019* |
| Inferior | 105.8 | 23.3 | 116.4 | 19.3 | 115.5 | 19.9 | 0.005* |
Rate of RNFL loss
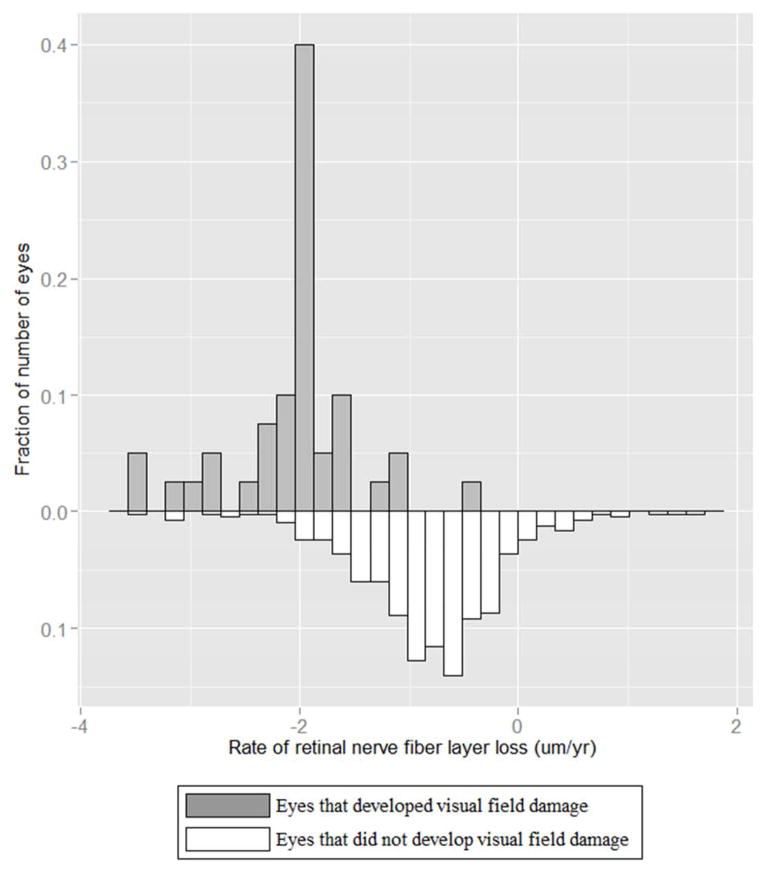
Figure 1 shows the wide distribution of the rate of global RNFL loss in eyes developing VFD and eyes that did not. It is important to note that the average rate of global and sectoral RNFL loss was significantly different from zero in both groups of eyes. (Table 3)
Figure 1.
Distribution of the rates of global retinal nerve fiber layer loss in eyes that developed visual field damage (upper panel) and in eyes that did not develop visual field damage (lower panel).
Table 3.
Summary table of retinal nerve fiber layer slope values (μm/year) in the Visual Field Damage and the non-Visual Field Damage group
| Visual Field Damage (n=40) | Non-Visual Field Damage (n=414) | Difference between groups | ||||
|---|---|---|---|---|---|---|
| Mean (SE) | P-value | Mean (SE) | P-value | Mean (SE) | P-value | |
| Global | −2.02 (0.30) | <0.001* | −0.82 (0.08) | <0.001* | −1.20 (0.31) | <0.001* |
| Temporal | −0.94 (0.42) | 0.0272* | −0.89 (0.10) | <0.001* | −0.05 (0.44) | 0.917 |
| Nasal | −2.15 (0.57) | <0.001* | −0.60 (0.14) | <0.001* | −1.55 (0.59) | 0.008* |
| Superior | −1.53 (0.52) | 0.003* | −0.30 (0.13) | 0.024* | −1.23 (0.53) | 0.021* |
| Inferior | −3.23 (0.52) | <0.001* | −1.46 (0.13) | <0.001* | −1.77 (0.54) | 0.001* |
Table 3 shows values of mean rates of change of global and sectoral RNFLT over time in eyes which developed VFD and in eyes which did not develop VFD. In each sector, the mean rate of global RNFL loss was significantly faster (all p<0.001) in patients developing VFD compared to those who did not (Table 3); (−2.02μm/year in the VFD group versus −0.82μm/year in the non-VFD group, P<0.001, Figure 1). The rate of RNFLT loss was also significantly greater in eyes developing VFD than eyes that did not develop VFD in the superior, inferior, and nasal but not temporal regions (Table 3).
Joint Longitudinal Survival Model
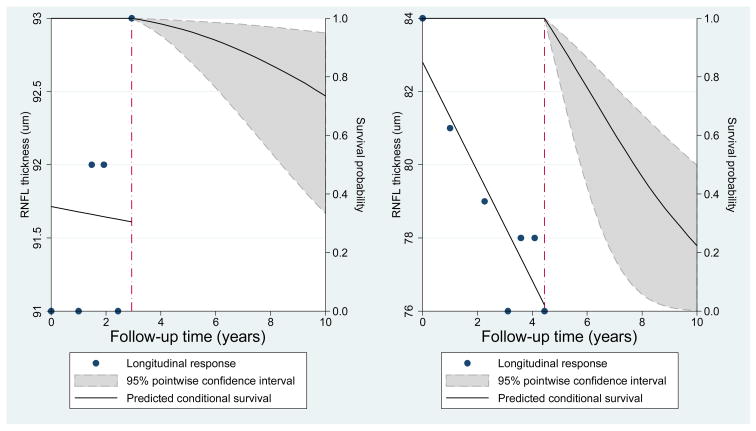
Table 4 shows the results of the joint longitudinal survival model. In the multivariable survival model, global rate of RNFL loss was significantly associated with risk of developing VFD. Each 1 μm/year faster rate of RNFLT loss was associated with 2.05 times higher risk of developing VFD (adjusted Hazard Ratio [HR]:2.05; 95% Confidence Interval [CI] =1.14 – 3.71; P =0.017). The baseline RNFLT (intercept) was also a significant predictive factor for developing VFD. Each 1 μm thinner RNFL at baseline was associated with 1.05 times higher risk of developing VFD (adjusted HR: 1.05; 95% CI = 1.02 – 1.09; P =0.001). Higher baseline PSD was also a significant predictive factor for developing VFD in the multivariable model. IOP, race, age, and central corneal thickness were not significantly associated with the risk of developing VFD (all p>0.05). Each 1 μm/year faster rate of inferior RNFL loss was also significantly associated with the risk of developing VFD (HR: 1.70; 95%CI = 1.16 – 2.49; P=0.007), whereas the rate of superior RNFL loss was not (HR: 1.19; 95%CI = 0.79 – 1.77; P=0.406). Figure 2 illustrates an impact of longitudinal RNFL thickness measurements on predicted survival (no VFD) probabilities in two representative eyes; one eye with steep RNFL thickness decline and the other eye with stable RNFL measurements. The predicted survival probabilities were relatively high for both eyes when only baseline measurements were considered. For an eye with steep decline of RNFL thickness, the model estimated much lower probabilities of survival as more information became available (right panel). An eye with slow rate of RNFL loss (stable eye, left panel) shows higher predicted probability of survival compared with an eye with faster rate of RNFL loss.
Table 4.
Results of the joint longitudinal survival model (N=454) investigating the effect of retinal nerve fiber layer slope on the risk of development of visual field damage, while adjusting for confounding factors
| Longitudinal Submodel | ||||
|---|---|---|---|---|
| Coefficient | 95% CI | P | ||
| Intercept | 98.909 | 91.373 – 106.445 | <0.001 | |
| Time | 1.243 | 0.430 – 2.056 | 0.003 | |
| IOP | 0.031 | −0.281 – 0.343 | 0.846 | |
| Race(white) | −3.100 | −5.771 – −0.429 | 0.023 | |
| Age | −0.036 | −0.117 – 0.044 | 0.372 | |
| Baseline PSD | −5.144 | −8.669 – −1.618 | 0.004 | |
| CCT (centered around the mean) per 100 μm thicker | 2.100 | −0.500 – 4.800 | 0.117 | |
| Time*IOP | −0.111 | −0.144 – −0.078 | <0.001 | |
| Time*Race | 0.198 | −0.0420 – 0.437 | 0.106 | |
| Time*Age | −0.008 | −0.019 – 0.002 | 0.125 | |
| Time*Baseline PSD | −0.104 | −0.511 – 0.303 | 0.616 | |
| Time*CCT | 0.500 | 0.200 – 0.800 | 0.002 | |
| Survival Submodel | ||||
| Coefficient | 95% CI | P | Hazard Ratio | |
| Intercept of RNFL (μm) | 0.053 | 0.0220–0.085 | 0.001 | 1.05 |
| Mean IOP during follow-up (per 1 mmHg higher) | −0.032 | −0.157 – 0.093 | 0.618 | 0.97 |
| Race (ED vs AD) | −0.121 | −0.894 – 0.652 | 0.759 | 0.89 |
| Age (per 1 year older) | 0.027 | −0.007 – 0.060 | 0.121 | 1.03 |
| Baseline PSD, per 1 dB higher | 0.907 | 0.087 – 1.727 | 0.030 | 2.48 |
| CCT (per 100 μm thicker) | −0.500 | −1.400 – 0.500 | 0.314 | 0.61 |
| RNFL slope (per 1 μm/year faster) | 0.720 | 0.128 – 1.312 | 0.017 | 2.05 |
IOP: intraocular pressure, MD: mean deviation, CCT: central corneal thickness, ART: automatic real time function, GON: glaucomatous optic neuropathy, OHT: ocular hypertension, IOP: intraocular pressure, PSD: pattern standard deviation, ED: European descent, AD: African descent
Figure 2.
Predicted survival curves for two eyes, one that showed a fast rate of retinal nerve fiber layer loss (right panel) and one that showed a stable measurements over time (left panel). Slopes are given as μm/year. A comparison of the predicted survival probabilities shows that the eye with fast progression had lower predicted probabilities of survival (i.e., retaining a normal visual field). The eye with a fast rate of loss in fact showed development of visual field damage during follow-up whereas the eye with stable measurements did not. RNFL: retinal nerve fiber layer. VFD: visual field damage
Discussion
In this cohort of glaucoma suspects followed for a relatively short period (2.2 years), eyes which developed VFD had an approximately 2.5 times faster rate of RNFLT loss compared to eyes which did not develop them. Moreover, a 1 μm/yr faster rate of RNFL loss resulted in a 2.05 times higher risk of developing VFD. These results suggest that the rate of RNFL loss measured with SD-OCT may be useful for identifying which glaucoma suspect patients are at the highest risk of developing VFD.
In our cohort of glaucoma suspects, the mean rate of global RNFL loss in eyes which developed visual field damage (the VFD group) was −2.02 μm/year, which was significantly faster than the mean global RNFLT slope in the non-VFD group (−0.82 μm/year). Sung and colleagues reported that the mean RNFL loss measured with SD-OCT in glaucomatous eyes with visual field progression was −2.08 μm/year, whereas the mean RNFL slope in eyes without visual field progression was −0.90 μm/year.37 The rates of RNFL loss in our study were very similar to their study, even though their study included advanced glaucoma patients whereas our study covered only glaucoma suspects at baseline. Results of the Sung study did differ from those of our study in that there was no significant difference in rate of RNFL loss between progressors and non-progressors in their study, probably at least in part due to the smaller number of the participants or the difference in study population. To date, there has been no report of longitudinal changes in RNFLT in a large cohort of glaucoma suspects using SD-OCT, possibly because of its relatively recent introduction to clinical practice. However, there are many reports on the rate of RNFL loss in progressing eyes versus non-progressing eyes using different imaging instruments. For example, we previously reported that the rate of RNFL loss was significantly faster in progressors compared with non-progressors using scanning laser polarimetry18 and time-domain OCT17 in a mixed population of glaucoma patients and glaucoma suspects. The mean slope values for progressors were pretty similar to each other; −0.70 μm/year in the scanning laser polarimetry study and −0.72 μm/year in the time-domain OCT study. Using Stratus time-domain OCT, Lee et al. also compared the rates of RNFL loss between progressors and non-progressors in a cohort of glaucoma patients with localized RNFL damage.38 The mean RNFL slope of progressors was −1.58 μm/year. The mean rate of global RNFL loss of the VFD group in our study (−2.02) was much steeper than those reported in these previous studies. Disagreement in the rate of RNFL loss estimates among these studies, however, is not surprising. Previous studies revealed that the RNFLT values measured with different devices are not interchangeable.23,39,40 The differences in mean RNFL loss among the different studies may also be influenced by the difference in the definition of progression among studies, length of follow-up and the eligibility criteria.
The rates of RNFL loss in eyes not developing VFD were also significantly different from zero. Moreover, the rate of RNFL loss in the non-VFD group in our cohort were much faster (more negative) compared with the rate of loss in glaucoma suspects without progression in previous studies.17,18,38 For example, we previously reported that the slopes were negative in both progressors and non-progressors using scanning laser polarimetry,18 but the mean slope in non-progressors (−0.14 μm/year, 95% confidence interval (CI): −0.26 to −0.02) was much smaller than the slope in the current study (−0.82 μm/year, 95% CI: −0.97 to −0.66). In another study using TD-OCT, most sectors did not show significant negative slopes of RNFLT in non-progressors.17 Lee et al. reported that the mean RNFL decline in non-progressors was −0.34 ± 1.41 μm/year (mean ± standard deviation).38 Because SD-OCT has better resolution, scan speed, and scan registration,33–45 it is possible that SD-OCT can detect the progressive structural damage in early stage glaucoma without detectable VFD more sensitively than earlier devices.
Another possible reason for a significant rate of RNFL loss in the non-VFD group is the age related decline. In a recent longitudinal study investigating the correlation between age and RNFLT measured using Cirrus SD-OCT, Leung et al. reported that the mean rate of change of global RNFLT in normal eyes was −0.52 μm/year,46 which was much steeper than the slopes reported in previous cross-sectional studies.47–52 Mean RNFL decline in eyes not developing VFD in our cohort was slightly faster, which could support the hypothesis that some of these eyes actually had glaucomatous progression which did not yet result in repeatable VFD. Many postmortem histologic studies53–55 and clinical imaging studies47–52 suggested the existence of age-related loss of RNFL with conflicting estimates of the magnitude of age-related change. Longer follow-up in a cohort of healthy eyes is needed to better estimate sectoral age-related RNFLT loss.
Earlier detection of progressing eyes may be one of the advantages of using SD-OCT in clinical practice. In progression analysis, RNFL loss significantly different from zero is generally considered as a significant progression. Therefore, the lower the variability of the instrument, the earlier it can detect significant loss of RNFLT. Previous studies demonstrated the significantly better reproducibility of SD-OCT devices compared to TD-OCT.41–45 A recent study reported that SD-OCT showed significantly higher detection rate of eyes with progressive RNFL loss than TD-OCT in a follow-up period of 24 to 33 months.23 Spectralis also incorporates two functions to improve the reproducibility further; the real-time eye tracking system and the retest function. The ART acquires multiple B-scans in the same location; these are then averaged to improve image quality. Retest function automatically places follow-up scans in the same location as the initial scan. These SD-OCT features facilitate the detection of glaucoma progression.
In contrast to earlier studies, we did not find a significant association between thinner central corneal thickness and an increased risk of the development of visual field damage.60,61,62 This may be due to difference in inclusion criteria, participant population, or endpoint definitions between studies. Furthermore, our findings may be a result of change in clinical practice as a result of the earlier findings. Clinicians in the 3 ADAGES academic centers may now treat patients with thin corneas more aggressively because they are aware of the studies indicating that patients with thinner central corneal are at higher risk of developing glaucoma. This cohort effect may explain at least in part the lack of an association of central corneal thickness and risk of developing glaucoma in the present study. For this reason, it is possible that the association between central corneal thickness and the risk of glaucomatous progression will also be reduced in future observational cohort studies.
Best linear unbiased prediction estimates (BLUP) derived from a mixed-effects model, with both random intercepts and slopes, were used to estimate the rates of RNFL loss. BLUP have many advantages over the naïve ordinary least squares (OLS) estimates.19 OLS estimates can be very imprecise in eyes with just a few measurements or with large intraindividual variability.56 OLS (i.e., individual regression lines) do not take into account the information provided by the whole population. BLUPs are shrinkage estimates that take into account the results obtained by evaluating the whole sample of eyes, giving less weight to estimates obtained in eyes with a small number of measurements and/or large intraindividual variability.57
One limitation of this study was the relatively short follow-up period (2.2 years) which could bias the analysis. Patients with longer follow-up have a higher chance of reaching visual field endpoint than eyes with shorter follow-up if these eyes have similar slope values. It is possible that patients with true progression did not reach the visual field end point in this study because of the short follow-up length. Despite this possible misclassification, we still found that eyes that developed VFD had approximately 2.5 times faster RNFL loss. Moreover, from the joint longitudinal survival model which includes follow-up time in the model, we showed that a 1 μm/yr faster slope was associated with a 2.0 higher risk of developing VFD. Longer follow-up is needed to determine whether the non-progressor eyes with faster RNFL loss will develop VFD in the future. Another limitation of this study was a relatively small sample size for each racial group. Several studies reported the significant differences in RNFLT and optic nerve topography by race.48,58,59 Race was also included in our model, but we did not find an association between race and the rates of RNFLT loss. However, the small sample size in each racial group could limit the power to evaluate the influence of race on the rate of RNFL thinning. A longer follow-up with larger sample size is needed to clarify whether there are racial differences in the rate of RNFLT loss. In addition, it should be noted that participants in this study were limited to glaucoma suspects. Therefore, we cannot directly extrapolate the results of this study to advanced glaucoma patients or normal subjects.
In conclusion, longitudinal trend-based analysis and joint longitudinal survival analysis of RNFL thickness measured with SD-OCT showed significantly faster rates of RNFL loss in glaucoma suspect eyes which developed VFD compared to eyes that did not develop VFD, and that a 1 μm faster rate of RNFL loss resulted in doubling the probability of developing VFD. These results obtained in a relatively short follow-up period, suggest that the rate of SD-OCT RNFL loss may be useful for managing glaucoma patients and for shortening the duration of clinical trials. Moreover, these results suggest that the rate of RNFL loss can be included in a model to predict the development of future visual field damage.
Acknowledgments
Financial Support: National Eye Institute, P30EY022589, U10EY14267, EY019869, EY021818, EY022039, EY08208, EY11008, and EY13959; Eyesight Foundation of Alabama; the Edith C. Blum Research Fund of the New York Glaucoma Research Institute, New York; Japan Eye Bank Association, Tokyo, Overseas Research Grant; Research to Prevent Blindness, New York, an unrestricted grant;
Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc. provided the participants’ glaucoma medications at no charge.
Dr. Miki reports grants from Japan Eye Bank Association, during the conduct of the study; personal fees from NIDEK, outside the submitted work; Dr. Medeiros reports grants from National Eye Institute, grants from Carl-Zeiss Meditec, grants from Heidelberg Engineering, grants from Topcon, Inc, during the conduct of the study; Dr. Weinreb reports grants from Nidek, non-financial support from Heidelberg Engineering, grants and personal fees from Topcon, grants and personal fees from Zeiss Meditec, during the conduct of the study; personal fees from Alcon, personal fees from Allergan, personal fees from Bausch + Lomb, outside the submitted work; In addition, Dr. Weinreb has a patent RGC Index pending; Dr. Liebmann reports grants from National Eye Institue, during the conduct of the study; grants from Carl ZEiss Meditec, grants from Topcon Inc, personal fees from Alcon Laboratories, personal fees from Allergan Inc, grants and personal fees from Diopsys Corporation, grants from Glaukos Corporation, grants from Heidelberg Engineering, personal fees from Merz Pharmaceutical Inc, grants from National Eye Institute, grants from New York GLaucoma Research Institute, grants and personal fees from Optovue Inc, personal fees from Quark Pharmaceuticals Inc, grants from SOLX Inc, outside the submitted work; Dr. Girkin reports grants from Carl Zeiss Meditech, Inc, grants from Heidelburg Engineering, outside the submitted work; Dr. Zangwill reports grants from National Eye Institue, non-financial support and other from Heidelberg Engineering, during the conduct of the study; non-financial support from Carl ZEiss Meditec, non-financial support from Topcon Inc, outside the submitted work;.
Footnotes
Conflict of Interest: No Disclosure (LS, PAS, NK, SJ, NH, HF)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Kass M, Heuer D, Higginbotham E, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 3.European Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Flammer J, Drance S, Zulauf M. Differential light threshold: short- and long-term fluctuation in patients with glaucoma, normal controls, and patients with suspected glaucoma. Arch Ophthalmol. 1984;102:704–6. doi: 10.1001/archopht.1984.01040030560017. [DOI] [PubMed] [Google Scholar]
- 5.Kutzko KE, Brito CF, Wall M. Effect of instructions on conventional automated perimetry. Invest Ophthalmol Vis Sci. 2000;41:2006–13. [PubMed] [Google Scholar]
- 6.Junoy Montolio FG, Wesselink C, Gordijn M, Jansonius NM. Factors that influence standard automated perimetry test results in glaucoma: test reliability, technician experience, time of day, and season. Invest Ophthalmol Vis Sci. 2012;53:7010–7. doi: 10.1167/iovs.12-10268. [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, Hitchings RA. Approach for identifying glaucomatous optic nerve progression by scanning laser tomography. Invest Ophthalmol Vis Sci. 2003;44:2621–6. doi: 10.1167/iovs.02-0850. [DOI] [PubMed] [Google Scholar]
- 8.Tan JC, Hitchings RA. Optimizing and validating an approach for identifying glaucomatous change in optic nerve topography. Invest Ophthalmol Vis Sci. 2004;45:1396–403. doi: 10.1167/iovs.03-0025. [DOI] [PubMed] [Google Scholar]
- 9.Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24:333–54. doi: 10.1016/j.preteyeres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Patterson AJ, Garway-Heath DF, Strouthidis NG, Crabb DP. A new statistical approach for quantifying change in series of retinal and optic nerve head topography images. Invest Ophthalmol Vis Sci. 2005;46:1659–67. doi: 10.1167/iovs.04-0953. [DOI] [PubMed] [Google Scholar]
- 11.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–70. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fayers T, Strouthidis NG, Garway-Heath DF. Monitoring glaucomatous progression using a novel Heidelberg Retina Tomograph event analysis. Ophthalmology. 2007;114:1973–80. doi: 10.1016/j.ophtha.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Kim TW, Park KH, et al. Ability of Stratus OCT to detect progressive retinal nerve fiber layer atrophy in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:662–8. doi: 10.1167/iovs.08-1682. [DOI] [PubMed] [Google Scholar]
- 14.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119:1858–66. doi: 10.1016/j.ophtha.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 15.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–10. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 16.See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116:840–7. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with Stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50:5741–8. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50:1675–81. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3531–9. doi: 10.1167/iovs.09-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung CK, Cheung CY, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010;51:217–22. doi: 10.1167/iovs.09-3468. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Kim TW, Weinreb RN, et al. Trend-based analysis of retinal nerve fiber layer thickness measured by optical coherence tomography in eyes with localized nerve fiber layer defects. Invest Ophthalmol Vis Sci. 2011;52:1138–44. doi: 10.1167/iovs.10-5975. [DOI] [PubMed] [Google Scholar]
- 22.Leung CK, Liu S, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011;118:1551–7. doi: 10.1016/j.ophtha.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Leung CK, Chiu V, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118:1558–62. doi: 10.1016/j.ophtha.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Kamal DS, Viswanathan AC, Garway-Heath DF, et al. Detection of optic disc change with the Heidelberg retina tomograph before confirmed visual field change in ocular hypertensives converting to early glaucoma. Br J Ophthalmol. 1999;83:290–4. doi: 10.1136/bjo.83.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan BC, Blanchard JW, Hamilton DC, Leblanc RP. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using scanning laser tomography. Invest Ophthalmol Vis Sci. 2000;41:775–82. [PubMed] [Google Scholar]
- 26.Kamal DS, Garway-Heath DF, Hitchings RA, Fitzke FW. Use of sequential Heidelberg retina tomograph images to identify changes at the optic disc in ocular hypertensive patients at risk of developing glaucoma. Br J Ophthalmol. 2000;84:993–8. doi: 10.1136/bjo.84.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan BC, McCormick TA, Nicolela MT, LeBlanc RP. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001;119:1492–9. doi: 10.1001/archopht.119.10.1492. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan BC, Hutchison DM, Artes PH, et al. Optic disc progression in glaucoma: comparison of confocal scanning laser tomography to optic disc photographs in a prospective study. Invest Ophthalmol Vis Sci. 2009;50:1682–91. doi: 10.1167/iovs.08-2457. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology. 2009;116:2110–8. doi: 10.1016/j.ophtha.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 30.van Velthoven MEJ, Faber DJ, Verbraak FD, et al. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros FA, Zangwill LM, Bowd C, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53:6939–46. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sample PA, Girkin CA, Zangwill LM, et al. ADAGES Study Group. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wulfsohn MS, Tsiatis AA. A joint model for survival and longitudinal data measured with error. Biometrics. 1997;53:330–9. [PubMed] [Google Scholar]
- 34.Crowther MJ, Abrams KR, Lambert PC. Joint modeling of longitudinal and survival data. Stata J. 2013;13:165–84. [Google Scholar]
- 35.Meira-Freitas D, Lisboa R, Tatham A, et al. Predicting progression in glaucoma suspects with longitudinal estimates of retinal ganglion cell counts. Invest Ophthalmol Vis Sci. 2013;51:4174–83. doi: 10.1167/iovs.12-11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros FA, Lisboa R, Zangwill L, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology. doi: 10.1016/j.ophtha.2013.06.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung KR, Sun JH, Na JH, et al. Progression detection capability of macular thickness in advanced glaucomatous eyes. Ophthalmology. 2012;119:308–13. doi: 10.1016/j.ophtha.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Lee EJ, Kim TW, Weinreb RN, et al. Trend-based analysis of retinal nerve fiber layer thickness measured by optical coherence tomography in eyes with localized nerve fiber layer defects. Invest Ophthalmol Vis Sci. 2011;52:1138–44. doi: 10.1167/iovs.10-5975. [DOI] [PubMed] [Google Scholar]
- 39.Seibold LK, Mandava N, Kahook MY. Comparison of retinal nerve fiber layer thickness in normal eyes using time-domain and spectral-domain optical coherence tomography. Am J Ophthalmol. 2010;150:807–14. doi: 10.1016/j.ajo.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Li S, Fu J, et al. Comparative study of retinal nerve fibre layer measurement by RTVue OCT and GDx VCC. Br J Ophthalmol. 2011;95:509–13. doi: 10.1136/bjo.2009.163493. [DOI] [PubMed] [Google Scholar]
- 41.Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with Stratus OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–6. doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 42.Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–63. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 43.Vizzeri G, Weinreb R, Gonzalez-Garcia AO, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol. 2009;93:775–81. doi: 10.1136/bjo.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langenegger SJ, Funk J, Töteberg-Harms M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Invest Ophthalmol Vis Sci. 2011;52:3338–44. doi: 10.1167/iovs.10-6611. [DOI] [PubMed] [Google Scholar]
- 45.Arthur SN, Smith SD, Wright MM, et al. Reproducibility and agreement in evaluating retinal nerve fibre layer thickness between Stratus and Spectralis OCT. Eye (Lond) 2011;25:192–200. doi: 10.1038/eye.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119:731–7. doi: 10.1016/j.ophtha.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119:627–36. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]
- 48.Poinoosawmy D, Fontana L, Wu JX, et al. Variation of nerve fibre layer thickness measurements with age and ethnicity by scanning laser polarimetry. Br J Ophthalmol. 1997;81:350–4. doi: 10.1136/bjo.81.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funaki S, Shirakashi M, Funaki H, et al. Relationship between age and the thickness of the retinal nerve fiber layer in normal subjects. Jpn J Ophthalmol. 1999;43:180–5. doi: 10.1016/s0021-5155(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 50.Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87:899–901. doi: 10.1136/bjo.87.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Da Pozzo S, Iacono P, Marchesan R, et al. The effect of ageing on retinal nerve fibre layer thickness: an evaluation by scanning laser polarimetry with variable corneal compensation. Acta Ophthalmol Scand. 2006;84:375–9. doi: 10.1111/j.1600-0420.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 52.Hirasawa H, Tomidokoro A, Araie M, et al. Peripapillary retinal nerve fiber layer thickness determined by spectral-domain optical coherence tomography in ophthalmologically normal eyes. Arch Ophthalmol. 2010;128:1420–6. doi: 10.1001/archophthalmol.2010.244. [DOI] [PubMed] [Google Scholar]
- 53.Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–30. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- 54.Balazsi AG, Rootman J, Drance SM, et al. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–6. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- 55.Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- 56.Beckett LA, Tancredi DJ, Wilson RS. Multivariate longitudinal models for complex change processes. Stat Med. 2004;23:231–9. doi: 10.1002/sim.1712. [DOI] [PubMed] [Google Scholar]
- 57.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci. 1991;6:15–32. [Google Scholar]
- 58.Zangwill LM, Weinreb R, Berry CC, et al. Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Racial differences in optic disc topography: baseline results from the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2004;122:22–8. doi: 10.1001/archopht.122.1.22. [DOI] [PubMed] [Google Scholar]
- 59.Girkin CA, McGwin G, Jr, Xie A, Deleon-Ortega J. Differences in optic disc topography between black and white normal subjects. Ophthalmology. 2005;112:33–9. doi: 10.1016/j.ophtha.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 60.Brandt JD, Beiser JA, Kass M, Gordon MO Ocular Hypertension Treatment Study (OHTS) Group. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108:1779–88. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 61.Gordon MO, Baiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 62.Leske MC, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114(11):1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]