Abstract
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is one of the most frequently disrupted tumor suppressors in cancer. The lipid phosphatase activity of PTEN antagonizes the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway to repress tumor cell growth and survival. In the nucleus, PTEN promotes chromosome stability and DNA repair. Consequently, loss of PTEN function increases genomic instability. PTEN deficiency is caused by inherited germline mutations, somatic mutations, epigenetic and transcriptional silencing, post-translational modifications, and protein-protein interactions. Given the high frequency of PTEN deficiency across cancer subtypes, therapeutic approaches that exploit PTEN loss-of-function could provide effective treatment strategies. Herein, we discuss therapeutic strategies aimed at cancers with loss of PTEN function, and the challenges involved in treating patients afflicted with such cancers. We review preclinical and clinical findings, and highlight novel strategies under development to target PTEN-deficient cancers.
Keywords: Phosphatase, cancer, tumor, targeted therapy, tumor suppressor, PI3K, mTOR, synthetic lethal
Introduction
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is as a well-known tumor suppressor that has both phosphatase-dependent and -independent roles. It was first identified in 1997 as a phosphatase that is mutated or lost in several cancers [1, 2]. We now know that lesions in the PTEN gene, located on chromosome 10q23, occur at a significant rate in the majority of human tumor subtypes, and this locus is thought to have the highest preference for loss in humans [3].
The best-characterized tumor suppressive role of PTEN is as a lipid phosphatase that antagonizes phosphatidylinositol 3-kinase (PI3K) signaling [4]. PI3K is a critical node in a major signaling pathway that regulates cancer cell growth, survival, and metabolism (Fig. 1). When activated, PI3K phosphorylates the 3’ (D3) position on the inositol ring of phosphatidylinositol (4,5)-bisphosphate (PIP2), which is present on the inner leaflet of the plasma membrane, to produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 serves as a second messenger and binds proteins containing pleckstrin homology (PH) domains. The recruitment of PH domain-containing proteins such as AKT to the plasma membrane facilitates their activation, and triggers downstream signaling cascades. Cytoplasmic PTEN negatively regulates this pathway by dephosphorylating PIP3 at its D3 position, thereby inhibiting downstream kinase activation and preventing cancer cell growth and survival (Fig. 1 and ref. [5]). Two recent studies have found that there is a translational variant(s) long form of PTEN secreted from cell that can enter neighboring cells. Like cytoplasmic PTEN, secreted PTEN has lipid phosphatase activity and antagonizes PI3K signaling in target cells [6, 7].
Fig. 1. PTEN exhibits tumor suppressive functions in the cytoplasm and nucleus.
The phosphatidylinositol 3-kinase (PI3K) pathway regulates cancer cell growth and survival. This pathway is activated by ligand binding to receptor tyrosine kinases (RTKs) and/or G protein coupled receptors (GPCRs). PI3K is then recruited to the membrane where it phosphorylates phosphatidylinositol (4,5)-bisphosphate (PIP2) to produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3), leading to activation of several signaling cascades including AKT/mTORC1. Cytoplasmic PTEN negatively regulates this pathway by dephosphorylating PIP3 at its D3 position. Nuclear PTEN promotes chromosome stability and regulates DNA double-strand break repair. Red star indicates a potential therapeutic target for which a drug(s) is in development.
PTEN has also been reported to exhibit protein phosphatase activity. In vitro studies showed that PTEN dephosphorylates tyrosine, serine, and threonine residues on phosphopeptides [8]. PTEN interacts with and dephosphorylates focal adhesion kinase and Shc [9, 10]. The protein phosphatase activity of PTEN also reduces cyclin D1 levels, preventing cell cycle progression [11]. Using a new bioassay to measure PTEN function in living tissue, it was recently shown that PTEN auto-dephosphorylates serine and/or threonine residues in its own C-terminal region; this event(s) appears to promote its lipid phosphatase activity [12, 13]. The protein phosphatase activity of PTEN also regulates secretion of hepatitis C virus particles in liver, possibly via regulation of cholesterol metabolism [14].
While cytoplasmic PTEN is primarily involved in regulating PI3K/PIP3 signaling, nuclear PTEN exhibits phosphatase-independent tumor suppressive functions, including regulation of chromosome stability, DNA repair, and apoptosis (Fig. 1; reviewed in refs. [15, 16]). Despite the fact that PTEN lacks a canonical nuclear localization sequence, ubiquitination in its C-terminal region may promote its nuclear import [17]. Studies in PTEN-null mouse embryonic fibroblasts revealed that 1) nuclear PTEN interacts with Centromere-Specific Binding Protein (CENP-C), an essential component for centromere stability, and 2) PTEN is crucial for the induction of RAD51, which regulates DNA double-strand break repair [18]. Nuclear PTEN binds to the anaphase-promoting complex or cyclosome (APC/C), and heightens the association of APC/C with the co-activator CDC20 homologue 1 (CDH1) [19]. In so doing, PTEN increases the chromosome-stabilizing activity of the APC/C-CDH1 complex [19]. Nuclear PTEN may also promote apoptosis [15]. Human glioblastoma cells with predominantly nuclear PTEN were more likely to have condensed nuclei in response to apoptosis induction, compared to cells with primarily cytoplasmic PTEN [20]. Hence, intracellular localization plays an important role(s) in the regulation of PTEN function(s) [16]. These various phosphatase-dependent and -independent functions of PTEN contribute to tumor suppression, and highlight the complexity of strategies to therapeutically target PTEN-deficient cancers.
Mechanisms of functional loss of PTEN
Loss of PTEN function is a major determinant that affects tumor development across tissues. PTEN function and expression are modulated by germline and somatic PTEN mutations, genomic deletion, epigenetic and transcriptional silencing, post-transcriptional regulation, post-translational regulation, and protein-protein interactions [3].
Inherited germline mutations
Patients with PTEN Hamartoma Tumor Syndrome (PHTS), which is rare in the general population, have germline mutations throughout much of the PTEN coding region [21]. PHTS includes the previously named Cowden Syndrome and Bannayan-Riley-Ruvalcaba Syndrome, and may include some individuals with Proteus Syndrome, Proteus-like Syndrome, and Autism Spectrum Disorder with Macrocephaly [22]. In PHTS, exon 5 encoding the PTEN phosphatase domain accounts for approximately 40% of germline mutations [21]. Some patients with Cowden Syndrome harbor germline mutations in the PTEN promoter, or in possibly splice donor and acceptor sites [23]. All types of germline mutations found in Cowden Syndrome lead to loss of expression or activity of PTEN [24]. PHTS patients have an increased lifetime risk of developing cancer [25–27].
Somatic Mutations
Missense, nonsense, insertion, and deletion mutations occur throughout PTEN and contribute to loss of PTEN expression and/or function. Although the distribution of these mutations is mostly sporadic, several mutational hotspots have been identified at amino acids Arg130, Arg173 and Arg233 (Fig. 2 and ref. [3]). However, PTEN mutations are not limited to a specific cancer subtype. Genomic sequence data from The Cancer Genome Atlas (TCGA) shows that mutations in PTEN occur across a wide range of cancers, with uterine cancer and glioblastoma multiforme having the highest percentages of PTEN mutations and homozygous loss (Table 1).
Fig. 2. Location and frequency of somatic mutations in PTEN.
Somatic mutations in PTEN occur throughout the coding region. 47.6% of these mutations form a premature STOP codon or cause a frameshift mutation leading to a truncated PTEN protein. Data was obtained from The Cancer Genome Atlas (TCGA) via the cBio Portal for Cancer Genomics [29, 44]. Domain mapping was obtained from UniProt [203].
Table 1. Frequencies of PTEN genetic lesions across cancer subtypes.
Genomic sequence data from The Cancer Genome Atlas (TCGA) [29] was interrogated using the cBioPortal for Cancer Genomics [44]. The percentages of tumors exhibiting PTEN mutations or homozygous loss are indicated, followed in parentheses by the numbers of cases containing such lesions / total number of cases analyzed.
| Cancer Type | % of tumors with PTEN mutation or homozygous loss (# altered/total) |
Reference(s) |
|---|---|---|
| Bladder | 4.1% (4/97) | [28] |
| Lower Grade Glioma | 5.3% (9/169) | Provisional [29] |
| Breast | 7.47% (57/760) | [30–33] |
| Cervical | 13.9% (5/36) | Provisional [29] |
| Colorectal | 6.3% (14/221) | [34, 35] |
| Glioblastoma Multiforme | 41.9% (99/236) | Provisional [29, 36] |
| Head & Neck SCC | 2.6% (8/302) | Provisional [29] |
| Renal clear cell | 3.4% (10/290) | Provisional [29] |
| Renal papillary | 3% (3/100) | Provisional [29] |
| Lung Adenocarcinoma | 3.9% (5/129) | [37, 38] |
| Lung SCC | 11.2% (20/179) | [39] |
| Ovarian | 7.28% (23/316) | [40] |
| Prostate | 13.6% (14/103) | [41] |
| Sarcoma | 2.9% (6/207) | [42] |
| Melanoma | 12.4% (28/225) | Provisional [29] |
| Stomach | 11.3% (13/115) | Provisional [29] |
| Thyroid | 1.9% (6/318) | Provisional [29] |
| Uterine | 66.3% (159/240) | [43] |
Epigenetic, transcriptional, and post-transcriptional silencing
PTEN loss-of-function can also result from epigenetic and transcriptional silencing. Several studies have shown that CpG islands in the PTEN promoter are hypermethylated in cancer, leading to silencing of PTEN transcription [45]. This hypermethylation has been observed in breast, colorectal, endometrial, gastric, hematological, liver, lung, skin (melanoma), and prostate cancers, and glioma [46–55]. Transcription of PTEN can be repressed by the epigenetic repressor complex Mi-2/NuRD that contains a chromatin-remodeling ATPase and a histone deacetylase (HDAC). This repression occurs when the transcription factor Sal-Like Protein 4 (SALL4) binds to the PTEN promoter and recruits Mi-2/NuRD [56]. PTEN transcription can also be repressed by the transcription factors NF-κB, c-JUN, and BM1 [57–59].
The p53 tumor suppressive transcription factor promotes PTEN expression. The oncogenic transcription factors Inhibitor of DNA-binding 1 (ID1) and SNAIL can repress transcription of PTEN by binding to its promoter region and preventing p53 binding [53, 60]. The ubiquitous transcription factor Specificity Protein 1 (Sp1) can also inhibit PTEN expression: acetylated Sp1 binds to the PTEN promoter and recruits HDAC1 to repress PTEN transcription [61]. Accordingly, Sp1 overexpression upregulated PI3K pathway activation (assessed by AKT phosphorylation), and promoted migration and invasion of human salivary adenoid cystic cancer cells [61].
MicroRNAs (miRNAs), have been shown to repress translation of PTEN mRNA by interacting with the 3’ untranslated region (reviewed in ref. [45]). Usually such miRNAs are specific to a particular cancer subtype; however, miR-21 represses PTEN expression in many cancer subtypes and metabolic diseases [45]. Intriguingly, it was proposed that miR-21 represses PTEN expression by increasing the expression of other miRNAs known to repress PTEN [62]. The transcription factor transforming growth factor beta (TGF-β), which inhibits PTEN expression in some models, upregulates miR-21 expression [63].
Post-translational regulation
Post-translational modifications including phosphorylation, acetylation, oxidation, and ubiquitylation have been shown to cause loss of PTEN function. The phosphatase activity of PTEN can be inhibited by phosphorylation of several serine and threonine resides in its C-terminal tail [64, 65], which may be driven by the kinase CK2 [66]. While such phosphorylation stabilizes PTEN, it reduces PTEN localization to the plasma membrane, thereby limiting its interaction with PIP3 [65].
PTEN can be also inhibited by oxidation and acetylation. PTEN contains a residue characteristic of protein tyrosine phosphatases termed a catalytic cysteine nucleophile which is prone to oxidation at Cys124 [67]. Reactive oxygen species can contribute to the formation of a disulfide bond between Cys71 and Cys124 in PTEN to inhibit its catalytic activity [68, 69]. Also, peroxiredoxin 1 (PRDX1) and thioredoxin-interacting protein (TXNIP) are involved in the oxidation and subsequent inactivation of PTEN [70, 71]. p300/CREB-binding protein (CBP)-associated factor (PCAF) and CBP are thought to acetylate PTEN at Lys125–128 and Lys402, respectively, to inhibit its phosphatase activity [72].
PTEN ubiquitylation and subsequent degradation are controlled by the ubiquitin E3 ligase NEDD4 [73]. PTEN monoubiquitination at Lys13 and Lys289 promotes its nuclear localization and suppresses its phosphatase activity [17].
Protein-protein interactions
Several proteins have been shown to interact with PTEN to repress its tumor suppressive functions [3]. Parkinson Protein 7 (PARK7, DJ-1) binds PTEN under conditions of oxidative stress, and this interaction is associated with increased AKT activation and poor clinical outcome in different cancer subtypes [74]. PIP3-dependent Rac Exchange Factor 2a (P-REX2a), Shank-Interacting Protein-Like 1 (SIPL1) and α-Mannosidase 2C1 (MAN2C1) have also been shown to bind PTEN and inhibit its phosphatase activity, leading to increased activation of AKT [75–77]. These proteins make attractive therapeutic targets to reverse loss of PTEN phosphatase activity in cancer cells.
Other PTEN-binding proteins promote PTEN stability, thereby increasing the potential for antagonism of PI3K signaling. The membrane-localized proteins E-cadherin and MAGI-2, which are lost in some cancers, promote PTEN stability [78, 79]. The p85 subunit of PI3K binds PTEN to promote stability. The genes encoding p85 isoforms (PIK3R1, PIK3R2) are frequently mutated in endometrial cancer, and some mutations destabilize PTEN and promote PI3K pathway activation [80].
Interplay between PTEN and p53 has led to the suggestion that PTEN can have tumor promoting effects in cells expressing mutant p53. Nuclear PTEN binds p53 in a phosphatase-independent manner to promote p53 stabilization, thus promoting PTEN transcription [81]. PTEN complexes with p300/CBP acetyltransferase to promote p53 acetylation in response to DNA damage, and p53 acetylation enhances PTEN-p53 interaction [82]. In cells expressing wild-type p53, PTEN inhibits cell proliferation and increases apoptosis. In contrast, PTEN promotes proliferation and suppresses apoptosis in cells expressing mutant p53. PTEN knockdown in mutant p53-expressing cells decreases proliferation and tumor growth in mice [83]. Thus, p53 status should be considered when PTEN may be involved in a pathway of therapeutic interest.
Challenges in determining tumor PTEN status
Loss-of-function mutations in PTEN occur in only a fraction of PTEN-deficient tumors. As described above, PTEN expression may be lost by many non-genomic mechanisms. It is therefore necessary to determine PTEN status in tumors by both protein quantification and DNA sequencing, as neither method alone will provide comprehensive information. Ideally, tumor PTEN phosphatase activity would also be quantified, although current technologies may not permit routine clinical implementation of such an assay.
Methods to determine PTEN status by IHC were historically difficult and continue to be refined, which may contribute to conflicting results between studies. These issues are likely due to antibody and scoring variability. The most commonly used control for PTEN positivity is adjacent normal cells (i.e., vascular endothelium) within a tumor section, which should be PTEN-positive. If the PTEN IHC signal is weaker in tumor cells compared to normal cells, the tumor is usually scored as “PTEN-deficient,” and tumors with malignant cells with no IHC signal are considered “PTEN-null.” This scoring system is subjective, prone to variability, and difficult to implement into routine clinical practice. Also, there are reports of PTEN heterogeneity by IHC within tumors [84, 85], which complicates scoring and interpretation. Reverse-phase protein array (RPPA) analysis, which is analogous to quantitative high-throughput dot-blotting, of lysates from 306 breast tumors showed that PTEN levels are strongly, inversely correlated with levels of activated AKT [86]. Efforts are underway to employ RPPA in routine clinical use; this method may be useful to accurately determine PTEN protein content, and is amenable to multiplex analysis of a panel of proteins and post-translational modifications [87].
Further confounding is the fact that tumors exhibited genetic heterogeneity. Biopsies of seven regions from a primary tumor and one from a liver metastasis within a patient with renal cell carcinoma were analyzed by DNA (exome) sequencing. A PTEN splice site mutation was detected in 3/6 regions of the primary tumor, and a PTEN missense mutation was detected in a fourth region of the primary tumor; the other two regions of the primary tumor, and the metastasis, were PTEN-wild-type [88]. In another study of multiple biopsies from 134 prostate cancers, PTEN exhibited genetic heterogeneity in 6 cases (5%), whereby one or both PTEN alleles were lost in different regions of a tumor [89]. Given that PTEN is often silenced at the transcriptional level, it is possible that the remaining intact PTEN allele in hemizygous prostate tumors was transcriptionally silenced. It should be considered that different regions within a tumor may exhibit convergent phenotypic evolution (i.e., different patterns of mutations to achieve the same phenotype). Hence, determination of PTEN status may not be as important as determination of the resultant phenotype (e.g., PHTS, Tuberous Sclerosis, and Progeria exhibit similar phenotypes; described below), which may be more amenable to therapeutic intervention, and capture a larger patient population.
Therapeutically targeting loss of PTEN phosphatase activity
Loss of PTEN lipid phosphatase activity leads to PIP3 accumulation at the plasma membrane, which activates the AKT/mTOR pathway to drive cell growth, proliferation, and survival [5]. As such, therapeutics targeting several nodes of this pathway are under development.
Pre-clinical findings on treatment of cancers with loss of PTEN phosphatase activity
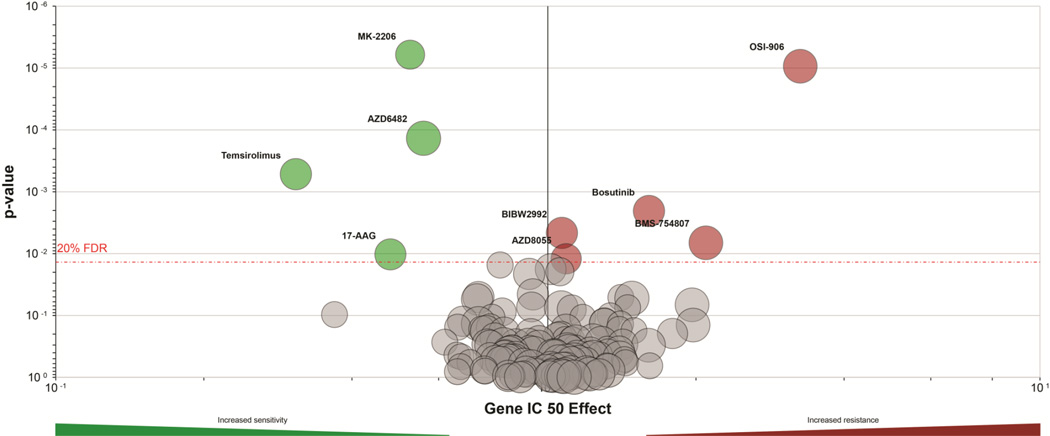
Mouse models with genetic loss of PTEN have proven valuable in the testing and development of therapies to target loss of PTEN phosphatase activity. Pten heterozygous mice (Pten+/−) exhibit several of the phenotypes displayed by patients with Cowden’s Syndrome, including increased susceptibility to endometrial and breast cancers [90]. Pten+/− mice treated with the pharmacological inhibitor of mTORC1 Temsirolimus (CCI-779) had reduced or no development of several tumor subtypes [91]. Another rapamycin analogue (Everolimus, Afinitor, RAD001) retarded the development of endometrial hyperplasia, and rapamycin decreased growth of prostate tumors in Pten+/− mice [92, 93]. In mice with prostate-specific deletion of Pten, heterozygous deletion of components of the TORC1/2 complexes (Mtor, Mlst8) increased lifespan [94]. In addition, deletion of Akt1 in prostate-specific Pten+/− mice prevented endometrial and prostate tumor growth [95]. These findings suggest that inhibitors of the AKT/mTOR pathway may be effective in preventing and controlling growth of PTEN-deficient tumors. Indeed, genetic lesions in PTEN are associated with increased sensitivity to Temsirolimus (allosteric mTORC1 inhibitor), AZD6482 (PI3K/p110β inhibitor), MK-2206 (allosteric AKT inhibitor), and 17-AAG (HSP90 chaperone inhibitor that induces degradation of many proteins including HER2 and AKT) (Fig. 3).
Fig. 3. Genetic lesions in PTEN are associated with increased sensitivity to PI3K, AKT, and mTOR inhibitors, and decreased sensitivity to RTK inhibitors.
As part of the Genomics of Drug Sensitivity in Cancer Project [204], >500 genetically characterized cell lines were profiled for sensitivity to a panel of 139 small molecules. This database (http://www.cancerrxgene.org) was queried to identify drugs with sensitivity/resistance profiles associated with PTEN genetic alterations. Data are presented as a volcano plot, where x-axis indicates magnitude of effect that PTEN alterations have on IC50 values in response to drug, y-axis indicates p-value from MANOVA of drug-PTEN interaction, size of a circle reflects number of PTEN alterations contributing to the analysis for a given drug, and red dashed line represents a Benjamini-Hochberg multiple testing correction threshold with a false discovery rate of 20%. Statistically significant PTEN-related sensitizing or resistance effects are indicated by green circles and red circles, respectively. PTEN alterations are associated with increased sensitivity to MK-2206 (AKT inhibitor), AZD6482 (PI3K/p110β inhibitor), Temsirolimus (allosteric mTORC1 inhibitor), and 17-AAG (Hsp90 inhibitor), and with increased resistance to BIBW2992 (Afatinib; EGFR/HER2 inhibitor), AZD8055 (ATP-competitive mTORC1/2 inhibitor), Bosutinib (Src and Abl inhibitor), BMS-754807 (IGF-1R/InsR inhibitor), and OSI-906 (IGF-1R/InsR inhibitor).
In addition to promoting tumorigenesis, loss of PTEN drives resistance to anti-cancer therapeutics. Knockdown of PTEN in estrogen receptor α (ER)-positive breast cancer cells conferred resistance to the anti-estrogens tamoxifen and fulvestrant, and to estrogen deprivation [4]. PTEN knockdown increased ER transcriptional activity, Insulin-like Growth Factor-1 Receptor (IGF-1R) and ErbB3 receptor tyrosine kinase activity, AKT activation, and cell proliferation [4]. Another study showed that PTEN knockdown in non-small cell lung cancer cells induced resistance to the EGFR inhibitor Gefitinib, but not to the VEGFR/EGFR dual inhibitor Vandetanib [96]. Genetic PTEN aberrations are associated with resistance to Bosutinib (BCR/Abl and Src inhibitor), BMS-754807 (IGF-1R/InsR and c-Met inhibitor), and OSI-906 (IGF-1R/InsR inhibitor) (Fig. 3). These data suggest that targeted agents acting at the level of PI3K or downstream may be most effective for treatment of PTEN-deficient cancers, while agents targeting signaling nodes upstream of PI3K will be less useful.
Most recently, PTEN loss was shown to reduce the sensitivity of melanoma cells to the γ-secretase inhibitor (GSI) RO4929097 [97]. GSIs are used to treat patients with melanoma because of their ability to prevent cleavage and activation of the Notch receptor, which is usually de-regulated in melanoma. RO4929097 was only effective at inducing senescence and apoptosis in human melanoma cell lines with wild-type PTEN. PTEN rescue in PTEN-null or -mutant melanoma cells restored sensitivity to RO4929097.
Post-translational modifications in PTEN also confer resistance to targeted therapies. PTEN phosphorylation at Tyr240, mediated by Src and Fibroblast Growth Factor Receptor (FGFR) 2 and 3 was shown to confer resistance to EGFR inhibitors in glioblastoma multiforme (GBM) in vitro and in vivo [98]. Although this modification in PTEN does not abrogate its ability to repress PI3K signaling, it occurs frequently in human GBM tumors and has been linked to shortened survival [98]. To corroborate their findings, the authors showed that a phospho-inhibiting Tyr240Phe mutation in PTEN enhanced sensitivity of xenograft-derived GBM cells to EGFR inhibitors [98].
Clinical findings on PTEN status and response to RTK/PI3K pathway-targeted therapeutics
Several drugs are currently in clinical trials for the treatment of patients with PTEN-deficient cancers (Table 2). Drug sensitivity information gleaned from preclinical studies has been partially validated in the clinic. The observation that PTEN deficiency is associated with resistance to RTK inhibitors in vitro makes mechanistic sense: if a PI3K antagonist (PTEN) is defective, then less PI3K activation from RTKs should be required to drive PIP3 signaling. However, whether PTEN loss is associated with resistance to RTK-directed therapies in cancer patients remains a matter of debate. Mature clinical data exist concerning the relationship between PTEN status and response to the anti-HER2 therapeutic antibody Trastuzumab in patients with breast cancers that overexpress the HER2 RTK protooncogene. First, we must consider the putative mechanisms of Trastuzumab anti-tumor action: Trastuzumab binds to the extracellular domain of HER2 on the cell surface, disrupts HER2-HER3 heterodimers (which activate PI3K) [99], inhibits cleavage of the HER2 extracellular domain (which removes the Trastuzumab-binding epitope from HER2) [100], and induces antibody-dependent cellular cytotoxicity [101, 102]. Several groups found that PTEN loss was generally associated with poor response to Trastuzumab therapy, whether this agent was administered in the neoadjuvant, adjuvant, or metastatic settings [103–105]. Oddly, PTEN loss was associated with improved response (assessed by pathological complete response) to neoadjuvant treatment with the EGFR/HER2 kinase inhibitor lapatinib followed by Trastuzumab [104]. Contrary to these smaller studies, results from a recent phase III trial (NCCTG N9831) with 1,201 patients treated with adjuvant chemotherapy plus Trastuzumab (sequential or concurrent) revealed that PTEN status did not have an impact on disease-free-survival [106]. Therefore, the role of PTEN status in sensitivity to HER2-directed therapy in HER2+ breast cancer remains unclear.
Table 2. Drugs under clinical development that may be useful to treat patients with PTEN-deficient cancers.
The phase of clinical development is indicated in the right column.
| Drug target | Drug | Mechanism of action | Clinical Phase | Reference(s) |
|---|---|---|---|---|
| Class IA PI3K | BKM120 | ATP-competitive | 3 | [107, 108] |
| XL-147 | ATP-competitive | 2 | [109] | |
| PX-866 | ATP-competitive | 2 | [110] | |
| GDC-0941 | ATP-competitive | 2 | [111] | |
| BAY80-6946 | ATP-competitive | 2 | [112] | |
| CH5132799 | ATP-competitive | 1 | [113, 114] | |
| GDC-0084 | ATP-competitive | 1 | ||
| ZSTK474 | ATP-competitive | 1 | [115] | |
| p110α/γ/δ/PI3K-specific | GDC-0032 | ATP-competitive | 1 | [116] |
| p110α/PI3K-specific | BYL719 | ATP-competitive | 2 | [117, 118] |
| MLN1117 | ATP-competitive | 1 | [119] | |
| p110β/PI3K-specific | GSK2636771 | ATP-competitive | 1 | [120] |
| PI3K/PLK | Rigosertib | ATP-competitive | 3 | [121] |
| PI3K/HDAC | CUDC-907 | Bifunctional | 1 | [122] |
| PI3K/mTOR | PKI-587 (PF-05212384) | ATP-competitive | 2 | [123, 124] |
| BEZ235 | ATP-competitive | 2 | [125] | |
| BGT226 | ATP-competitive | 2 | [126, 127] | |
| GDC-0980 | ATP-competitive | 2 | [128–130] | |
| XL-765 | ATP-competitive | 2 | ||
| SF1126 | ATP-competitive* | 1 | [131] | |
| LY3023414 | ATP-competitive | 1 | ||
| TORC1 | Everolimus (RAD001) | Indirect, FKBP12-mediated | Approved | [132, 133] |
| Temsirolimus (CCI-779) | Indirect, FKBP12-mediated | Approved | [134] | |
| Ridaforolimus (AP-23573) | Indirect, FKBP12-mediated | 3 | [135] | |
| TORC1/TORC2 | MLN0128 | ATP-competitive | 1/2 | [136] |
| AZD-2014 | ATP-competitive | 1 | [137] | |
| CC-223 | ATP-competitive | 1 | [138] | |
| AKT/p70S6K/PKA | AZD-5363 | ATP-competitive | 1 | [139] |
| AKT/p70S6K | LY2780301 | ATP-competitive | 1 | [140] |
| AKT | GDC-0068 | ATP-competitive | 1 | [141] |
| GSK2110183 | ATP-competitive | 1 | [142] | |
| MK-2206 | Allosteric | 2 | [143] | |
| PARP | Olaparib | Inhibits DNA repair | 2 | [144] |
| Veliparib | Inhibits DNA repair | 2 | [145] | |
| Iniparib | Inhibits DNA repair | 3 | [146] | |
| Rucaparib | Inhibits DNA repair | 2 | [147] | |
| CEP-9722 | Inhibits DNA repair | 2 | [148, 149] | |
| E7016 | Inhibits DNA repair | 2 | [150] | |
| E7449 | Inhibits DNA repair | 2 | [151] |
indicates peptide conjugate.
If PTEN loss confers resistance to Trastuzumab, adding a drug that blocks a signaling node downstream of PI3K may abrogate the effects of PTEN loss. In a phase I/II clinical study, patients with metastatic breast cancer that progressed on Trastuzumab were treated with Trastuzumab plus the TORC1 inhibitor Everolimus. Patients with PTEN-deficient tumors had decreased overall survival compared to those with PTEN-normal tumors, and progression-free survival was not affected by PTEN status [152]. In two phase I trials, patients with metastatic breast cancer that had progressed on Trastuzumab were treated with Trastuzumab plus Everolimus, with or without Vinorelbine chemotherapy. These studies did not report PTEN status of tumors, but encouraging anti-tumor activity was observed [153, 154]. These data indicate that PTEN deficiency may not sensitize tumors to TORC1 inhibition, but a fraction of patients with PTEN-deficient, HER2+, Trastuzumab-resistant breast cancer may benefit from the combination of Trastuzumab and Everolimus.
Several clinical studies are ongoing to evaluate the benefit of PI3K/AKT/mTOR pathway inhibitors in patients with advanced HER2+ breast cancer. Most trials thus far with PI3K inhibitors only include patients with tumors harboring PIK3CA mutations or PTEN deficiency. However, early data suggest that PTEN and PIK3CA status are not associated with response to the PI3K/mTOR dual inhibitor BEZ235 in patients with HER2+, metastatic, Trastuzumab-resistant breast cancer, or to the PI3K inhibitor Buparlisib (BKM120) in patients with advanced ER+ breast cancer [155, 156]. Notably, all of the aforementioned trials have been conducted with patients with advanced breast cancer. The benefit of PI3K/AKT/mTOR pathway inhibitors in early-stage breast cancer is being tested in ongoing studies [e.g., NeoPHOEBE, NCT01816594, testing neoadjuvant Trastuzumab, Buparlisib (BKM120), and paclitaxel].
EGFR is a major oncogenic driver in colorectal cancer (CRC) and squamous cell carcinomas of the head and neck (HNSCC), and patients with EGFR-expressing tumors are often treated with an EGFR antibody-based regimen (i.e., Cetuximab or Panitumumab). PTEN deficiency has been linked with Cetuximab resistance in preclinical studies in CRC [157]. In a meta-analysis of five small clinical studies, PTEN loss in tumors was associated with decreased objective response, shorter progression-free survival, and shorter overall survival in patients with primary or metastatic CRC treated with Cetuximab-based therapy [158–163]. While clinical studies suggest that PTEN deficiency is associated with poor disease outcome following EGFR antibody therapy for CRC, PTEN loss/mutation often co-exists with mutations in BRAF and KRAS, two oncogenes that play significant roles in determining drug sensitivity/resistance; so mutational overlap confounds interpretation of the effects of PTEN loss. Results from early-phase clinical studies show encouraging anti-tumor activity of the combination of an EGFR antibody and a TORC1 inhibitor (Everolimus or Temsirolimus) [164, 165]. Ongoing phase I/II studies testing combinations of EGFR antibodies and PI3K/AKT/mTOR pathway inhibitors (e.g., NCT01256385, NCT00522665, NCT01283334, NCT01816984, NCT01602315, NCT01252628, NCT01719380) will provide larger numbers of patients to assess the role of PTEN status in therapeutic response.
While PTEN deficiency has been associated with increased sensitivity to PI3K pathway inhibitors in preclinical studies in select cancer subtypes [139, 166–171], early clinical data from patients receiving single-agent therapies give mixed results. A phase I study with the PI3K inhibitor Buparlisib (BKM120) in patients with advanced solid tumors showed no association between PTEN status and clinical response [108]; these data imply that selection of patients for Buparlisib therapy based on PTEN status is not beneficial, and that some PTEN-deficient tumors will respond to Buparlisib. In contrast, PTEN deficiency may sensitize tumors to TORC1 inhibition. In a phase II study with patients with metastatic castration-resistant prostate cancer treated with Everolimus, PTEN loss (determined by FISH) was associated with improved response and longer progression-free survival [172]. Patients with advanced pancreatic neuroendocrine tumors treated with standard-of-care plus Everolimus exhibited significantly increased progression-free survival compared to placebo/standard-of-care [173]; while the association between PTEN status and clinical benefit remains to be determined, it is likely that at least a fraction of patients with PTEN-deficient pancreatic cancers benefited from Everolimus.
A body of evidence suggests that PTEN-deficient cancer cells rely on the p110β isoform of Class IA PI3K to drive phosphatidylinositol signaling [174–177]. PI3K/p110β is thought to be activated primarily by G protein-coupled receptors (GPCRs). In contrast, PTEN-wild-type cells seem to engage the p110α or p110δ isoforms, depending on tumor subtype (p110δ is thought to be more important in hematologic malignancies). As such, clinical testing of p110β-specific inhibitors in patients with PTEN-deficient cancers is ongoing (e.g., NCT01458067).
Treatment of patients with PTEN Hamartoma Tumor Syndrome (PHTS)
Germline mutations in PTEN predispose to PHTS. Such individuals exhibit macrocephaly, delayed mental development, skin lesions, vascular abnormalities, and cancer predisposition. The lifetime risks for cancer among PHTS individuals versus the general population are: female breast (85.2% vs. 12.4%); colorectal (9% vs. 5%); thyroid (35.2% vs. 1%); kidney (33.6% vs. 1.6%); endometrial (28.2% vs. 2.6%); melanoma (6% vs. 2%) [25–27]. A gain-of-function somatic mutation in AKT1 was recently discovered in patients with Proteus Syndrome [178]. Individuals with Tuberous Sclerosis carry germline mutations in TSC1 or TSC2, exhibit benign tumors, and have an elevated risk of developing subependymal giant cell astrocytoma [179]. These observations indicate that similar phenotypes can result from mutations in genes encoding proteins present at distinct signaling nodes, all of which converge to activate the AKT/mTOR pathway (Fig. 1). Such signaling is known to promote cell growth and proliferation, leading to tissue overgrowth and the formation of benign tumors. However, DNA replication promotes the acquisition of additional genetic lesions, PTEN deficiency can increase genomic instability, and tissue overgrowth promotes inflammation. These processes likely contribute to malignant transformation and cancer development.
A logical target for treatment of these syndromes is TORC1, which lies downstream of the signaling nodes known to be mutated/lost in afflicted individuals. Trials testing TORC1 inhibitors (Rapamycin, Everolimus) in patients with Tuberous Sclerosis have shown promising results and tumor shrinkage in many cases [180–185]. Given that the penetrance of PHTS in individuals with germline PTEN mutations is ~80%, preventive therapy may be beneficial prior to cancer development. This presents a challenge with the use of TORC1 inhibitors because the effects of long-term treatment are unknown, and these drugs elicit significant adverse events (e.g., mucositis, rash, ulcers, fatigue, anorexia, diarrhea, nausea, arthralgia, thrombocytopenia, and immunosuppression) that will decrease quality of life. Early clinical data indicate that short-term TORC1 inhibition is beneficial in patients with PHTS [186], and the results of recently completed phase I/II studies with TORC1 and PI3K inhibitors are pending (e.g., NCT00971789, NCT00600275, NCT00620594).
Therapeutically targeting loss of PTEN nuclear/genomic activity
Nuclear PTEN is important for the regulation of genome stability, homologous recombination, and apoptosis [15, 16]. PTEN loss or disruption of nuclear import leads to severe chromosomal alterations [16]. Several recent studies have proposed that the role of PTEN in regulation of homologous recombination can be harnessed therapeutically. PTEN deficiency incites a defect in homologous recombination in tumor cells. This defect sensitizes tumor cells to inhibitors of polyadenosine diphosphate ribose polymerase (PARP), an enzyme important for repair of DNA double-strand breaks [187]. Moreover, PTEN loss in endometrial cancer cells decreases homologous recombination and sensitizes cells to PARP inhibitors [188]. In line with these findings, a mouse model with T cell-specific deletion of Pten develops T cell receptor (Tcr)-Myc translocations caused by a defect in Tcr recombination [189]. These findings suggest that PARP inhibitors may be beneficial for the treatment of tumors with loss of PTEN nuclear/genomic activity.
Since PTEN deficiency causes a defect in homologous recombination, cells rely on PARP for repair of double-strand DNA breaks. PTEN deficiency therefore sensitizes cancer cells to PARP inhibition [188, 190, 191]. PARP inhibitors are in widespread clinical testing for a variety of cancer subtypes with deficiencies in genome integrity, mainly in patients with breast or ovarian cancers harboring mutations in BRCA1 or BRCA2. Such genetic lesions can co-occur with PTEN aberrations, confounding interpretation of the contribution of PTEN deficiency to response to PARP inhibitors. Similarly, prostate cancers exhibiting PTEN loss often harbor a genetic rearrangement leading to a TMPRSS22-ERG fusion. The TMPRSS22-ERG protein product promotes the formation of DNA double-strand breaks and interacts with PARP, thus sensitizing cells to PARP inhibition [192, 193].
Inference from early clinical results suggests that some PTEN-deficient cancers may be sensitive to PARP inhibition. In a phase I trial with the PARP inhibitor Niraparib, tumors in 7/10 patients with advanced prostate cancer exhibited clinical response [194]. Given that the majority of prostate cancers are PTEN-deficient, it is likely that a fraction of Niraparib-sensitive prostate tumors were PTEN-deficient in this study. A case report also described significant tumor response to the PARP inhibitor Olaparib in a patient with PTEN-null, BRCA1/2-wild-type, metastatic endometrial cancer [195]. Retrospective analysis of genetic alterations and PTEN status in ongoing studies with PARP inhibitors will help resolve the question of whether PTEN deficiency, in the absence of co-existing defects in DNA repair, is associated with sensitivity to PARP inhibition.
Novel strategies to develop therapeutics targeting PTEN-deficient cancers
Synthetic lethality occurs when aberrations in two genes cause cell death, but individually these aberrations do not. This approach can be used to discover PTEN synthetic lethal interactions in PTEN-deficient tumors [196]. A recent study by Ashworth and colleagues identified Nemo-Like Kinase (NLK), Polo-Like Kinase 4 (PLK4) and MonoPolar Spindle 1 (MLK) as synthetic lethal genes in PTEN-deficient cancer cells [197]. In addition, the synthetic lethal effect of NLK can be abolished by knockdown of Forkhead Box O1 (FOXO1), which suggests that FOXO1 mediates the effects of NLK.
Two recent reports describing cellular export of functional PTEN that can enter other cells and suppress PI3K/PIP3 signaling suggest that PTEN itself may be therapeutic. Putz et al. found that PTEN is exported in exosomes in a Ndfip1-dependent manner. Ndfip1 is an adaptor for Nedd4 E3 ubiquitin ligases, and the ubiquitination site Lysine-13 of PTEN is required for PTEN export [198]. Hopkins et al. reported that a translational variant gives rise to a longer PTEN protein (“PTEN-Long”) that is secreted from cells. This membrane-permeable lipid phosphatase enters other cells and antagonizes PI3K signaling [199]. It is therefore conceivable that PTEN may be delivered to cancer cells to treat patients with PTEN-deficient cancers.
Other therapeutic strategies have been proposed to target tumors with PTEN loss. It was suggested that statins may be used to increase PTEN expression since they promote the activity of the transcription factor peroxisome proliferator-activated receptor-gamma (PPARγ), known to upregulate PTEN mRNA levels [200]. Since PTEN can be silenced by methylation, demethylating agents may reverse epigenetic silencing of PTEN [24, 201]. Furthermore, it was proposed that the mRNA of the PTEN pseudogene PTENP1 can be used to sequester miRNAs that repress PTEN translation [202]. Finally, therapies that target P-REX2a and SIPL1 may be useful to derepress PTEN phosphatase activity.
Summary
The recent development of targeted anti-cancer therapeutics has been focused primarily on oncogenes and tumor promoters, most commonly in the form of kinase inhibitors. Designing anti-cancer therapeutics directed at loss of tumor suppressors has traditionally been more difficult. Even now, most of the strategies to target PTEN-deficient cancers utilize inhibitors of kinases that lie at the level of PI3K or downstream. With the relatively recent implementation of synthetic lethal screens in cancer drug target studies, novel therapeutic angles may be uncovered that will expand our thinking of PTEN deficiency beyond the PI3K pathway and genome instability. Retrospective analysis of PTEN status in tumors from patients participating in ongoing clinical trials will provide much needed support for the idea that PTEN deficiency modulates drug sensitivity and resistance.
Acknowledgments
Funding
Funding for this manuscript was supported by NCI R00CA142899.
Abbreviations
- PI3K
phosphatidylinositol 3-kinase
- RTK
receptor tyrosine kinase
- miRNA
microRNA
- PARP
polyadenosine diphosphate ribose polymerase
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PHTS
PTEN Hamartoma Tumor Syndrome
- PLK
polo-like kinase
- HDAC
histone deacetylase
- FISH
fluorescence in situ hybridization
Footnotes
Conflict of interest
T.W.M. has received research funding from Piramal Life Sciences, Ltd.
References
- 1.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 2.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 3.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 4.Miller TW, Perez-Torres M, Narasanna A, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69(10):4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TW, Rexer BN, Garrett JT, et al. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13(6):224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins BD, Fine B, Steinbach N, et al. A Secreted PTEN Phosphatase that Enters Cells to Alter Signaling and Survival. Science. 2013 doi: 10.1126/science.1234907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putz U, Howitt J, Doan A, et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5(243):ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 8.Leslie NR, Downes CP. PTEN: The down side of PI 3-kinase signalling. Cell Signal. 2002;14(4):285–295. doi: 10.1016/s0898-6568(01)00234-0. [DOI] [PubMed] [Google Scholar]
- 9.Gu J, Tamura M, Pankov R, et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146(2):389–403. doi: 10.1083/jcb.146.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura M, Gu J, Matsumoto K, et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280(5369):1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 11.Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10(6):599–604. doi: 10.1093/hmg/10.6.599. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XC, Piccini A, Myers MP, et al. Functional analysis of the protein phosphatase activity of PTEN. Biochem J. 2012;444(3):457–464. doi: 10.1042/BJ20120098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibarewal P, Zilidis G, Spinelli L, et al. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal. 2012;5(213):ra18. doi: 10.1126/scisignal.2002138. [DOI] [PubMed] [Google Scholar]
- 14.Peyrou M, Clement S, Maier C, et al. PTEN protein phosphatase activity regulates hepatitis C virus secretion through modulation of cholesterol metabolism. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121(Pt 3):249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 16.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27(41):5443–5553. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 17.Trotman LC, Wang X, Alimonti A, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128(1):141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Song MS, Carracedo A, Salmena L, et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144(2):187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil A, Andres-Pons A, Fernandez E, et al. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell. 2006;17(9):4002–4013. doi: 10.1091/mbc.E06-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27(41):5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 22.Mester J, Eng C. When Overgrowth Bumps Into Cancer: The PTEN-Opathies. Am J Med Genet C Semin Med Genet. 2013 doi: 10.1002/ajmg.c.31364. [DOI] [PubMed] [Google Scholar]
- 23.Pezzolesi MG, Li Y, Zhou XP, et al. Mutation-positive and mutation-negative patients with Cowden and Bannayan-Riley-Ruvalcaba syndromes associated with distinct 10q haplotypes. Am J Hum Genet. 2006;79(5):923–934. doi: 10.1086/508943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11(4):289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18(2):400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mester J, Eng C. When Overgrowth Bumps Into Cancer: The PTEN-Opathies. American Journal of Medical Genetics Part C-Seminars in Medical Genetics. 2013;163C(2):114–121. doi: 10.1002/ajmg.c.31364. [DOI] [PubMed] [Google Scholar]
- 27.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) Research Data (1973–2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission
- 28.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and Co-Occurrence of Actionable Genomic Alterations in High-Grade Bladder Cancer. J Clin Oncol. 2013;31(25):3133–3140. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.TCGA. The Cancer Genome Atlas ( http://cancergenome.nih.gov/)
- 30.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerji S, Cibulskis K, Rangel-Escareno C, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486(7403):405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens PJ, Tarpey PS, Davies H, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seshagiri S, Stawiski EW, Durinck S, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488(7413):660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leslie NR, Foti M. Non-genomic loss of PTEN function in cancer: not in my genes. Trends Pharmacol Sci. 2011;32(3):131–140. doi: 10.1016/j.tips.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Khan S, Kumagai T, Vora J, et al. PTEN promoter is methylated in a proportion of invasive breast cancers. Int J Cancer. 2004;112(3):407–410. doi: 10.1002/ijc.20447. [DOI] [PubMed] [Google Scholar]
- 47.Goel A, Arnold CN, Niedzwiecki D, et al. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64(9):3014–3021. doi: 10.1158/0008-5472.can-2401-2. [DOI] [PubMed] [Google Scholar]
- 48.Salvesen HB, MacDonald N, Ryan A, et al. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int J Cancer. 2001;91(1):22–26. doi: 10.1002/1097-0215(20010101)91:1<22::aid-ijc1002>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 49.Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82(3):285–291. doi: 10.1038/labinvest.3780422. [DOI] [PubMed] [Google Scholar]
- 50.Wiencke JK, Zheng S, Jelluma N, et al. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro Oncol. 2007;9(3):271–279. doi: 10.1215/15228517-2007-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahia PL, Aguiar RC, Alberta J, et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanismsin haematological malignancies. Hum Mol Genet. 1999;8(2):185–193. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Wang WL, Zhang Y, et al. Epigenetic and genetic alterations of PTEN in hepatocellular carcinoma. Hepatol Res. 2007;37(5):389–396. doi: 10.1111/j.1872-034X.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 53.Soria JC, Lee HY, Lee JI, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8(5):1178–1184. [PubMed] [Google Scholar]
- 54.Mirmohammadsadegh A, Marini A, Nambiar S, et al. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res. 2006;66(13):6546–6552. doi: 10.1158/0008-5472.CAN-06-0384. [DOI] [PubMed] [Google Scholar]
- 55.Whang YE, Wu X, Suzuki H, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci U S A. 1998;95(9):5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu J, Jeong HW, Kong N, et al. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS One. 2009;4(5):e5577. doi: 10.1371/journal.pone.0005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song LB, Li J, Liao WT, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119(12):3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hettinger K, Vikhanskaya F, Poh MK, et al. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14(2):218–229. doi: 10.1038/sj.cdd.4401946. [DOI] [PubMed] [Google Scholar]
- 59.Xia D, Srinivas H, Ahn YH, et al. Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through an NF kappa B-dependent pathway. J Biol Chem. 2007;282(6):3507–3519. doi: 10.1074/jbc.M610141200. [DOI] [PubMed] [Google Scholar]
- 60.Escriva M, Peiro S, Herranz N, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28(5):1528–1540. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kou XX, Hao T, Meng Z, et al. Acetylated Sp1 inhibits PTEN expression through binding to PTEN core promoter and recruitment of HDAC1 and promotes cancer cell migration and invasion. Carcinogenesis. 2013;34(1):58–67. doi: 10.1093/carcin/bgs336. [DOI] [PubMed] [Google Scholar]
- 62.Kato M, Putta S, Wang M, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11(7):881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis BN, Hilyard AC, Lagna G, et al. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Odriozola L, Singh G, Hoang T, et al. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem. 2007;282(32):23306–23315. doi: 10.1074/jbc.M611240200. [DOI] [PubMed] [Google Scholar]
- 65.Rahdar M, Inoue T, Meyer T, et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci U S A. 2009;106(2):480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276(2):993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 67.Ross SH, Lindsay Y, Safrany ST, et al. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19(7):1521–1530. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Lee SR, Yang KS, Kwon J, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277(23):20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 69.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118(11):3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao J, Schulte J, Knight A, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28(10):1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hui ST, Andres AM, Miller AK, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008;105(10):3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okumura K, Mendoza M, Bachoo RM, et al. PCAF modulates PTEN activity. J Biol Chem. 2006;281(36):26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Trotman LC, Koppie T, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128(1):129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim RH, Peters M, Jang Y, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7(3):263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Fine B, Hodakoski C, Koujak S, et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325(5945):1261–1265. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He L, Fan C, Kapoor A, et al. alpha-Mannosidase 2C1 attenuates PTEN function in prostate cancer cells. Nat Commun. 2011;2(307) doi: 10.1038/ncomms1309. [DOI] [PubMed] [Google Scholar]
- 77.He L, Ingram A, Rybak AP, et al. Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. J Clin Invest. 2010;120(6):2094–2108. doi: 10.1172/JCI40778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu X, Hepner K, Castelino-Prabhu S, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci U S A. 2000;97(8):4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fournier MV, Fata JE, Martin KJ, et al. Interaction of E-cadherin and PTEN regulates morphogenesis and growth arrest in human mammary epithelial cells. Cancer Res. 2009;69(10):4545–4552. doi: 10.1158/0008-5472.CAN-08-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheung LW, Hennessy BT, Li J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1(2):170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang Y, Eng C. PTEN autoregulates its expression by stabilization of p53 in a phosphatase-independent manner. Cancer Res. 2006;66(2):736–742. doi: 10.1158/0008-5472.CAN-05-1557. [DOI] [PubMed] [Google Scholar]
- 82.Li AG, Piluso LG, Cai X, et al. Mechanistic insights into maintenance of high p53 acetylation by PTEN. Molecular Cell. 2006;23(4):575–587. doi: 10.1016/j.molcel.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 83.Li YQ, Guessous F, Kwon S, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Research. 2008;68(6):1723–1731. doi: 10.1158/0008-5472.CAN-07-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garg K, Broaddus RR, Soslow RA, et al. Pathologic Scoring of PTEN Immunohistochemistry in Endometrial Carcinoma is Highly Reproducible. International Journal of Gynecological Pathology. 2012;31(1):48–56. doi: 10.1097/PGP.0b013e3182230d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gumuskaya B, Gurel B, Fedor H, et al. Assessing the order of critical alterations in prostate cancer development and progression by IHC: further evidence that PTEN loss occurs subsequent to ERG gene fusion. Prostate Cancer Prostatic Dis. 2013;16(2):209–215. doi: 10.1038/pcan.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Espina V, Liotta LA, Petricoin EF., 3rd Reverse-phase protein microarrays for theranostics and patient tailored therapy. Methods Mol Biol. 2009;520(89–105) doi: 10.1007/978-1-60327-811-9_7. [DOI] [PubMed] [Google Scholar]
- 88.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshimoto M, Ding K, Sweet JM, et al. PTEN losses exhibit heterogeneity in multifocal prostatic adenocarcinoma and are associated with higher Gleason grade. Mod Pathol. 2013;26(3):435–447. doi: 10.1038/modpathol.2012.162. [DOI] [PubMed] [Google Scholar]
- 90.Stambolic V, Tsao MS, Macpherson D, et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60(13):3605–3611. [PubMed] [Google Scholar]
- 91.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001;98(18):10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milam MR, Celestino J, Wu W, et al. Reduced progression of endometrial hyperplasia with oral mTOR inhibition in the Pten heterozygote murine model. Am J Obstet Gynecol. 2007;196(3):247 e1–247 e5. doi: 10.1016/j.ajog.2006.10.872. [DOI] [PubMed] [Google Scholar]
- 93.Blando J, Portis M, Benavides F, et al. PTEN deficiency is fully penetrant for prostate adenocarcinoma in C57BL/6 mice via mTOR-dependent growth. Am J Pathol. 2009;174(5):1869–1879. doi: 10.2353/ajpath.2009.080055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guertin DA, Stevens DM, Saitoh M, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15(2):148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen ML, Xu PZ, Peng XD, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20(12):1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takeda H, Takigawa N, Ohashi K, et al. Vandetanib is effective in EGFR-mutant lung cancer cells with PTEN deficiency. Exp Cell Res. 2013;319(4):417–423. doi: 10.1016/j.yexcr.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 97.Nair JS, Sheikh T, Ho AL, et al. PTEN regulates sensitivity of melanoma cells to RO4929097, the gamma-secretase inhibitor. Anticancer Res. 2013;33(4):1307–1316. [PubMed] [Google Scholar]
- 98.Fenton TR, Nathanson D, Ponte de Albuquerque C, et al. Resistance to EGF receptor inhibitors in glioblastoma mediated by phosphorylation of the PTEN tumor suppressor at tyrosine 240. Proc Natl Acad Sci U S A. 2012;109(35):14164–14169. doi: 10.1073/pnas.1211962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 100.Molina MA, Codony-Servat J, Albanell J, et al. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Research. 2001;61(12):4744–4749. [PubMed] [Google Scholar]
- 101.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clinical Cancer Research. 2004;10(17):5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 102.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. Journal of Clinical Oncology. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 103.Razis E, Bobos M, Kotoula V, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Research and Treatment. 2011;128(2):447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 104.Dave B, Migliaccio I, Gutierrez MC, et al. Loss of Phosphatase and Tensin Homolog or Phosphoinositol-3 Kinase Activation and Response to Trastuzumab or Lapatinib in Human Epidermal Growth Factor Receptor 2-Overexpressing Locally Advanced Breast Cancers. Journal of Clinical Oncology. 2011;29(2):166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Esteva FJ, Guo H, Zhang SY, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K Status Association with Trastuzumab Response and Survival in Patients with HER2-Positive Metastatic Breast Cancer. American Journal of Pathology. 2010;177(4):1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perez EA, Dueck AC, McCullough AE, et al. Impact of PTEN Protein Expression on Benefit From Adjuvant Trastuzumab in Early-Stage Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer in the North Central Cancer Treatment Group N9831 Trial. J Clin Oncol. 2013;31(17):2115–2122. doi: 10.1200/JCO.2012.42.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11(2):317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 108.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 109.Reynolds CP, Kang MH, Carol H, et al. Initial testing (stage 1) of the phosphatidylinositol 3 kinase inhibitor, SAR245408 (XL147) by the pediatric preclinical testing program. Pediatric Blood & Cancer. 2013;60(5):791–798. doi: 10.1002/pbc.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Molecular Cancer Therapeutics. 2004;3(7):763–772. [PubMed] [Google Scholar]
- 111.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. Journal of Medicinal Chemistry. 2008;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 112.Glauer J, Pletz N, Schon M, et al. A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo. Blood Cancer J. 2013;3(e141) doi: 10.1038/bcj.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohwada J, Ebiike H, Kawada H, et al. Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg Med Chem Lett. 2011;21(6):1767–1772. doi: 10.1016/j.bmcl.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 114.Tanaka H, Yoshida M, Tanimura H, et al. The selective class I PI3K inhibitor CH5132799 targets human cancers harboring oncogenic PIK3CA mutations. Clin Cancer Res. 2011;17(10):3272–3281. doi: 10.1158/1078-0432.CCR-10-2882. [DOI] [PubMed] [Google Scholar]
- 115.Yaguchi S, Fukui Y, Koshimizu I, et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98(8):545–556. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 116.Ndubaku CO, Heffron TP, Staben ST, et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem. 2013;56(11):4597–4610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 117.Furet P, Guagnano V, Fairhurst RA, et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett. 2013;23(13):3741–3748. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 118.Elkabets M, Vora S, Juric D, et al. mTORC1 Inhibition Is Required for Sensitivity to PI3K p110 alpha Inhibitors in PIK3CA-Mutant Breast Cancer. Science Translational Medicine. 2013;5(196) doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.So LM, Yea SS, Oak JS, et al. Selective Inhibition of Phosphoinositide 3-Kinase p110 alpha Preserves Lymphocyte Function. Journal of Biological Chemistry. 2013;288(8):5718–5731. doi: 10.1074/jbc.M112.379446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weigelt B, Warne PH, Lambros MB, et al. PI3K Pathway Dependencies in Endometrioid Endometrial Cancer Cell Lines. Clinical Cancer Research. 2013;19(13):3533–3544. doi: 10.1158/1078-0432.CCR-12-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma WW, Messersmith WA, Dy GK, et al. Phase I Study of Rigosertib, an Inhibitor of the Phosphatidylinositol 3-Kinase and Polo-like Kinase 1 Pathways, Combined with Gemcitabine in Patients with Solid Tumors and Pancreatic Cancer. Clinical Cancer Research. 2012;18(7):2048–2055. doi: 10.1158/1078-0432.CCR-11-2813. [DOI] [PubMed] [Google Scholar]
- 122.Qian CG, Lai CJ, Bao RD, et al. Cancer Network Disruption by a Single Molecule Inhibitor Targeting Both Histone Deacetylase Activity and Phosphatidylinositol 3-Kinase Signaling. Clinical Cancer Research. 2012;18(15):4104–4113. doi: 10.1158/1078-0432.CCR-12-0055. [DOI] [PubMed] [Google Scholar]
- 123.Mallon R, Feldberg LR, Lucas J, et al. Antitumor Efficacy of PKI-587, a Highly Potent Dual PI3K/mTOR Kinase Inhibitor. Clinical Cancer Research. 2011;17(10):3193–3203. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- 124.Venkatesan AM, Dehnhardt CM, Delos Santos E, et al. Bis(morpholino-1,3,5-triazine) Derivatives: Potent Adenosine 5'-Triphosphate Competitive Phosphatidylinositol-3-kinase/Mammalian Target of Rapamycin Inhibitors: Discovery of Compound 26 (PKI-587), a Highly Efficacious Dual Inhibitor. Journal of Medicinal Chemistry. 2010;53(6):2636–2645. doi: 10.1021/jm901830p. [DOI] [PubMed] [Google Scholar]
- 125.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008 doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 126.Chang KY, Tsai SY, Wu CM, et al. Novel Phosphoinositide 3-Kinase/mTOR Dual Inhibitor, NVP-BGT226, Displays Potent Growth-Inhibitory Activity against Human Head and Neck Cancer Cells In Vitro and In Vivo. Clinical Cancer Research. 2011;17(22):7116–7126. doi: 10.1158/1078-0432.CCR-11-0796. [DOI] [PubMed] [Google Scholar]
- 127.Markman B, Tabernero J, Krop I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Annals of Oncology. 2012;23(9):2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 128.Bendell JC, Wagner AJ, Dolly S, et al. A First-in-Human Phase I Study to Evaluate the Dual Pi3k/Mtor Inhibitor Gdc-0980 Administered Qd in Patients with Advanced Solid Tumors or Non-Hodgkin S Lymphoma (Nhl) Annals of Oncology. 2010;21(162–163) [Google Scholar]
- 129.Wagner AJ, Jayson GC, Gomez-Roca C, et al. A phase I study evaluating the pharmacokinetics (PK) and pharmacodynamic (PD) activity of the dual PI3K/mTor inhibitor GDC-0980 administered QW. Ejc Supplements. 2010;8(7):123–124. [Google Scholar]
- 130.Sutherlin DP, Bao L, Berry M, et al. Discovery of a Potent, Selective, and Orally Available Class I Phosphatidylinositol 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Kinase Inhibitor (GDC-0980) for the Treatment of Cancer. Journal of Medicinal Chemistry. 2011;54(21):7579–7587. doi: 10.1021/jm2009327. [DOI] [PubMed] [Google Scholar]
- 131.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Research. 2008;68(1):206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 132.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 134.Elit L. CCI-779 Wyeth. Curr Opin Investig Drugs. 2002;3(8):1249–1253. [PubMed] [Google Scholar]
- 135.Demetri GD, Chawla SP, Ray-Coquard I, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31(19):2485–2492. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 136.Gokmen-Polar Y, Liu Y, Toroni RA, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat. 2012;136(3):673–682. doi: 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- 137.Pike KG, Malagu K, Hummersone MG, et al. Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett. 2013;23(5):1212–1216. doi: 10.1016/j.bmcl.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 138.Shih KC, et al. Phase I trial of an oral TORC1/TORC2 inhibitor (CC-223) in advanced solid and hematologic cancers. J. Clin. Oncol. 2012;30(suppl) abstr 3006. [Google Scholar]
- 139.Davies BR, Greenwood H, Dudley P, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11(4):873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 140.Calvo E, et al. First-in-human phase I study of LY2780301, an oral P70S6K/AKT inhibitor, in patients with refractory solid tumors. J. Clin. Oncol. 2012;30(suppl) abstr 3005. [Google Scholar]
- 141.Lin J, Sampath D, Nannini MA, et al. Targeting Activated Akt with GDC-0068, a Novel Selective Akt Inhibitor That Is Efficacious in Multiple Tumor Models. Clinical Cancer Research. 2013;19(7):1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 142.Yoon S-S, et al. Novel AKT Inhibitor GSK2110183 Shows Favorable Safety, Pharmacokinetics, and Clinical Activity in Multiple Myeloma. Preliminary Results From a Phase I First-Time-In-Human Study American Society of Hematology Annual Meeting. 2011 Abstract 1856. [Google Scholar]
- 143.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an Allosteric Akt Inhibitor, Enhances Antitumor Efficacy by Standard Chemotherapeutic Agents or Molecular Targeted Drugs In vitro and In vivo. Molecular Cancer Therapeutics. 2010;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 144.Fong PC, Boss DS, Yap TA, et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. New England Journal of Medicine. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 145.Donawho CK, Luo Y, Luo YP, et al. ABT-888, an orallyactive poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clinical Cancer Research. 2007;13(9):2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 146.Liang HY, Tan AR. Iniparib, a PARP1 inhibitor for the potential treatment of cancer, including triple-negative breast cancer. Idrugs. 2010;13(9):646–656. [PubMed] [Google Scholar]
- 147.Plummer R, Lorigan P, Steven N, et al. A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemotherapy and Pharmacology. 2013;71(5):1191–1199. doi: 10.1007/s00280-013-2113-1. [DOI] [PubMed] [Google Scholar]
- 148.Campone M, Plummer R, Stephens P, et al. Phase I dose-escalation study to evaluate the safety, pharmacokinetics, and pharmacodynamics of CEP-9722 (a PARP1>2 inhibitor) as single-agent and in combination with temozolomide in patients with advanced solid tumors ( NCT00920595) Journal of Clinical Oncology. 2012;30(15) [Google Scholar]
- 149.Miknyoczki S, Chang H, Grobelny J, et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Molecular Cancer Therapeutics. 2007;6(8):2290–2302. doi: 10.1158/1535-7163.MCT-07-0062. [DOI] [PubMed] [Google Scholar]
- 150.Russo AL, Kwon HC, Burgan WE, et al. In vitro and In vivo Radiosensitization of Glioblastoma Cells by the Poly (ADP-Ribose) Polymerase Inhibitor E7016. Clinical Cancer Research. 2009;15(2):607–612. doi: 10.1158/1078-0432.CCR-08-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McGonigle S, et al. E7449: A novel PARP inhibitor enhances the efficacy of radiotherapy and chemotherapy and has potent single agent anticancer activity in BRCA-deficient tumors. Cancer Res. 2012;72(8 suppl.):4688. [Google Scholar]
- 152.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II Study of Trastuzumab in Combination With Everolimus (RAD001) in Patients With HER2-Overexpressing Metastatic Breast Cancer Who Progressed on Trastuzumab-Based Therapy. Journal of Clinical Oncology. 2011;29(23):3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125(2):447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 154.Andre F, Campone M, O'Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28(34):5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 155.Krop IE, Saura C, Rodon-Ahnert J, Becerra C, Britten CD, Isakoff SJ, Demanse D, Hackl W, Quadt C, Silva AP, Burris HA, Abu-Khalaf MM, Baselga J. A phase I/IB dose-escalation study of BEZ235 in combination with trastuzumab in patients with PI3-kinase or PTEN altered HER2+ metastatic breast cancer. J. Clin. Oncol. 2013;30(Suppl) Abstract 508. [Google Scholar]
- 156.Mayer IA, Balko JM, Kuba MG, Sanders ME, Yap J, Li Y, Winer E, Arteaga CL. SU2C phase Ib study of pan-PI3K inhibitor BKM120 plus aromatase inhibitor letrozole in ER+/HER2− metastatic breast cancer. Cancer Res; Presented at the San Antonio Breast Cancer Symposium, Dec. 2011; 2011. (Abstract PD09-05. [Google Scholar]
- 157.Jhawer M, Goel S, Wilson AJ, et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68(6):1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen Y, Yang J, Xu Z, et al. Phosphatase and tensin homolog expression related to cetuximab effects in colorectal cancer patients: a meta-analysis. World J Gastroenterol. 2012;18(21):2712–2718. doi: 10.3748/wjg.v18.i21.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4(10):e7287. doi: 10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Negri FV, Bozzetti C, Lagrasta CA, et al. PTEN status in advanced colorectal cancer treated with cetuximab. Br J Cancer. 2010;102(1):162–164. doi: 10.1038/sj.bjc.6605471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Loupakis F, Pollina L, Stasi I, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27(16):2622–2629. doi: 10.1200/JCO.2008.20.2796. [DOI] [PubMed] [Google Scholar]
- 162.Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97(8):1139–1145. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Razis E, Briasoulis E, Vrettou E, et al. Potential value of PTEN in predicting cetuximab response in colorectal cancer: an exploratory study. BMC Cancer. 2008;8(234) doi: 10.1186/1471-2407-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saba NF, Kono SA, Robin-Mendel J, Hurwitz SJ, Kunle-Owonikoko T, Lewis CM, Harvey D, Willemann-Rogerio J, et al. Carboplatin (C), cetuximab (Cet), and everolimus (E) in recurrent or metastatic squamous cell carcinoma of the head and neck (RMSCCHN). Results of a phase Ib study. J. Clin. Oncol. 2013;31(suppl) abstr 6083. [Google Scholar]
- 165.Shahda S, Yu M, Picus J, Bufill JA, Harb WA, Burns M, Spittler AJ, Flynn J, Zeng Y, Vance GH, Wu J, Currie CR, Loehrer PJ, Chiorean EG. Phase I study of everolimus (RAD001) with irinotecan (Iri) and cetuximab (C) in second-line metastatic colorectal cancer (mCRC): Hoosier Oncology Group GI05-102—Final report. J. Clin. Oncol. 2011;29(suppl) abstr 3587. [Google Scholar]
- 166.Spoerke JM, O'Brien C, Huw L, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18(24):6771–6783. doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- 167.Shoji K, Oda K, Kashiyama T, et al. Genotype-dependent efficacy of a dual PI3K/mTOR inhibitor, NVP-BEZ235, and an mTOR inhibitor, RAD001, in endometrial carcinomas. PLoS One. 2012;7(5):e37431. doi: 10.1371/journal.pone.0037431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meric-Bernstam F, Akcakanat A, Chen H, et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res. 2012;18(6):1777–1789. doi: 10.1158/1078-0432.CCR-11-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66(20):10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 170.Lin J, Sampath D, Nannini MA, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19(7):1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 171.Jiang X, Chen S, Asara JM, et al. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. J Biol Chem. 2010;285(20):14980–14989. doi: 10.1074/jbc.M109.085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Templeton AJ, Dutoit V, Cathomas R, et al. Phase 2 Trial of Single-agent Everolimus in Chemotherapy-naive Patients with Castration-resistant Prostate Cancer (SAKK 08/08) Eur Urol. 2013;64(1):150–158. doi: 10.1016/j.eururo.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 173.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen H, Mei L, Zhou L, et al. PTEN restoration and PIK3CB knockdown synergistically suppress glioblastoma growth in vitro and in xenografts. J Neurooncol. 2011;104(1):155–167. doi: 10.1007/s11060-010-0492-2. [DOI] [PubMed] [Google Scholar]
- 175.Kim J, Hong S, Hong S. Discovery of new aminopyrimidine-based phosphoinositide 3-kinase beta (PI3Kbeta) inhibitors with selectivity over PI3Kalpha. Bioorg Med Chem Lett. 2011;21(23):6977–6981. doi: 10.1016/j.bmcl.2011.09.118. [DOI] [PubMed] [Google Scholar]
- 176.Edgar KA, Wallin JJ, Berry M, et al. Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res. 2010;70(3):1164–1172. doi: 10.1158/0008-5472.CAN-09-2525. [DOI] [PubMed] [Google Scholar]
- 177.Ni J, Liu Q, Xie S, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer Discov. 2012;2(5):425–433. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lindhurst MJ, Sapp JC, Teer JK, et al. A Mosaic Activating Mutation in AKT1 Associated with the Proteus Syndrome. New England Journal of Medicine. 2011;365(7):611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hallett L, Foster T, Liu Z, et al. Burden of disease and unmet needs in tuberous sclerosis complex with neurological manifestations: systematic review. Curr Med Res Opin. 2011;27(8):1571–1583. doi: 10.1185/03007995.2011.586687. [DOI] [PubMed] [Google Scholar]
- 180.Davies DM, de Vries PJ, Johnson SR, et al. Sirolimus Therapy for Angiomyolipoma in Tuberous Sclerosis and Sporadic Lymphangioleiomyomatosis: A Phase 2 Trial. Clinical Cancer Research. 2011;17(12):4071–4081. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- 181.Krueger DA, Care MM, Holland K, et al. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. New England Journal of Medicine. 2010;363(19):1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 182.McCormack FX, Inoue Y, Moss J, et al. Efficacy and Safety of Sirolimus in Lymphangioleiomyomatosis. New England Journal of Medicine. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pressey JG, Wright JM, Geller JI, et al. Sirolimus Therapy for Fibromatosis and Multifocal Renal Cell Carcinoma in a Child With Tuberous Sclerosis Complex. Pediatric Blood & Cancer. 2010;54(7):1035–1037. doi: 10.1002/pbc.22401. [DOI] [PubMed] [Google Scholar]
- 184.Hofbauer GFL, Marcollo-Pini A, Corsenca A, et al. The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. British Journal of Dermatology. 2008;159(2):473–475. doi: 10.1111/j.1365-2133.2008.08677.x. [DOI] [PubMed] [Google Scholar]
- 185.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 186.Komiya T, Blumenthal GM, Ballas MS, Dechowdhury R, Manu M, Fioravanti S, Hornyak TJ, Morrow B. A pilot study of sirolimus (S) in subjects with Cowden syndrome (CS) with germ-line mutations in PTEN. J. Clin. Oncol. 2013;31(suppl) doi: 10.1634/theoncologist.2019-0514. abstr 2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1(6-7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN Deficiency in Endometrioid Endometrial Adenocarcinomas Predicts Sensitivity to PARP Inhibitors. Science Translational Medicine. 2010;2(53) doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 189.Liu X, Karnell JL, Yin B, et al. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J Clin Invest. 2010;120(7):2497–2507. doi: 10.1172/JCI42382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. Embo Molecular Medicine. 2009;1(6–7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McEllin B, Camacho CV, Mukherjee B, et al. PTEN Loss Compromises Homologous Recombination Repair in Astrocytes: Implications for Glioblastoma Therapy with Temozolomide or Poly(ADP-Ribose) Polymerase Inhibitors. Cancer Research. 2010;70(13):5457–5464. doi: 10.1158/0008-5472.CAN-09-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nature Genetics. 2009;41(5):524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brenner JC, Ateeq B, Li Y, et al. Mechanistic Rationale for Inhibition of Poly(ADP-Ribose) Polymerase in ETS Gene Fusion-Positive Prostate Cancer. Cancer Cell. 2011;19(5):664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ogilvie-Michie C, Sandhu SK, Schelman WR, Molife LR, Wilding G, Omlin AG, Kansra V, Wenham RM. Final results of the phase I trial of niraparib (MK4827), a poly(ADP)ribose polymerase (PARP) inhibitor incorporating proof of concept biomarker studies and expansion cohorts involving BRCA1/2 mutation carriers, sporadic ovarian, and castration resistant prostate cancer (CRPC) J. Clin. Oncol. 2013;31(suppl) abstr 2513. [Google Scholar]
- 195.Forster MD, Dedes KJ, Sandhu S, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nature Reviews Clinical Oncology. 2011;8(5):302–306. doi: 10.1038/nrclinonc.2011.42. [DOI] [PubMed] [Google Scholar]
- 196.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 197.Mendes-Pereira AM, Lord CJ, Ashworth A. NLK is a novel therapeutic target for PTEN deficient tumour cells. PLoS One. 2012;7(10):e47249. doi: 10.1371/journal.pone.0047249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Putz U, Howitt J, Doan A, et al. The Tumor Suppressor PTEN Is Exported in Exosomes and Has Phosphatase Activity in Recipient Cells. Sci Signal. 2012;5(243) doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 199.Hopkins BD, Fine B, Steinbach N, et al. A Secreted PTEN Phosphatase That Enters Cells to Alter Signaling and Survival. Science. 2013;341(6144):399–402. doi: 10.1126/science.1234907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Teresi RE, Shaiu CW, Chen CS, et al. Increased PTEN expression due to transcriptional activation of PPARgamma by Lovastatin and Rosiglitazone. Int J Cancer. 2006;118(10):2390–2398. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- 201.Nguyen TTP, Kim SK, Lim SC, et al. Role of PTEN promoter methylation in tamoxifen-resistant breast cancer cells. Breast Cancer Research and Treatment. 2011;130(1):73–83. doi: 10.1007/s10549-010-1304-2. [DOI] [PubMed] [Google Scholar]
- 202.Poliseno L, Salmena L, Zhang J, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.UniProt C. The Universal Protein Resource (UniProt) in 2010. Nucleic Acids Res. 2010;38(Database issue):D142–D148. doi: 10.1093/nar/gkp846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(Database issue):D955–D961. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]