Abstract
Mammalian pheromones control a myriad of innate social behaviors and acutely regulate hormone levels. Responses to pheromones are highly robust, reproducible, and stereotyped and likely involve developmentally predetermined neural circuits. Here, I review several facets of pheromone transduction in mammals, including (a) chemosensory receptors and signaling components of the main olfactory epithelium and vomeronasal organ involved in pheromone detection; (b) pheromone-activated neural circuits subject to sex-specific and state-dependent modulation; and (c) the striking chemical diversity of mammalian pheromones, which range from small, volatile molecules and sulfated steroids to large families of proteins. Finally, I review (d ) molecular mechanisms underlying various behavioral and endocrine responses, including modulation of puberty and estrous; control of reproduction, aggression, suckling, and parental behaviors; individual recognition; and distinguishing of own species from predators, competitors, and prey. Deconstruction of pheromone transduction mechanisms provides a critical foundation for understanding how odor response pathways generate instinctive behaviors.
Keywords: olfaction, VNO, hypothalamus, sexual behavior
INTRODUCTION
Animal behavior is controlled by sensory input through poorly understood mechanisms. Sensory systems that mediate touch, taste, vision, hearing, and smell activate dedicated neural circuits in the brain. Of these circuits, olfactory circuits that process pheromones are highly streamlined, targeting brain regions of the limbic system that orchestrate behavioral and endocrine responses in only a few synapses. The molecular characterization of pheromones, and the identification of responding receptors and neural circuits, builds an important foundation for understanding how different sensory inputs are selectively routed in the brain to control behavior.
In 1959, Karlson & Luscher (1) first coined the term pheromone, defined as a chemical released by one organism that modulates the behavior or physiology of a second organism of the same species. Pheromones were first identified as sexual attractants in insect species but are now known across the animal kingdom. This review discusses pheromone signaling in mammals, with many principles emerging from studies involving the powerful mouse model system. In mice, pheromones regulate endocrine status; signal individual identity; and evoke instinctive sexual, nurturing, and fighting behaviors.
MOLECULAR BASIS OF PHEROMONE AND ODOR SENSATION
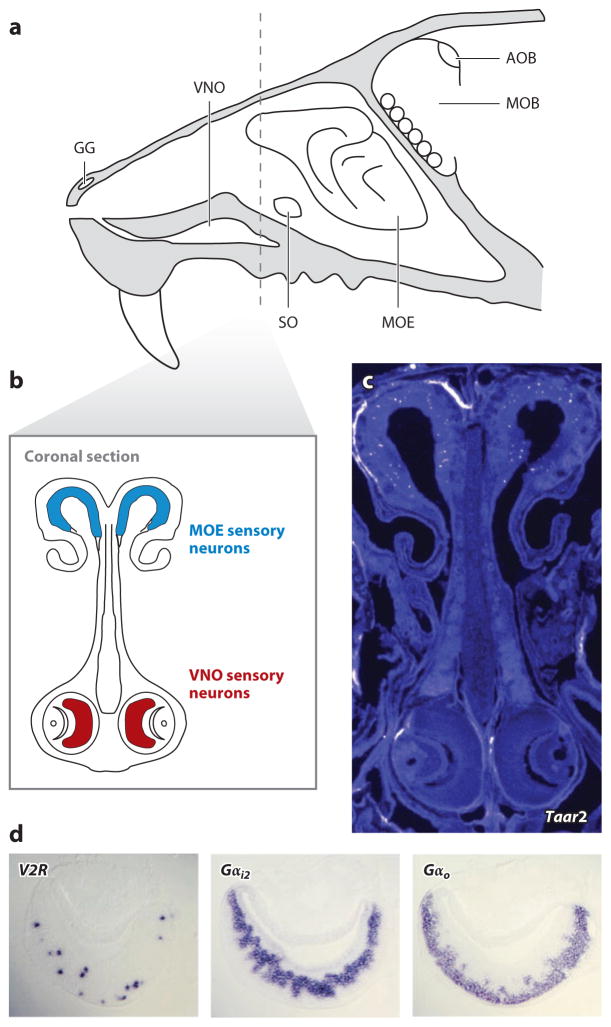
Mammalian pheromones are detected by the olfactory system, which consists of several sensory substructures (Figure 1), including the main olfactory epithelium (MOE), vomeronasal organ (VNO), Grueneberg ganglion (GG), and septal organ (SO). Sensory neurons located in these various olfactory tissues express different repertoires of chemosensory receptors and signaling molecules, project axons to discrete target regions of the olfactory bulb, and stimulate distinct limbic circuits. Despite these differences, sensory mechanisms in the MOE, VNO, and GG have been implicated in pheromone transduction.
Figure 1.
(a) The anatomy of the mouse nose, including the main olfactory epithelium (MOE), vomeronasal organ (VNO), Grueneberg ganglion (GG), septal organ (SO), main olfactory bulb (MOB), and accessory olfactory bulb (AOB). (b) A cartoon depiction of a coronal section containing MOE (blue) and VNO (red) neuroepithelium. (c) Taar2 cRNA riboprobes label MOE but not VNO sensory neurons by in situ hybridization, with pseudocoloring to facilitate visualization (author’s own data). (d ) Expression patterns of genes indicated in VNO sections determined by using in situ hybridization. Adapted from References 53 and 154 with permission.
Anatomy and Function of the Main Olfactory Epithelium
The MOE is the largest olfactory structure in mice, containing millions of sensory neurons that detect airborne odorants and pheromones that enter the nasal cavity through the nostrils. Olfactory sensory neurons are bipolar neurons that extend a solitary sensory dendrite into the nasal mucosa, detecting odorants at specialized cilia that contain the requisite receptors and signaling components. Olfactory sensory neurons also project very long axons that cross the cribriform plate of the skull and enter the brain, where they form synaptic connections in the olfactory bulb. The architecture of the MOE is particularly conducive for detecting volatile odors, although nonvolatile chemicals reportedly gain access as well (2).
The MOE plays an important role in pheromone signaling. Olfactory sensory neurons detect mammalian pheromones and other natural products with high affinity, including sex-specific urinary volatiles, steroids, biogenic amines, and major histocompatibility complex (MHC) peptides (2–6). Loss of MOE signaling by genetic ablation of key olfactory signaling molecules or by surgical disruption impairs behaviors that include mating, fighting, nurturing, and predator escape (7–11). Furthermore, MOE-derived signals are eventually transmitted to the hypothalamus, amygdala, and bed nucleus of the stria terminalis (BNST) (7, 12–14)—key neuronal structures that influence innate neuroendocrine responses associated with stress, aggression, and reproduction.
Chemosensory receptors in the MOE
Two families of G protein–coupled receptors (GPCRs) function as chemosensory receptors in olfactory sensory neurons: odorant receptors (ORs) (15), which are encoded by the largest mammalian gene family, and trace amine–associated receptors (TAARs) (5), a smaller family of GPCRs distantly related to biogenic amine receptors. In addition, rare olfactory sensory neurons utilize a noncanonical guanylyl cyclase-D (GC-D)-mediated pathway (16). Urine-derived chemicals activate a small percentage of olfactory sensory neurons (6), including neurons expressing ORs, TAARs, and GC-D (3, 5, 17–19).
Odorant receptors
The vast majority of olfactory sensory neurons express ORs, an enormous family of rhodopsin-like GPCRs. Humans have ~350 intact OR genes, whereas rodents have >1,000 OR genes dispersed on almost every chromosome in clusters varying in size from one gene to hundreds of genes (20). OR genes are expressed in striking patterns in the olfactory epithelium, as each sensory neuron expresses one receptor (20). The response field of a sensory neuron is dictated by the ligand-binding properties of its expressed receptor. OR ligands are volatile odors with highly divergent structural motifs and functional groups including, but not limited to, thiols, alcohols, esters, ethers, ketones, aldehydes, terpenes, camphors, and macrocyclic alkanes (21, 22). Consistent with this tremendous functional heterogeneity, OR ligand–binding pockets display high sequence variability. Furthermore, the olfactory system encodes odor identity by using combinations of ORs, as a single odor activates multiple ORs, and a single OR detects multiple ligands (20). OR-ligand interactions are generally low affinity, as ORs sacrifice ligand specificity for promiscuity; however, some ORs instead display highly tuned ligand preferences, as might be expected for the detection of salient cues (22).
Several ORs detect mammalian odors, consistent with MOE-mediated pheromone transduction. For example, one mouse OR detects a male preputial gland–derived aliphatic alcohol that enhances urine attractiveness to females (19), whereas a human OR detects sweat-derived steroids (4). Other ORs likely mediate attraction responses to the male urine thiol (methylthio)-methylthiol (MTMT) (6), aversion responses to the fox odor 2,5-dihydro-2,4,5-trimethylthiazole (TMT) (7), and suckling responses to the rabbit mammary pheromone 2-methylbut-2-enal (23). Furthermore, various volatile urinary pheromones activate the MOE with high affinity (2, 24), and some of these likely activate ORs (25). However, the ORs required for particular pheromone responses are unknown.
Trace amine–associated receptors
TAARs are distantly related to biogenic amine receptors and are evolutionarily distinct from ORs. There are 15 mouse and 6 human TAARs, and all except TAAR1 function as chemosensory receptors in the olfactory system (5). Numerous rodent TAARs detect volatile amines (26), some of which are urinary chemicals that evoke behavioral responses in rodents (3, 7, 18). TAAR ligands include 2-phenylethylamine, an aversive carnivore odor that activates TAAR4 (3), and trimethylamine, a sexually dimorphic mouse odor that activates TAAR5 (5, 18). TAAR5 knockout mice lose behavioral attraction to trimethylamine and display a decreased attraction to mouse scent, providing the first example of an altered odor-evoked behavior in mice lacking a single MOE receptor (18). TAAR4 and TAAR5 are encoded by immediately adjacent genes in the mouse genome and are localized to adjacent glomeruli in the olfactory bulb (27), yet respond to odors that evoke opposing behaviors. TAARs provide a powerful model system by which to understand how sensory cues generate aversion and attraction responses.
TAARs are not simply a family of amine receptors, as most TAARs in zebrafish have lost key amine recognition motifs and likely recognize other odor types. In addition, other chemosensory receptors detect amines (21, 28). Ancestral TAARs likely were amine detectors, but as the TAAR family expanded and mutated, some TAARs seemingly acquired the ability to recognize novel odors, providing an evolutionary advantage (26). TAARs, like ORs, recognize diverse chemicals and evoke divergent behaviors, highlighting the evolutionary flexibility of the olfactory system to adapt to the unique environmental niche of a species.
Guanylyl cyclase-D
Rare olfactory sensory neurons located predominantly in posterior MOE cul-de-sacs express membrane-associated GC-D rather than ORs, TAARs, and canonical MOE signaling molecules. GC-D neurons respond to diverse stimuli, including environ mental CO2, which is membrane permeable and reacts with intracellular carbonic anhydrase to form bicarbonate (29, 30). Bicarbonate directly activates the intracellular catalytic domain of GC-D, leading to increased cGMP synthesis and neuron depolarization. Adding complexity, GC-D is also activated by extracellular peptides such as urine-derived guanylin and uroguanylin (17). Finally, GC-D neurons detect CS2, another volatile gas and carbonic anhydrase substrate that has been implicated in socially transmitted food preference (31). GC-D knockout mice fail to display electrophysiological responses to peptides and show muted responses to CS2, but display largely normal sexual and suckling behaviors (17, 31).
Signaling mechanisms in the main olfactory epithelium
Olfactory sensory neurons use a canonical signaling pathway to convert chemical stimuli from the environment into electrical impulses that are transmitted to the brain. Activated ORs couple with heterotrimeric G proteins containing the conserved subunit Gαolf (32), which in turn activates a cAMP-directed signaling cascade involving type III adenylyl cyclase, cyclic nucleotide–gated ion channel A2, and a calcium-activated chloride channel (20). Activation of this signaling pathway results in membrane depolarization and action potential generation in the vast majority of olfactory sensory neurons. TAARs, likewise, are coexpressed with Gαolf and stimulate cAMP production in heterologous cells, suggesting that they couple to a similar pathway (5). GC-D neurons, as discussed above, utilize an alternative cGMP-mediated cascade (16).
Anatomy and Function of the Vomeronasal Organ
The VNO is a sensory organ that is found in most mammals and that plays an important role in pheromone detection. Humans lack a functional VNO, a finding that has ignited debate as to whether humans produce and perceive pheromones (see sidebar, Do Humans Have Pheromones?). The VNO consists of a pair of cylindrical structures located above the palate that connects to the nasal cavity via a water-filled duct. During social investigation behaviors, stimuli are pumped through this duct and into the VNO lumen, where they are detected by VNO sensory neurons. VNO sensory neurons reside within a crescent-shaped sensory neuroepithelium and extend a single apical dendrite into the aqueous lumen to detect pheromones. The apical and basal layers of the VNO neuroepithelium form distinct zones that respond to different ligands, express distinct receptor families and signaling components, and project to discrete regions of the accessory olfactory bulb (AOB).
DO HUMANS HAVE PHEROMONES?
Human legends and myths have long depicted chemicals that provide an easy path to romance. In Shakespeare’s “A Midsummer Night’s Dream,” applying a potion made from wild pansy extracts to the sleeping eyelids of a man or woman will cause the person to fall madly in love with the first creature seen upon waking. [For the record, this has been tested and does not work (148).] Human pheromones derived from axillary sweat and tears reportedly control ovulation, mood, hormone secretion, and attraction to genetically dissimilar mates (149). The most scrutinized effect, odor-mediated menstrual synchrony in female roommates or college dormitories, was observed in some studies, but not in others (150, 151). Strong anatomical evidence indicates that adult humans lack a functional VNO or accessory olfactory bulb (152, 153). Human TRPC2 is a pseudogene, and the human genome encodes a small number of likely vestigial V1Rs and does not encode any V2Rs, MUPs, ESPs, or ABPs. However, the MOE could in principle detect human pheromones. A human OR detects sweat-derived androgens (4), which are proposed—but debated—to function as pheromones (149). For now, the existence of human pheromones remains controversial.
Both VNO layers detect natural products secreted externally in pheromone sources such as urine, tears, and saliva, often in age-, sex-, or physiology-specific patterns (33–38). The architecture of the VNO is particularly conducive for detecting water-soluble macromolecules, such as proteins and sulfated steroids, that are transmitted during direct animal-stimulus contact (24). Small, volatile molecules also activate VNO sensory neurons with remarkably high affinity when applied directly to sensory neuron dendrites (28, 39) and may be escorted to the VNO lumen during social interactions by binding to protein carriers. VNO activators elicit instinctive behaviors such as mating, aggression, and fear; influence hormone levels related to puberty or estrous; and serve as cues for individual recognition (37, 38, 40–44). Mice with disrupted VNO signaling, by surgical removal or knockout of signaling components, exhibit aberrant social behaviors (28, 45–48). VNO signals target key limbic system areas that control innate drives and endocrine responses, such as the hypothalamus and amygdala, although MOE- and VNO-derived neuronal pathways that target these brain regions are distinct (49).
Chemosensory receptors in the VNO
VNO sensory neurons detect stimuli by using three families of GPCRs: vomeronasal receptors type 1 and 2 (V1Rs and V2Rs) and formyl peptide receptors (FPRs) (50–55). Each receptor type is discussed in turn below.
V1Rs
V1Rs, a large family of ~180 GPCRs in mouse, are distantly related to bitter taste receptors (50). Each V1R is monoallelically expressed in a small subset of sensory neurons confined to the apical VNO zone (50, 56, 57). Apical VNO sensory neurons are highly tuned to detect urinary volatiles and sulfated steroids (36, 39, 58), with steroid-responsive neurons accounting for a large percentage of urine-activated neurons (59). The lack of a tissue culture–based V1R expression system has hampered V1R ligand identification. However, recent studies using VNO immediate early genes implicate particular V1Rs in detecting estrogen-, androgen-, corticosterone-, and pregnane-derived metabolites (58). Furthermore, V1Rb2-expressing neurons, labeled by GFP knock-in, respond to 2-heptanone, a urinary ketone (60). Knockout mice lacking a cluster of 16 V1Rs lose vomeronasal responses to three other urinary volatiles and display deficits in maternal aggression and male sexual behavior (28).
V2Rs
V2Rs, a similarly large family of ~120 GPCRs in mouse, are distantly related to the sweet taste receptor (51, 53, 55). Unlike other olfactory receptors, V2Rs have a large N-terminal extracellular domain involved in ligand binding (61). Most V2Rs are expressed in dispersed subsets of basal VNO sensory neurons (51, 53, 55), but some are broadly expressed across the basal neuroepithelium (62). Individual neurons can express multiple V2Rs, suggesting that V2Rs, like the sweet taste receptor complex, function as heterodimers. Furthermore, many V2Rs are coexpressed with nonclassical class I MHC receptors that are proposed, but debated, to function as V2R chaperones (63–65). Protein pheromones activate the basal VNO layer with strikingly high affinity (40, 41, 43, 45), and several V2Rs are tuned to detect naturally occurring odors from males, females, and predators (34, 58). One V2R mediates sensory neuron responses to an MHC peptide (66), whereas another is required for female sexual responses to a male tear pheromone, exocrine gland–secreting peptide 1 (ESP1) (41). Adding complexity, neurons expressing a single V2R display heterogeneous peptide responses, suggesting that V2R binding preference is modulated by coexpression of other factors (66). Recently developed in vitro and in vivo assays for V2R function should expedite ligand discovery and analysis of molecular recognition properties (58, 67). It will be exciting to determine how specific V2R-expressing cells couple with neural circuits that mediate aggression, sex, and fear.
Formyl peptide receptors
In most mammals, FPRs function in the immune system, recognizing formylated peptides secreted by bacteria or mitochondria during infection or tissue damage. In rodents, recent gene expansions increased the size of the FPR family, with two mouse FPRs that function in the immune system and five that function as VNO sensory receptors (52, 54). Vomeronasal FPRs (vFPRs) are expressed in subsets of sensory neurons that do not express V1Rs or V2Rs and are confined to either apical (FPR-rs3/4/6/7) or basal (FPR-rs1) VNO layers (52, 54). Ligand-binding analyses for vFPRs produced conflicting results; two studies report that vFPRs detect bacterial peptides, although activating peptides and structural motifs are divergent (54, 68). Furthermore, a maternally inherited formyl peptide from mitochondria activates VNO neurons in the basal neuroepithelium (45). Specific roles for vFPRs in mouse behavior remain elusive, but vFPRs, like other chemosensory receptors (18, 69), may detect metabolites produced by endogenous, invasive, or environmental microbes.
The recent neofunctionalization of FPRs as sensory receptors highlights the intrinsic developmental flexibility of the olfactory system. vFpr genes are embedded in a cluster of V1r and V2r genes, and recent gene duplication events in rodents likely placed an Fpr gene duplicate in proximity to a VNO receptor promoter, leading to VNO-specific expression. Incorporation of a new receptor into the olfactory system seems to require only proper gene expression control and G protein coupling. This developmental flexibility enables the rapid expansion of existing receptor families, and the incorporation of new receptor families, with relative ease.
Signaling mechanisms in the VNO
The G protein–mediated signaling pathway in VNO sensory neurons that couples chemical stimulation to neuronal depolarization is distinct from the canonical MOE pathway but instead shares similarity with bitter and sweet taste transduction mechanisms. Apical and basal VNO neurons utilize different Gα subunits (70, 71)—Gαi2 and Gαo, respectively—but common downstream transduction molecules, including phospholipase C, diacylglycerol, and the ion channel TrpC2 (46, 47, 72–74). Knockout mice lacking Gαi2 or TrpC2 globally, or Gαo specifically in sensory neurons, display aberrant aggression and/or sexual behaviors (45–47). Various TrpC2-independent currents are also reported in VNO sensory neurons that involve arachidonic acid–gated ion channels, calcium-activated chloride channels, and potassium channels (75–77). Knockout mice lacking VNO potassium channels showed social behavior deficits that were distinct from those observed in TrpC2 knockout mice, suggesting that different classes of VNO sensory neurons display unique ion channel dependencies (75).
Anatomy and Function of the Grueneberg Ganglion and Septal Organ
The GG, a small olfactory structure at the tip of the nose, is composed of 300–500 cells. GG neurons detect odors and express olfactory receptors, including one V2R, one OR, several TAARs, and a guanylyl cyclase (78). However, GG sensory dendrites appear to lack lumenal access, so the site of odor detection remains unclear (78). Expression of some GG receptors is enriched in neonates, suggesting a role in maternal interactions; however, suckling behaviors are normal following GG axon transection (79). GG neurons are reportedly activated by threatening odors like alarm pheromones and predator odors with common thiazoline-containing or thiol-containing structures (79, 80), whereas other GG neurons detect cold temperature through a noncanonical TrpM8-independent mechanism (78). Future studies are needed to clarify the sensory functions of GG neurons.
The SO is a thin layer of ciliated sensory neurons located in the ventral nasal septum. SO neurons resemble MOE neurons, as they express a small number of ORs and GC-D, utilize a cAMP-mediated signaling cascade, and respond to odorants of diverse structure (81). A role for SO neurons in pheromone transduction has not been reported.
NEURAL CIRCUITS THAT PROCESS PHEROMONES
Sensory Neuron Projections to the Olfactory Bulb
The axons of olfactory sensory neurons form synaptic connections in the main olfactory bulb (MOB) with second-order neurons termed mitral and tufted cells (13). These synapses occur in spatially defined spherical structures termed glomeruli. There are >2,000 glomeruli that collectively envelop a mouse olfactory bulb, and each is a coalescence point for ~5,000 axons of sensory neurons expressing a single receptor (20). Thus, although input from a single OR is spatially dispersed in the olfactory epithelium, such input becomes highly organized and spatially confined in the olfactory bulb. The position of a glomerulus for a particular receptor is stereotyped in different individuals and is determined by sensory neuron position within the olfactory epithelium, as well as by axon guidance cues whose expression is controlled by receptor-mediated cAMP signaling (82).
TAAR neurons target a discrete MOB domain (27, 83), but it is unknown whether TAAR-activated mitral cells have unique connectivity within higher-brain structures. GC-D neurons, in contrast, project to a string of glomeruli with a characteristic necklace-like distribution (so-called necklace glomeruli) (84). The large number of glomeruli receiving GC-D neuron input suggests either response heterogeneity or response integration with input from other olfactory receptors.
VNO sensory neuron axons, which are bundled in the ascending vomeronasal nerve, target the glomerular layer of an adjacent but discrete structure termed the AOB (49). Projections from apical and basal VNO neurons are segregated into distinct AOB domains, with partial integration of these inputs occurring in higher limbic system structures (71, 85, 86). Sensory neurons expressing a particular VR target numerous glomeruli, suggesting a distinct stimulus-coding logic in the AOB (56, 57). Glomeruli receiving input from highly related V1Rs are intermingled in an anatomical stripe of the AOB, indicating a clade-based grid of receptor input (87).
Glomerulus position within the MOB may be associated with the evoked behavioral response and/or chemical structure of activating odorants (7, 88). Mice lacking a large dorsal domain of the olfactory epithelium lose innate avoidance responses to certain odors, including some predator odors, suggesting that perception of odors as aversive or attractive is established early in olfactory circuitry (7). Interestingly, TAAR5 glomeruli are also located in the dorsal MOB, yet TAAR5 mediates attraction behavior in mouse (18, 27). It is not known whether VNO receptors that trigger distinct behavioral responses activate discrete information channels in the AOB.
Third-Order Neurons in Olfactory Circuits
Mitral cells from the MOB and AOB have distinct projection patterns deeper in the brain. MOB mitral cells target several brain regions, with dense innervation of the piriform cortex, cortical amygdala, and olfactory tubercle (13). In contrast, AOB mitral cells directly target limbic circuits, including the medial amygdala (MeA) and posteromedial cortical nucleus, collectively termed the vomeronasal amygdala, as well as the BNST and nucleus of the accessory olfactory tract (49). VNO inputs can be hormonally regulated in limbic circuits (see discussion of particular pheromone effects below), resulting in state-dependent or sex-dependent responses (41, 89, 90). VNO circuits bypass the olfactory cortex and provide highly streamlined targeting of hypothalamic neurons that release circulating hormones such as luteinizing hormone (LH), dopamine, and glucocorticoids and that orchestrate acute behavioral responses such as sex, aggression, and parental behavior. Although the small number of synaptic connections between the VNO and the limbic system suggests a hardwired circuit diagram, experience and learning are clearly evident in the accessory olfactory system (see discussion of the Bruce effect below) (91).
CHEMICAL DIVERSITY OF MAMMALIAN PHEROMONES
Pheromones display tremendous structural diversity and include small, volatile molecules, steroid derivatives, peptides, and large protein-ligand complexes. Natural pheromone sources such as urine, sweat, saliva, and tears contain natural product blends that vary with species, sex, age, genotype, and endocrine state. These pheromone blends can be quite complex; for example, male mice do not release a single pheromone that signals maleness but rather release a cocktail of androgen-dependent volatiles and peptides (6, 18, 19, 33, 40, 41, 44, 92). Different male-derived odors may be important in various behavioral paradigms, or alternatively, each individual male may produce a unique odor gestalt that conveys multifaceted information about sex, age, individuality, species, and physiological state.
Urinary Volatiles
Numerous sex-specific urinary volatiles reportedly regulate reproductive physiology, scent attraction, and aggression behaviors. Novotny and colleagues, in pioneering work, identified structurally diverse urinary pheromones (herein termed Novotny pheromones), including male-enriched 2-(sec-butyl)-dihydrothiazole, dehydro-exo-brevicomin, and farnesenes, as well as female-enriched 2,5-dimethylpyrazine, aliphatic ketones, and acetates (37, 38, 93). Novotny pheromones evoke high-affinity responses in both MOE and apical VNO sensory neurons (2, 24, 39), although the relative roles of these detection systems are unclear. Olfactory receptors for Novotny pheromones are largely unknown, but one cue, 2-heptanone, activates one OR, one V1R, and other receptors (25, 39, 60). Furthermore, three volatile odors, 6-hydroxy-6-methyl-3-heptanone, n-pentylacetate, and isobutylamine, fail to activate the vomeronasal system of knockout mice lacking a V1R cluster (28). Behavioral responses to some Novotny pheromones require copresentation with other urinary constituents (37); volatile urinary pheromones may require a lipocalin escort through the nasopalatine duct to access the VNO lumen of a behaving animal.
Investigators have since identified other male-enriched urinary volatiles, including MTMT, trimethylamine, and (Z)-5-tetradecen-1-ol (6, 18, 19). MTMT activates MOB mitral cells (6); (Z)-5-tetradecen-1-ol activates Olfr288 (19); and behavioral attraction to trimethylamine requires a single MOE receptor, TAAR5 (18).
Steroid Derivatives
Externally released steroids function as pheromones that provide direct information about internal hormone state. For example, the androgenic steroid 5α-androst-16-en-3-one (androstenone) functions as a sex pheromone in the domestic pig (Sus scrofa) (94). Androstenone is produced in male pig saliva and is aerosolized during courtship displays by a stereotyped chomping behavior. Female pigs in estrous are attracted to androstenone and respond by adopting a characteristic mating posture. Androstenone-mediated sexual behavior in pigs requires MOE rather than VNO signaling (95), and a human OR detects androstenone and a related steroid present in human sweat (4).
Steroid derivatives are also found in mouse urine following the addition of polar moieties (glycine, taurine, and sulfate) that promote water solubility. Sulfated steroids are nonvolatile cues that induce potent, TrpC2-dependent responses in a high percentage of apical VNO sensory neurons and anterior AOB mitral cells (36, 59). Different classes of steroids—androgens, estrogens, pregnenolones, and glucocorticoids—activate particular V1R clades (58) and stimulate discrete processing streams in the AOB (59).
Furthermore, steroids released into mouse saliva and tears can be bound by androgen-binding proteins (ABPs) (96). ABPs are secretoglobins that form heterodimeric complexes with steroids bound at the dimer interface (96). The steroid-binding capacity of ABPs is reminiscent of the pheromone-binding properties of lipocalins (see below). The Abp gene family has undergone strikingly rapid evolution, with numerous species-specific gene expansions, as is observed for families of protein pheromones (96). Furthermore, ABPs are produced with striking sex specificity in various exocrine glands and are proposed to influence mate choice (96, 97). The steroid-binding capacities of different ABPs remain poorly studied, and whether ABPs activate VNO sensory neurons is unknown.
MHC Peptides
MHC molecules function in intracellular pathogen defense, displaying proteasome-derived peptides on the cell surface for surveillance by cytotoxic T cells. The evolutionary battle between hosts and pathogens led to extensive natural variation at MHC loci, with MHC receptors from different individuals binding to unique peptide repertoires on the basis of specific anchor residues. It has been proposed that the mouse olfactory system utilizes MHC locus heterogeneity to discriminate individuals during social interactions, by recognizing either receptor fragments, bound peptides, or other associated odors (91, 98). MHC peptides that bind to the H-2b MHC haplotype of C57BL/6 mice (AAPDNRETF) and the H-2d haplotype of BALBc mice (SYFPEITHI) stimulate high-affinity electrical and calcium responses in VNO and MOE sensory neurons (2, 43). Altering anchor residue identity eliminates these responses or reduces their sensitivity (2, 43). These two peptides activate a high percentage of sensory neurons in the VNO (2.6%) and the MOE (2.9%), suggesting detection by many receptors (2, 43). In the VNO, activated neurons are confined to the basal neuroepithelium, the site of V2R expression, and responses require Gαo but not TrpC2 (43, 45, 99). Neurons expressing a particular V2R, V2R1b, display heterogeneous responses to structurally divergent peptides, and knockout of V2R1b eliminates peptide responses in these cells (66). Adding complexity, most basal VNO sensory neurons express noncanonical class I MHC receptors (63, 65).
Major Urinary Proteins and Other Lipocalins
Mice excrete milligrams of protein every day, at enormous energetic cost and predation risk. Members of a single ~18–20 kDa protein family termed major urinary proteins (MUPs) account for the vast majority (>99%) of mouse urinary protein (100). Urinary MUPs are synthesized in liver, whereas other MUPs are produced in exocrine glands and are passed externally into saliva, tears, and milk (100). In liver, MUP production can be sex specific, a result of a Stat5b-mediated signaling cascade responsive to male-typical cycles of pituitary growth hormone (101). The Mup gene family has undergone strikingly rapid evolution; the last common ancestor of mouse and rat likely had 1 MUP (humans have 0), but recent gene duplications led to 21 MUPs in mice and ~20 in rats (termed α2u-globulins) (102). Furthermore, Mup genes are highly polymorphic in outbred mouse populations, with different individuals producing unique MUP codes readily distinguished by isoelectric-focusing electrophoresis (42, 100). An individual’s MUP signature in scent marks may provide a mechanism for genotype discrimination (42, 100).
MUPs are members of the lipocalin superfamily and adopt a characteristic β-barrel structure, with structurally diverse small molecules bound in the large, hydrophobic interior (103). MUPs display the highest binding affinity for the mouse pheromone 2-(sec-butyl)-dihydrothiazole and lower affinity for other urinary volatiles (104). Pheromone binding by MUPs was proposed to prevent pheromone degradation or evaporation from scent marks or to escort pheromones to hydrophilic environments such as urine or the VNO lumen. However, MUPs encoded by different genes or individuals display largely similar binding preferences for urinary volatiles (104, 105), raising the question of why so many MUPs are needed if they function only as pheromone chaperones.
MUPs devoid of ligands are now thought to function independently as mouse pheromones. Recombinant MUPs stimulate calcium and electrical responses in VNO sensory neurons located in the basal layer of the neuroepithelium (40, 45, 106) and induce cFos expression in the AOB (106). MUP responses require Gαo; TrpC2; and β2-microglobulin (40, 45, 106), a component of MHC class I molecules proposed to function as a V2R chaperone. V2Rs that detect MUPs are unknown, and discovery of such V2Rs would enable analysis of MUP recognition features and testing of whether individual receptors discriminate MUPs from different individuals. MUPs and other lipocalins are implicated in numerous pheromone effects, including sexual attraction, male-male aggression, hormone modulation, individuality recognition, spatial learning, and predator odor–induced fear (40, 42, 44, 106–109).
Exocrine Gland–Secreting Peptides
ESPs are a family of 7–10 kDa peptides that are major constituents of mouse tears and saliva (34), rich but poorly studied pheromone sources. There are 24 ESPs in mouse, 10 in rat, and 0 in human (35), with one, mouse ESP1, functioning as a male sex pheromone that enhances female receptivity for copulation (41). ESPs are produced in mouse exocrine glands, with most (13 out of 24) expressed in the extraorbital lacrimal gland, which produces tear fluid (35). Expression of some ESPs is sexually dimorphic and strain dependent; for example, in BALBc mice, testosterone induces expression of male-enriched ESP1 but represses expression of female-enriched ESP36 (35).
Recombinant ESPs activate VNO sensory neurons, as determined by cFos immunostaining, electrophysiology, and calcium imaging of single neurons in dissociated cultures and tissue slices (34, 35, 41, 110). Furthermore, ESP1 activates cFos expression in brain nuclei that receive VNO input, with some nuclei of the limbic system displaying sexually dimorphic responses (41). Behavioral and neuronal responses to ESP1 require a single vomeronasal receptor, V2Rp5, as well as Gαo and TrpC2 (41, 45). β2-Microglobulin and MHC class I receptors facilitate cell surface trafficking of ESP5- and ESP6-detecting V2Rs (67), but neuronal responses to ESP1 are preserved in β2-microglobulin knockout mice (41).
Crystallographic analysis of ESP1 indicates a core structure consisting of three α-helices and an intramolecular disulfide bond (61). V2Rp5 does not recognize a linear ESP1 epitope but rather recognizes a conformational epitope that requires tertiary structure formation, with the highly charged ESP1 surface likely forming several electrostatic salt bridges to the receptor (61).
BEHAVIORAL AND ENDOCRINE RESPONSES TO MAMMALIAN PHEROMONES
Primer Pheromones and Estrous Modulation
In 1956, Whitten (111) reported the first pheromone effect in mammals: Urinary cues from male mice promote estrous synchronization in group-housed females. Isolated female mice typically undergo first estrous at ~4 weeks of age and then display ~4 day estrous cycles in adulthood. At least three different types of pheromone input acutely modulate the kinetics of the estrous cycle. First, the aforementioned Whitten effect ensures that female mice are fertile at the time of a male encounter. Second, male odors can accelerate the onset of puberty in juvenile females, promoting growth of the uterus and initiating estrous (the Vandenbergh effect) (112). Third, female mice housed in groups of eight to ten per cage, or exposed to the odors of group-housed females, display estrous cycle delay (38). The effects of male and female odors on the estrous cycle involve a class of pheromones termed primer pheromones, olfactory cues that modulate endocrine effects. Primer pheromones are conceptually distinguished from releaser pheromones that induce rapid behavioral responses such as mating, aggression, and fear.
The identities of the male mouse primer pheromones that underlie the Vandenbergh and Whitten effects have been controversial. Primer pheromones are reported in urine of intact males, but not in that of females or male castrates, indicating testosterone-regulated production. Several Novotny pheromones, including 2-(sec-butyl)-dihydrothiazole, dehydro-exo-brevicomin, urinary ketones, and preputial gland–derived farnesenes, reportedly function as puberty-accelerating and/or estrous-synchronizing pheromones (113, 114). Biogenic amines, MUPs stripped of bound ligands, and a MUP-derived hexapeptide have also been reported, and debated, to function as primer pheromones (108, 115). Furthermore, a recent study argues that none of the reported primer pheromones accelerate puberty and that the relevant pheromones are other, unidentified chemicals (116). Clarifying the chemical identity of mouse primer pheromones that accelerate puberty and synchronize estrous will provide an important basis for identifying responding receptors and neural circuits.
Female primer pheromones that delay estrous in group-housed females have also been reported. The production of female primer pheromones depends on housing density and is blocked by adrenalectomy, but not by ovariectomy. Urine fractionation studies identified a blend of adrenal gland–derived metabolites, including 2,5-dimethylpyrazine and various aliphatic acetates, that suppress female estrous (38). Adrenal gland–derived ketones found in female urine, including 2-heptanone, are enriched during pregnancy and promote rather than delay estrous (117). Finally, VNO-activating corticosterone-21-sulfate is also enriched in female urine (36, 58), but a role for sulfated steroids as primer pheromones has not been reported.
The hypothalamic-pituitary-gonadal axis controls the timing of puberty and estrous. Hypothalamic neurons that produce gonadotropin-releasing hormone (GnRH) are master regulators of this circuit, controlling pituitary release of LH and follicle-stimulating hormone (FSH), gonadal release of sex steroids, and ultimately the reproductive cycle and sexual behavior. Many factors influence the timing of puberty in mice, including age, weight, and olfactory cues, and GnRH neurons accordingly receive input from many brain regions (12, 14). Male primer pheromones are thought to exert endocrine effects by direct control of GnRH neuron activity (49). The opposing effects of female and male primer pheromones on estrous indicate that responsive VNO circuits display different modes of connectivity to the hypothalamic-pituitary-gonadal axis. Transynaptic tracing techniques revealed that GnRH neurons receive strong input from both the main and accessory olfactory pathways (12, 14), and surgical lesions to the VNO or AOB abolish the Whitten and Vandenbergh effects (118).
Aggression Toward Intruder Males
Aggressive attack behavior underlies defense of territory, offspring, and mates from intruders. In mice, the decision to attack requires olfactory cues, as knockout of signaling molecules in the MOE, the apical VNO, or the basal VNO impairs male-male aggression and maternal aggression (8, 9, 45–47, 119, 120). Aggression-promoting pheromones were identified by using a resident-intruder behavioral paradigm (37, 40), in which resident males attack foreign males, but not females or male castrates. Painting castrates with intact male urine, or its purified constituents, restores resident aggression and provides a robust bioassay for pheromone identification. Aggression-promoting pheromones include two Novotny pheromones that also function as primer pheromones, 2-(sec-butyl)-dihydrothiazole and dehydro-exo-brevicomin (37), as well as MUPs (40). Urinary volatiles are not sufficient to induce aggression when presented individually but require copresentation with other cues (37). The notion that aggressive behavior is triggered by a pheromone blend is consistent with the requirement for multiple olfactory subsystems in evoking this behavior.
Olfactory circuits trigger aggressive behavior by activating limbic circuits in the amygdala and hypothalamus. A hypothalamic attack locus has been identified in a subdivision of the ventromedial hypothalamus (VMHvl) (121, 122). VMHvl activation causes rapid attack behavior toward males, female mates, and even inanimate objects, whereas lesions or pharmacogenetic silencing suppresses aggressive behavior (121, 122). The VMHvl receives input from the MeA, a major target site of bulbar mitral cells (123). Males and lactating mothers, but not virgin females, display aggression behavior toward intruder males, suggesting that hormonal signaling can modulate the activity of these neural circuits. The mechanisms involved are unknown, but IRS4 is one gene with sexually dimorphic expression in the VMHvl, and knockout of IRS4 impairs maternal aggression (90). Olfactory receptors that respond to aggression-promoting cues are unknown and would provide a powerful inroad toward understanding how olfactory inputs that promote aggression are organized differently from other olfactory inputs.
Sexual Attraction
Opposite-sex odors are highly attractive in many species, driving social interaction, courtship display, and mating behavior. MOE deficits eliminate sexual activity (8, 9), whereas VNO deficits impair sex specificity of copulation and odor attraction responses (46, 47, 124). Different effects of VNO removal and TrpC2 knockout on male sexual behavior are reported (46, 47, 124) and may be due to (a) social experience prior to VNO surgery, (b) TrpC2-independent VNO function, (c) TrpC2 expression in other tissues, or (d ) side effects of VNO surgery.
Male pheromones that induce stereotyped lordosis responses in females include salivary androstenone in pig (94) and tear-derived ESP1 in mouse (41). Other chemicals in male mouse urine are highly attractive to females; such chemicals include urinary volatiles such as trimethylamine, MTMT, tetradecen-1-ol, 2-(sec-butyl)-dihydrothiazole, dehydro-exo-brevicomin, farnesenes, and a nonvolatile MUP termed darcin (6, 18, 19, 44, 92). Attraction to urinary odors likely involves blend recognition, and consistently, enzymatic depletion of one scent constituent, trimethylamine, impairs but does not eliminate scent attraction (18). Urinary volatiles may function as homing signals to locate sources of nonvolatile VNO activators, which would explain the striking requirement of main olfactory signaling in many odor-driven social behaviors. It has been proposed that darcin confers the unconditioned attractive properties of urine and that attraction to volatile odors is enhanced by darcin-mediated learning (44).
Less is known about the female cues that induce male attraction. Male mice are attracted to estrous female–derived urinary volatiles (125), whereas male hamsters display sexual behavior toward anesthetized males painted with female vaginal fluid (107). Fractionation studies identified a hamster lipocalin termed aphrodisin that is sufficient to induce such male sexual behavior (107).
Olfactory receptors are known for some male odors, including ESP1, trimethylamine, and tetradecen-1-ol (18, 19, 41). Knockout of a single VNO receptor, V2Rp5, abolishes ESP1-induced neuronal and behavioral responses (41), whereas knockout of a single MOE receptor, TAAR5, eliminates attraction to trimethylamine and impairs attraction to mouse urine, an abundant natural source of trimethylamine (18). Other VNO receptors detect sex-specific odors (34, 58), but the associated ligands and behaviors remain uncharacterized.
Male and female odors activate sex-specific neural circuits in the amygdala, hypothalamus, and BNST. Sexually dimorphic nuclei of males and females differ in terms of neuron number and projections, spine density, and gene expression patterns (126). Furthermore, these regions express estrogen and androgen receptors, consistent with an important role for sex hormones in shaping the architecture or function of the limbic system (126). Masculinization of the mouse brain involves a perinatal testosterone surge, followed by local conversion of testosterone to estradiol by aromatase (126, 127). Estradiol then activates, perhaps counterintuitively, estrogen receptor signaling in the male brain to promote male-patterned behaviors (126, 127). These perinatal changes in neural circuitry are thought to enable sexually dimorphic behaviors that materialize after puberty in adulthood. Furthermore, different reproductive behaviors are impacted by knockout of different sex hormone–regulated genes expressed in the limbic system, suggesting modular control of sexual behavior (90). Additional studies are needed to clarify the mechanisms by which sex hormone–driven molecular and cellular changes alter mammalian behavior.
Mammary Pheromones and the Suckling Response
Newborn mammals, immediately after birth, display an instinctive suckling response to locate their first meal. Mice are born blind and use olfactory cues to navigate toward the nipple by a stereotyped chemotactic search mechanism termed rooting behavior. Elimination of olfactory cues by washing the nipple hinders suckling behavior (128–130), and disruption of MOE signaling results in neonatal lethality due to an inability to feed (9, 11, 32, 131). A mammary pheromone from rabbit milk, 2-methylbut-2-enal, induces an innate suckling response in neonates that have not nursed previously (23). Neonatal mice instead rely on odors present in amniotic fluid and rapidly learn to suckle in response to novel cues present in milk or maternal saliva (128, 130). Moreover, changing the composition of amniotic fluid by maternal diet modification alters odor-induced suckling preferences in neonates (128, 129), suggesting that mice learn in utero to enable this first instinctive behavior.
Neonatal Pheromones and Maternal Behavior
In rodents, endocrine changes following pregnancy facilitate the development of maternal behaviors important for offspring survival, including nursing, nest building, retrieval of wandering pups to the nest, grooming behavior, and defensive aggression toward intruders. Rodents rely on multisensory input to recognize pups, such as pheromones and ultrasonic vocalizations, as well as other visual, tactile, and thermal cues (132). In rats, neonatal pheromones are sufficient but are not independently required to evoke certain maternal behaviors (132). However, mice with impaired MOE signaling, through surgery or gene knockout, display severe deficits in maternal behavior (9, 11, 132); mice lacking VNO function display largely normal pup retrieval behavior but have deficits in maternal aggression and decreased nest occupancy (119).
The identities of neonatal pheromones in mammals are largely unknown. Dodecyl propionate is a preputial gland–derived pheromone of neonatal rats that stimulates maternal grooming through a VNO-mediated pathway (133). Neonatal pheromones have not been characterized in the mouse and would provide reagents by which to probe the dynamic relationship between the olfactory system and the limbic system nuclei that control parental behavior.
In the rat, olfactory circuits that respond to neonatal pheromones are dramatically rewired following pregnancy (132). The medial preoptic area (MPOA) of the hypothalamus together with adjacent ventral areas of the BNST (vBNST) forms a critical brain region important for the development of maternal behavior (132). Lesions of the MPOA/vBNST abolish or impair several maternal behaviors, whereas electrical stimulation has the opposite effect, promoting the development of maternal behavior in virgin animals (132). Neonatal cues that activate the rat olfactory system (the MOE or the VNO) can evoke responses of different valence in sexually naive females and mothers (132). A change in odor valence from aversion to attraction following pregnancy has been attributed to modified amygdalar outputs (132). Neonatal odors stimulate an aversion circuit involving the ventromedial hypothalamus (VMH) and periaqueductal gray in naive females but stimulate an attraction circuit involving the MPOA and the ventral tegmental area in lactating mothers (132). Naive lab mice, unlike rats, display a less pronounced pup aversion response and are more prone to develop maternal behavior toward foster pups (132).
Rewiring of olfactory circuits responsive to neonatal pheromones involves a series of pregnancy-associated surges of circulating hormones, including estrogen, progesterone, prolactin, and oxytocin (132). The ordered release of pregnancy hormones orchestrates systemic responses that include neuronal changes critical for the appropriate onset of maternal behavior in advance of parturition. Neurons in the MPOA and vBNST express receptors for pregnancy hormones (132), although the cellular basis by which pregnancy hormones reroute pup odor–responsive neural circuits is not clear. Possible mechanisms for neural circuit modulation include changes in intracellular signaling, gene expression, cell survival, synapse function, and neurogenesis (132, 134–136). Maternal behavior is disrupted in knockout mice lacking FosB, a transcription factor rapidly induced in the MPOA by maternal behavior and pup-associated stimuli (134). Furthermore, mice lacking the signaling protein Irs4 display performance impairment in pup retrieval assays and decreased maternal aggression (90).
Recognition of Individuals
Social animals must recognize specific individuals of the same species to remember mates, offspring, and parents; to defend territory and resources from intruders; and to avoid inbreeding with kin. In many mammals, olfactory cues are critical for distinguishing individuals, although relevant chemicals are debated.
Several behavioral paradigms have been used to query the mechanisms underlying individuality recognition, including various odor discrimination tasks, mating preference, scent countermarking behavior, and a pheromone-mediated pregnancy block effect named the Bruce effect (42, 91, 98). The Bruce effect occurs when a newly mated female mouse encounters a stranger male, or his odor, and terminates pregnancy (91). This pregnancy termination is beneficial, as it enables copulation with the newer, dominant male that effectively countermarked the territory with his scent. The Bruce effect is induced when the odor of a new male, but not that of a familiar male, is present, indicating that the female can effectively establish an olfactory memory of her mate and distinguish his odor from that of a stranger. Furthermore, the Bruce effect is maximally evoked by odors of intact males compared with those of castrated males or females, suggesting the integration of information related to sex and genotype. The Bruce effect requires a functional VNO but not a functional MOE, although it is maintained in TrpC2 knockout mice (91, 99).
The Bruce effect is triggered by the activation of a neuroendocrine circuit involving the tuberoinfundibular dopamine system in the arcuate nucleus of the hypothalamus (91). Dopamine released from the median eminence into the hypophysial portal vasculature inhibits pituitary prolactin release and thus the ability to sustain pregnancy. Prolactin release normally occurs in distinct temporal waves early in pregnancy, so synchronizing odor exposure with prolactin release is crucial for successful induction of the Bruce effect. Furthermore, noradrenaline signaling in the AOB is required for mate memory formation (118).
MHC-associated odors reportedly govern mate choice, inbreeding avoidance, parent-offspring interactions, and the Bruce effect (91, 98). MHC peptides are potent activators of both VNO and MOE sensory neurons (2, 43), although it is debated whether urinary levels of MHC peptides are sufficient for sensory detection (33, 43). A recent study indicates that MHC-binding peptides can be identified in urine, albeit at low levels compared with levels of other urinary peptides, and that peptide production is not linked to MHC haplotype (137). MHC receptors bind a spectrum of peptides on the basis of preference for several key anchor residues, and VNO receptors would have to disambiguate this peptide mixture to infer MHC haplotype. Studies involving two peptides suggested that similar anchor residues are required for MHC receptor binding and VNO sensory neuron activation (43, 66). The number of naturally occurring MHC alleles far exceeds the number of V2Rs, and a relatively high percentage of neurons is activated by a single MHC peptide. One possibility is that peptide recognition by basal VNO sensory neurons is combinatorial such that a large number of structurally diverse peptides can be recognized and distinguished.
MUPs are also proposed to function in genotype recognition, as they are highly abundant in urine and are polymorphic in wild strains (100). MUPs have not been reported to stimulate the Bruce effect, with lower-molecular-weight urinary cues instead being implicated (138). However, MUPs reportedly mediate scent countermarking behavior, inbreeding avoidance, and female mating preference for dominant-scent owners (42, 139, 140). VNO sensory neurons or receptors that distinguish MUPs from different individuals are yet to be characterized.
Finally, ESPs are VNO activators that provide integrated information about sex and strain (35). Intriguingly, in mouse, the Esp gene cluster is near the Mhc class I locus (35). Furthermore, although urine is sufficient to induce the Bruce effect, a role for ESPs or other male pheromones found in tears or saliva has not been similarly studied.
Recognition of Foreign Species: Predators, Competitors, and Prey
Pheromones, by definition, are chemosignals transmitted between members of the same species. Mammals, in addition, can detect cues emitted by foreign species termed kairomones and allomones. Kairomones are foreign species–derived olfactory cues that benefit the detecting animal, such as odors from predators and prey (141). Allomones, in contrast, benefit the emitting animal and include the aversive thiol cocktail released by skunks for escape behavior.
Detection and avoidance of predator odors provide a classic paradigm for analysis of innate odor-driven behavior. Predator odors produce powerful fear-like responses in prey species that facilitate escape, inducing stereotyped freezing and avoidance behaviors, as well as acute changes in the levels of circulating stress hormones (141). The active kairomones in predator odors can be derived from different odor sources, including urine, feces, saliva, fur, and dander. Investigators have chemically identified several kairomones, including TMT, a volatile component of fox odor (7); lipocalins found in rat urine and cat saliva (106); and 2-phenylethylamine, a biogenic amine produced in many carnivore urines (3). Predator-derived lipocalins are produced with high species specificity, and their highly tuned detection by rodents may reflect a long evolutionary history with particular predators (106). In contrast, 2-phenylethylamine is generally produced by many carnivore species and may enable avoidance of novel and dangerous predators (3).
Different predator odors are detected by the MOE and VNO, and relevant detection mechanisms may depend on stimulus volatility. Innate responses to TMT are lost in mice lacking a large dorsal domain of the MOE (7), and responses to TMT and to putative alarm pheromones also reportedly occur in the GG (80). 2-Phenylethylamine is a high-affinity agonist of the MOE receptor TAAR4 (3), which is a candidate kairomone receptor, and 2-phenylethylamine avoidance persists in TrpC2 knockout mice (3). Predator-derived lipocalins instead stimulate basal VNO sensory neurons, and behavioral responses to these cues require TrpC2 (106). In addition, many VNO receptors detect predator odors derived from raptors, snakes, and carnivores (58). Olfactory receptors in the MOE, VNO, and GG that detect predator odors may provide a selective advantage for rodents.
Predator odors activate second-order neurons in the MOB and AOB (7, 106, 142). VNO-activating predator odors, like cat collar odor, stimulate the hypothalamic defensive system, a characteristic neural circuit that includes the MeA, the dorsomedial VMH, the premammillary nucleus, periaqueductal gray, and other brain regions (141). Interestingly, predator odor–responsive neurons in this circuit are intermingled with sex pheromone–responsive neurons that have distinct projections and gene expression patterns (123). TMT, which instead stimulates the MOE, induces the hypothalamic-pituitary axis through a different neural circuit involving the BNST (7). Intriguingly, infection with Toxoplasma parasite in rodents can acutely alter predator odor–responsive neural circuits, causing predator attraction rather than aversion and promotion of the parasite life cycle (143).
In addition to detecting predators and prey, animals must distinguish the odors of closely related species. At speciation events, strong selective pressure acts on pheromone biosynthesis pathways and pheromone response pathways to ensure that mating and other stereotyped behaviors are properly directed. Indeed, many volatile odors proposed to function as pheromones are similarly produced by other species (144), consistent with pheromones functioning as part of an odor blend. Recent evolutionary changes in the biosynthesis pathway of one mouse-enriched odor, trimethylamine, provide insight into how pheromone biosynthesis pathways may change during speciation (18). Abundant and sexually dimorphic trimethylamine production arose de novo in Mus, a result of male-specific repression of the principal trimethylamine oxidase, FMO3 (18). Synchronous with this evolving biosynthesis pathway, mice display species-specific attraction responses to trimethylamine that require TAAR5 (18). These studies highlight how rapid evolution of olfactory signaling may occur to ensure species-appropriate social interactions. Interestingly, TAAR4 is present in both carnivores and rodents (145), and TAAR4 may mediate distinct behavioral responses to 2-phenylethylamine in predators and prey.
Other Pheromone Effects
Researchers have reported other odor-driven behaviors, including responses to sickness cues, alarm pheromones, social dominance cues, nest pheromones, and odors that underlie the social transmission of food preference (31, 79, 92, 146, 147). Preputial gland–derived farnesenes are enriched in dominant male urine (92), whereas the Novotny pheromone 2-(sec-butyl)-dihydrothiazole, among its many functions, is a putative alarm pheromone (80). Numerous bacterial metabolites can be detected by the olfactory system (18, 54, 68, 69), but the particular cues and receptors involved in sickness detection are unknown. Finally, CS2 detection by GC-D neurons is implicated in the social transmission of food preference (31).
CONCLUSION
Olfactory circuits that respond to pheromones provide a powerful model system for understanding the molecular basis of behavior. Pheromones and predator odors are highly controllable stimuli that trigger robust, developmentally programmed responses. The chemicals underlying many pheromone effects remain unknown or unclear, and generating a collection of pheromones that activate different instinctive behaviors would provide a valuable toolbox for dissecting the basic function and organization of olfactory circuits. Receptors for pheromones that evoke different innate behaviors likely couple with discrete hardwired neural circuits, yet differences between receptor-specific neural circuits remain poorly understood. Finally, delineating classes of hypothalamic and amygdalar neurons that respond to behaviorally salient stimuli would help elucidate basic organizational principles of how sensory and limbic systems interact. It will be interesting to determine whether VNO-derived inputs are integrated into a small number of behaviorally relevant channels, indicating a decoding scheme different from that of the main olfactory system, which is instead wired for diversity recognition. Characterizing the connectivity between different classes of VNO receptors and particular subsets of hypothalamic neurons would provide mechanistic insight into how sensory cues control different social behaviors.
SUMMARY POINTS.
Mammalian pheromones display tremendous structural diversity and range from small, volatile molecules to large protein complexes.
Sensory neurons in the mouse olfactory epithelium and the vomeronasal organ detect odors and pheromones using five large families of GPCRs and, in rare neurons, GC-D.
Chemicals that function as primer pheromones; individuality cues; and releaser pheromones that control aggression, sex, suckling, and maternal behaviors have been reported. However, the importance of some proposed pheromones remains controversial.
Pheromones activate neural circuits in the limbic system that can be hormone modulated, resulting in sex-specific or state-dependent responses. The mechanisms by which different types of pheromone input are channeled in limbic circuits to exact appropriate behavioral outcomes remain poorly understood.
Acknowledgments
I thank David Strochlic and Rui Chang for comments on this manuscript. This work was supported by a grant from the NIH (award number R01 DC010155).
Glossary
- Pheromone
a chemical released by one organism that modulates the behavior or physiology of a second organism of the same species
- Main olfactory epithelium (MOE)
the largest olfactory structure in many mammals
- Vomeronasal organ (VNO)
an olfactory structure that detects some pheromones
- Grueneberg ganglion (GG)
a small olfactory structure at the tip of the nose
- Odorant receptors (ORs)
an enormous family of GPCRs expressed in the vast majority of olfactory sensory neurons
- Trace amine–associated receptors (TAARs)
a second family of GPCRs that function as MOE sensory receptors
- Guanylyl cyclase-D (GC-D)
expressed in rare olfactory sensory neurons that do not utilize canonical olfactory transduction molecules
- TMT (2, 5-dihydro-2,4,5-trimethylthiazole)
a fox-derived predator odor that causes fear-like responses in rodents
- Accessory olfactory bulb (AOB)
a forebrain structure that is adjacent to the MOB and that receives direct input from VNO sensory neuron axons
- MHC peptides
peptide ligands for the major histocompatibility complex (MHC)
- Exocrine gland–secreting peptides (ESPs)
a family of mouse pheromones released predominantly into tears and saliva
- Transient receptor potential cation channel C2 (TrpC2)
TrpC2 knockout mice display significant deficits in VNO function
- Main olfactory bulb (MOB)
a forebrain structure that receives direct input from MOE sensory neuron axons
- Glomeruli
spherical structures in the olfactory bulb where synapses occur between first-order sensory neurons and second-order mitral cells
- Major urinary proteins (MUPs)
a family of mouse pheromones
- cFos
a protein rapidly induced by electrical depolarization in many neuron types, providing a stable marker for recent neural activity
- Primer pheromones
pheromones that exert long-lasting changes in endocrine state
- Bruce effect
a pheromone-mediated pregnancy termination that provides evidence of olfaction-mediated individuality recognition
- Kairomones
olfactory cues, such as predator odors, that are transmitted between species and that benefit the detecting organism
- Allomones
olfactory cues, such as skunk-derived thiols, that are transmitted between species and that benefit the emitting organism
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Karlson P, Luscher M. “Pheromones”: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 2.Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–70. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, et al. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci USA. 2011;108:11235–40. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–72. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 5.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–50. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 6.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–77. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 7.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–8. doi: 10.1038/nature06281. Different TMT-responsive glomeruli support innate and learned behaviors. [DOI] [PubMed] [Google Scholar]
- 8.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–62. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–79. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacology. 2011;36:772–81. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, et al. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature. 2011;472:186–90. doi: 10.1038/nature09975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–95. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30:123–76. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–82. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–87. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 16.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3388–95. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, et al. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–12. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Korzan WJ, Ferrero DM, Chang RB, Roy DS, et al. Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr Biol. 2013;23:11–20. doi: 10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshikawa K, Nakagawa H, Mori N, Watanabe H, Touhara K. An unsaturated aliphatic alcohol as a natural ligand for a mouse odorant receptor. Nat Chem Biol. 2013;9:160–62. doi: 10.1038/nchembio.1164. [DOI] [PubMed] [Google Scholar]
- 20.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–40. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 21.Nara K, Saraiva LR, Ye X, Buck LB. A large-scale analysis of odor coding in the olfactory epithelium. J Neurosci. 2011;31:9179–91. doi: 10.1523/JNEUROSCI.1282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaal B, Coureaud G, Langlois D, Ginies C, Semon E, Perrier G. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- 24.Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard I, Rouquier S, Pin JP, Mollard P, Richard S, et al. A single olfactory receptor specifically binds a set of odorant molecules. Eur J Neurosci. 2002;15:409–18. doi: 10.1046/j.0953-816x.2001.01871.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrero DM, Wacker D, Roque MA, Baldwin MW, Stevens RC, Liberles SD. Agonists for 13 trace amine–associated receptors provide insight into the molecular basis of odor selectivity. ACS Chem Biol. 2012;7:1184–89. doi: 10.1021/cb300111e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MA, Tsai L, Roy DS, Valenzuela DH, Mosley C, et al. Neurons expressing trace amine–associated receptors project to discrete glomeruli and constitute an olfactory subsystem. Proc Natl Acad Sci USA. 2012;109:13410–15. doi: 10.1073/pnas.1206724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, et al. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Zhong C, Ding C, Chi Q, Walz A, et al. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–57. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;106:2041–46. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, et al. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol. 2010;20:1438–44. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 33.He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–38. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 35.Kimoto H, Sato K, Nodari F, Haga S, Holy TE, Touhara K. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol. 2007;17:1879–84. doi: 10.1016/j.cub.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 36.Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, et al. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–18. doi: 10.1523/JNEUROSCI.1425-08.2008. The first characterization of sulfated steroids as mouse urine–derived VNO activators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novotny M, Harvey S, Jemiolo B, Alberts J. Synthetic pheromones that promote inter-male aggression in mice. Proc Natl Acad Sci USA. 1985;82:2059–61. doi: 10.1073/pnas.82.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novotny M, Jemiolo B, Harvey S, Wiesler D, Marchlewska-Koj A. Adrenal-mediated endogenous metabolites inhibit puberty in female mice. Science. 1986;231:722–25. doi: 10.1126/science.3945805. [DOI] [PubMed] [Google Scholar]
- 39.Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–96. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- 40.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 41.Haga S, Hattori T, Sato T, Sato K, Matsuda S, et al. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–22. doi: 10.1038/nature09142. Knockout of V2Rp5 abolishes behavioral responses to the mouse sex pheromone ESP1. [DOI] [PubMed] [Google Scholar]
- 42.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, et al. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–34. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 43.Leinders-Zufall T, Brennan P, Widmayer P, Chandramani Shivalingappa P, Maul-Pavicic A, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–37. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- 44.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, et al. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, et al. G protein Gαo is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci USA. 2011;108:12898–903. doi: 10.1073/pnas.1107770108. Gαo knockout specifically in sensory neurons disrupts basal VNO function and aggression behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–81. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 48.Wysocki CJ, Lepri JJ. Consequences of removing the vomeronasal organ. J Steroid Biochem Mol Biol. 1991;39:661–69. doi: 10.1016/0960-0760(91)90265-7. [DOI] [PubMed] [Google Scholar]
- 49.Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–67. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- 50.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 51.Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–73. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- 52.Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, et al. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci USA. 2009;106:9842–47. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–84. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- 54.Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor–like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–77. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 55.Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–79. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 56.Belluscio L, Koentges G, Axel R, Dulac C. A map of pheromone receptor activation in the mammalian brain. Cell. 1999;97:209–20. doi: 10.1016/s0092-8674(00)80731-x. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 58.Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, et al. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–45. doi: 10.1038/nature10437. Particular vomeronasal receptors detect sulfated steroids and natural odors that signal sex and species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meeks JP, Arnson HA, Holy TE. Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci. 2010;13:723–30. doi: 10.1038/nn.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boschat C, Pelofi C, Randin O, Roppolo D, Luscher C, et al. Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci. 2002;5:1261–62. doi: 10.1038/nn978. [DOI] [PubMed] [Google Scholar]
- 61.Yoshinaga S, Sato T, Hirakane M, Esaki K, Hamaguchi T, et al. Structure of the mouse sex peptide pheromone ESP1 reveals a molecular basis for specific binding to the class-C G-protein-coupled vomeronasal receptor. J Biol Chem. 2013;288:16064–72. doi: 10.1074/jbc.M112.436782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martini S, Silvotti L, Shirazi A, Ryba NJ, Tirindelli R. Co-expression of putative pheromone receptors in the sensory neurons of the vomeronasal organ. J Neurosci. 2001;21:843–48. doi: 10.1523/JNEUROSCI.21-03-00843.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishii T, Hirota J, Mombaerts P. Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Curr Biol. 2003;13:394–400. doi: 10.1016/s0960-9822(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 64.Ishii T, Mombaerts P. Expression of nonclassical class I major histocompatibility genes defines a tripartite organization of the mouse vomeronasal system. J Neurosci. 2008;28:2332–41. doi: 10.1523/JNEUROSCI.4807-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loconto J, Papes F, Chang E, Stowers L, Jones EP, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–18. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 66.Leinders-Zufall T, Ishii T, Mombaerts P, Zufall F, Boehm T. Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nat Neurosci. 2009;12:1551–58. doi: 10.1038/nn.2452. [DOI] [PubMed] [Google Scholar]
- 67.Dey S, Matsunami H. Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc Natl Acad Sci USA. 2011;108:16651–56. doi: 10.1073/pnas.1018140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bufe B, Schumann T, Zufall F. Formyl peptide receptors from immune and vomeronasal system exhibit distinct agonist properties. J Biol Chem. 2012;287:33644–55. doi: 10.1074/jbc.M112.375774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–15. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berghard A, Buck LB. Sensory transduction in vomeronasal neurons: evidence for Gαo, Gαi2, and adenylyl cyclase II as major components of a pheromone signaling cascade. J Neurosci. 1996;16:909–18. doi: 10.1523/JNEUROSCI.16-03-00909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia C, Halpern M. Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Giα2 and Goα) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–28. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- 72.Inamura K, Kashiwayanagi M, Kurihara K. Blockage of urinary responses by inhibitors for IP3-mediated pathway in rat vomeronasal sensory neurons. Neurosci Lett. 1997;233:129–32. doi: 10.1016/s0304-3940(97)00655-1. [DOI] [PubMed] [Google Scholar]
- 73.Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–96. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–61. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- 75.Kim S, Ma L, Jensen KL, Kim MM, Bond CT, et al. Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat Neurosci. 2012;15:1236–44. doi: 10.1038/nn.3173. Different VNO sensory neurons may display unique ion channel dependencies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S, Ma L, Yu CR. Requirement of calcium-activated chloride channels in the activation of mouse vomeronasal neurons. Nat Commun. 2011;2:365. doi: 10.1038/ncomms1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spehr M, Hatt H, Wetzel CH. Arachidonic acid plays a role in rat vomeronasal signal transduction. J Neurosci. 2002;22:8429–37. doi: 10.1523/JNEUROSCI.22-19-08429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fleischer J, Breer H. The Grueneberg ganglion: a novel sensory system in the nose. Histol Histopathol. 2010;25:909–15. doi: 10.14670/HH-25.909. [DOI] [PubMed] [Google Scholar]
- 79.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–95. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- 80.Brechbuhl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, et al. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci USA. 2013;110:4762–67. doi: 10.1073/pnas.1214249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma M, Grosmaitre X, Iwema CL, Baker H, Greer CA, Shepherd GM. Olfactory signal transduction in the mouse septal organ. J Neurosci. 2003;23:317–24. doi: 10.1523/JNEUROSCI.23-01-00317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakano H. Neural map formation in the mouse olfactory system. Neuron. 2010;67:530–42. doi: 10.1016/j.neuron.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Pacifico R, Dewan A, Cawley D, Guo C, Bozza T. An olfactory subsystem that mediates high-sensitivity detection of volatile amines. Cell Rep. 2012;2:76–88. doi: 10.1016/j.celrep.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cockerham RE, Puche AC, Munger SD. Heterogeneous sensory innervation and extensive intra-bulbar connections of olfactory necklace glomeruli. PLoS ONE. 2009;4:e4657. doi: 10.1371/journal.pone.0004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–80. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- 86.von Campenhausen H, Mori K. Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur J Neurosci. 2000;12:33–46. doi: 10.1046/j.1460-9568.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 87.Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron. 2006;50:697–709. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 88.Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–33. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- 89.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–90. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- 90.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, et al. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. Identifies sexually dimorphic genes that mediate different aspects of reproductive behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brennan PA. Outstanding issues surrounding vomeronasal mechanisms of pregnancy block and individual recognition in mice. Behav Brain Res. 2008;200:287–94. doi: 10.1016/j.bbr.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 92.Jemiolo B, Xie TM, Novotny M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiol Behav. 1991;50:1119–22. doi: 10.1016/0031-9384(91)90570-e. [DOI] [PubMed] [Google Scholar]
- 93.Jemiolo B, Alberts J, Sochinski-Wiggins S, Novotny M. Behavioural and endocrine responses of female mice to synthetic analogues of volatile compounds in male urine. Anim Behav. 1985;33:1114–18. [Google Scholar]
- 94.Melrose DR, Reed HC, Patterson RL. Androgen steroids associated with boar odour as an aid to the detection of oestrus in pig artificial insemination. Br Vet J. 1971;127:497–502. doi: 10.1016/s0007-1935(17)37337-2. [DOI] [PubMed] [Google Scholar]
- 95.Dorries KM, Adkins-Regan E, Halpern BP. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- 96.Laukaitis CM, Dlouhy SR, Emes RD, Ponting CP, Karn RC. Diverse spatial, temporal, and sexual expression of recently duplicated androgen-binding protein genes in Mus musculus. BMC Evol Biol. 2005;5:40. doi: 10.1186/1471-2148-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laukaitis CM, Critser ES, Karn RC. Salivary androgen binding protein (ABP) mediates sexual isolation in Mus musculus. Evolution. 1997;51:2000–5. doi: 10.1111/j.1558-5646.1997.tb05121.x. [DOI] [PubMed] [Google Scholar]
- 98.Restrepo D, Lin W, Salcedo E, Yamazaki K, Beauchamp G. Odortypes and MHC peptides: complementary chemosignals of MHC haplotype? Trends Neurosci. 2006;29:604–9. doi: 10.1016/j.tins.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 99.Kelliher KR, Spehr M, Li XH, Zufall F, Leinders-Zufall T. Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur J Neurosci. 2006;23:3385–90. doi: 10.1111/j.1460-9568.2006.04866.x. [DOI] [PubMed] [Google Scholar]
- 100.Beynon RJ, Hurst JL. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochem Soc Trans. 2003;31:142–46. doi: 10.1042/bst0310142. [DOI] [PubMed] [Google Scholar]
- 101.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–28. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Logan DW, Marton TF, Stowers L. Species specificity in major urinary proteins by parallel evolution. PLoS ONE. 2008;3:e3280. doi: 10.1371/journal.pone.0003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bocskei Z, Groom CR, Flower DR, Wright CE, Phillips SE, et al. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature. 1992;360:186–88. doi: 10.1038/360186a0. [DOI] [PubMed] [Google Scholar]
- 104.Sharrow SD, Vaughn JL, Zidek L, Novotny MV, Stone MJ. Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci. 2002;11:2247–56. doi: 10.1110/ps.0204202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwak J, Grigsby CC, Rizki MM, Preti G, Koksal M, et al. Differential binding between volatile ligands and major urinary proteins due to genetic variation in mice. Physiol Behav. 2012;107:112–20. doi: 10.1016/j.physbeh.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 106.Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. Identifies predator-derived lipocalins that evoke fear responses in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Briand L, Trotier D, Pernollet JC. Aphrodisin, an aphrodisiac lipocalin secreted in hamster vaginal secretions. Peptides. 2004;25:1545–52. doi: 10.1016/j.peptides.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 108.Mucignat-Caretta C, Caretta A, Cavaggioni A. Acceleration of puberty onset in female mice by male urinary proteins. J Physiol. 1995;486(Pt. 2):517–22. doi: 10.1113/jphysiol.1995.sp020830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal induction of spatial learning in mice. Science. 2012;338:1462–65. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- 110.He J, Ma L, Kim S, Schwartz J, Santilli M, et al. Distinct signals conveyed by pheromone concentrations to the mouse vomeronasal organ. J Neurosci. 2010;30:7473–83. doi: 10.1523/JNEUROSCI.0825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol. 1956;13:399–404. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- 112.Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–60. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- 113.Jemiolo B, Harvey S, Novotny M. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc Natl Acad Sci USA. 1986;83:4576–79. doi: 10.1073/pnas.83.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Novotny MV, Ma W, Wiesler D, Zidek L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc Biol Sci. 1999;266:2017–22. doi: 10.1098/rspb.1999.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishimura K, Utsumi K, Yuhara M, Fujitani Y, Iritani A. Identification of puberty-accelerating pheromones in male mouse urine. J Exp Zool. 1989;251:300–5. doi: 10.1002/jez.1402510306. [DOI] [PubMed] [Google Scholar]
- 116.Flanagan KA, Webb W, Stowers L. Analysis of male pheromones that accelerate female reproductive organ development. PLoS ONE. 2011;6:e16660. doi: 10.1371/journal.pone.0016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jemiolo B, Andreolini F, Xie TM, Wiesler D, Novotny M. Puberty-affecting synthetic analogs of urinary chemosignals in the house mouse, Mus domesticus. Physiol Behav. 1989;46:293–98. doi: 10.1016/0031-9384(89)90270-9. [DOI] [PubMed] [Google Scholar]
- 118.Keverne EB. Pheromonal influences on the endocrine regulation of reproduction. Trends Neurosci. 1983;6:381–84. [Google Scholar]
- 119.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–14. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 120.Norlin EM, Gussing F, Berghard A. Vomeronasal phenotype and behavioral alterations in Gαi2 mutant mice. Curr Biol. 2003;13:1214–19. doi: 10.1016/s0960-9822(03)00452-4. [DOI] [PubMed] [Google Scholar]
- 121.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–26. doi: 10.1038/nature09736. Characterizes the hypothalamic attack locus using elegant genetic techniques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kruk MR. Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev. 1991;15:527–38. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- 123.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–60. doi: 10.1016/j.neuron.2005.04.011. Maps the connectivity of genetically defined medial amygdala neurons responsive to reproductive and defensive stimuli. [DOI] [PubMed] [Google Scholar]
- 124.Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–57. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnston RE, Bronson F. Endocrine control of female mouse odors that elicit luteinizing hormone surges and attraction in males. Biol Reprod. 1982;27:1174–80. doi: 10.1095/biolreprod27.5.1174. [DOI] [PubMed] [Google Scholar]
- 126.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Curr Biol. 2012;22:1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev Psychobiol. 1982;15:349–55. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- 130.Teicher MH, Blass EM. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–36. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- 131.Singh PJ, Tucker AM, Hofer MA. Effects of nasal ZnSO4 irrigation and olfactory bulbectomy on rat pups. Physiol Behav. 1976;17:373–82. doi: 10.1016/0031-9384(76)90094-9. [DOI] [PubMed] [Google Scholar]
- 132.Numan M, Fleming AS, Levy F. Maternal behavior. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. St. Louis, MO: Elsevier Acad; 2006. pp. 1921–93. [Google Scholar]
- 133.Brouette-Lahlou I, Godinot F, Vernet-Maury E. The mother rat’s vomeronasal organ is involved in detection of dodecyl propionate, the pup’s preputial gland pheromone. Physiol Behav. 1999;66:427–36. doi: 10.1016/s0031-9384(98)00334-5. [DOI] [PubMed] [Google Scholar]
- 134.Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 135.Kopel H, Schechtman E, Groysman M, Mizrahi A. Enhanced synaptic integration of adult-born neurons in the olfactory bulb of lactating mothers. J Neurosci. 2012;32:7519–27. doi: 10.1523/JNEUROSCI.6354-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–20. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 137.Sturm T, Leinders-Zufall T, Macek B, Walzer M, Jung S, et al. Mouse urinary peptides provide a molecular basis for genotype discrimination by nasal sensory neurons. Nat Commun. 2013;4:1616. doi: 10.1038/ncomms2610. [DOI] [PubMed] [Google Scholar]
- 138.Peele P, Salazar I, Mimmack M, Keverne EB, Brennan PA. Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur J Neurosci. 2003;18:622–28. doi: 10.1046/j.1460-9568.2003.02790.x. [DOI] [PubMed] [Google Scholar]
- 139.Cheetham SA, Thom MD, Jury F, Ollier WE, Beynon RJ, Hurst JL. The genetic basis of individual-recognition signals in the mouse. Curr Biol. 2007;17:1771–77. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 140.Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WE, et al. The genetic basis of inbreeding avoidance in house mice. Curr Biol. 2007;17:2061–66. doi: 10.1016/j.cub.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–44. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 142.Ben-Shaul Y, Katz LC, Mooney R, Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci USA. 2011;107:5172–77. doi: 10.1073/pnas.0915147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vyas A, Kim S-K, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. 2007;104:6442–47. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelly DR. When is a butterfly like an elephant? Chem Biol. 1996;3:595–602. doi: 10.1016/s1074-5521(96)90125-8. [DOI] [PubMed] [Google Scholar]
- 145.Staubert C, Boselt I, Bohnekamp J, Rompler H, Enard W, Schoneberg T. Structural and functional evolution of the trace amine–associated receptors TAAR3, TAAR4 and TAAR5 in primates. PLoS ONE. 2010;5:e11133. doi: 10.1371/journal.pone.0011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Honeycutt H, Alberts JR. Housing pregnant mice (Mus musculus) in small groups facilitates the development of odor-based homing in offspring. J Comp Psychol. 2005;119:418–29. doi: 10.1037/0735-7036.119.4.418. [DOI] [PubMed] [Google Scholar]
- 147.Zala SM, Potts WK, Penn DJ. Scent-marking displays provide honest signals of health and infection. Behav Ecol. 2004;15:338–44. [Google Scholar]
- 148.Derbyshire D. Midsummer night love potion proves a work of fiction. Telegraph. 2002 Feb 14; http://www.telegraph.co.uk/news/uknews/1384809/Midsummer-night-love-potion-proves-a-work-of-fiction.html.
- 149.Wysocki CJ, Preti G. Facts, fallacies, fears, and frustrations with human pheromones. Anat Rec A. 2004;281:1201–11. doi: 10.1002/ar.a.20125. [DOI] [PubMed] [Google Scholar]
- 150.Stern K, McClintock MK. Regulation of ovulation by human pheromones. Nature. 1998;392:177–79. doi: 10.1038/32408. [DOI] [PubMed] [Google Scholar]
- 151.Weller L, Weller A. Human menstrual synchrony: a critical assessment. Neurosci Biobehav Rev. 1993;17:427–39. doi: 10.1016/s0149-7634(05)80118-6. [DOI] [PubMed] [Google Scholar]
- 152.Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci USA. 2003;100:3328–32. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang J, Webb DM. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc Natl Acad Sci USA. 2003;100:8337–41. doi: 10.1073/pnas.1331721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ferrero DM, Liberles SD. The secret codes of mammalian scents. Wiley Interdiscip Rev Syst Biol Med. 2010;2:23–33. doi: 10.1002/wsbm.39. [DOI] [PubMed] [Google Scholar]
RELATED RESOURCES
- 1.Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–60. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- 2.Burger BV. Mammalian semiochemicals. Top Curr Chem. 2005;240:231–78. [Google Scholar]
- 3.Chamero P, Leinders-Zufall T, Zufall F. From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci. 2012;35:597–606. doi: 10.1016/j.tins.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 5.Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev. 2009;89:921–56. doi: 10.1152/physrev.00037.2008. [DOI] [PubMed] [Google Scholar]