Abstract
Cellular senescence is a process that results from a variety of stresses, leading to a state of irreversible growth arrest. Senescent cells accumulate during aging and have been implicated in promoting a variety of age-related diseases. Mitochondrial stress is an effective inducer of cellular senescence, but the mechanisms by which mitochondria regulate permanent cell growth arrest are largely unexplored. Here, we review some of the mitochondrial signaling pathways that participate in establishing cellular senescence. We discuss the role of mitochondrial reactive oxygen species (ROS), mitochondrial dynamics (fission and fusion), the electron transport chain (ETC), bioenergetic balance, redox state, metabolic signature, and calcium homeostasis in controlling cellular growth arrest. We emphasize that multiple mitochondrial signaling pathways, besides mitochondrial ROS, can induce cellular senescence. Together, these pathways provide a broader perspective for studying the contribution of mitochondrial stress to aging, linking mitochondrial dysfunction and aging through the process of cellular senescence.
Keywords: aging, bioenergetics, cellular senescence, electron transport chain, metabolism, mitochondria, NAD, reactive oxygen species
Introduction
Mitochondria generate reactive oxygen species (ROS) in the form of superoxides as byproducts of the inefficient transfer of electrons across the electron transport chain (ETC) (Quinlan et al., 2013). Superoxide radicals can further react to form other ROS, such as hydrogen peroxides and hydroxyl radicals. These superoxides and other ROS can damage the mitochondria and further decrease the efficiency of the mitochondrial ETC, resulting in a positive feedback loop of mitochondrial ROS generation and mitochondrial oxidative damage (Balaban et al., 2005). For decades, this accumulation of mitochondrial oxidative damage with age has been proposed to contribute to aging and age-related phenotypes (Harman, 1956). This is the basis for the free radical theory of aging. However, while several studies support the free radical theory of aging (Melov et al., 2001; Kirby et al., 2002), other reports are now showing that increased ROS production does not always shorten lifespan (Van Raamsdonk & Hekimi, 2012), and can even promote longevity (Van Raamsdonk & Hekimi, 2009; Yee et al., 2014). This suggests that steady increase in ROS generation as described by the free radical theory of aging may not be sufficient to explain the phenotypes associated with aging. Hence, other factors may contribute to the aging process.
Cellular senescence, which is a biological process that causes cells to reach a state of irreversible growth arrest (Hayflick & Moorhead, 1961), may be an important factor that contributes to the aging phenotype. Indeed, senescent cells accumulate with age (Jeyapalan et al., 2007) and are thought to promote age-related phenotypes. Elimination of senescent cells delays age-related pathologies in a mouse model of aging (Baker et al., 2011). Senescent cells can contribute to aging by accelerating loss of tissue regeneration through depletion of stem cells and progenitors cells (Campisi & D'Adda di Fagagna, 2007). They also secrete several cytokines, growth factors, and proteases, collectively termed as senescence-associated secretory phenotype (SASP) (Coppé et al., 2008). These SASP factors have multiple autocrine and paracrine activities, which are capable of altering tissue homeostasis (Krtolica et al., 2001; Coppé et al., 2008). Hence, cellular senescence is implicated in several pathological conditions associated with aging (Campisi, 2013).
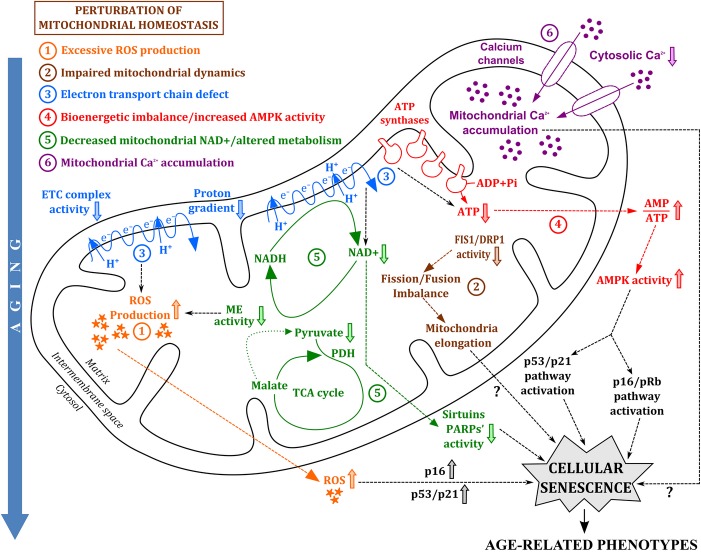
Cellular senescence is accompanied by an increase in cell size (Hayflick & Moorhead, 1961), lysosomal content (Kurz et al., 2000), and senescence-associated β-galactosidase (SA-βgal) activity (Dimri et al., 1995; Kurz et al., 2000). It is associated with decreased nuclear expression of lamin B1 (Freund et al., 2012) and release of high-mobility group box 1 (HMGB1) proteins (Davalos et al., 2013). It is often correlated with the presence of nuclear DNA damage foci (Rodier et al., 2009) and chromatin alterations (Narita et al., 2003). It is induced by multiple factors, such as repeated cell culture, telomere attrition, irradiation, oncogene activation, and oxidative damage (Hayflick & Moorhead, 1961; Campisi & D'Adda di Fagagna, 2007). It can also be caused by the perturbation of mitochondrial homeostasis (Fig. 1), which may accelerate age-related phenotypes (Sahin & DePinho, 2010, 2012). While several studies already show that mitochondrial defects can promote cellular senescence (Passos et al., 2007; Moiseeva et al., 2009; Velarde et al., 2012), the mechanisms involved in this regulation are poorly understood. Because mitochondria can generate ROS (Quinlan et al., 2013), it is proposed that excessive mitochondrial ROS is important to establish cellular senescence. This has been an attractive model because of its consistency with the free radical theory of aging. However, other mitochondrial factors may be equally or even more important to induce cellular senescence.
Figure 1.
Perturbation of mitochondrial homeostasis promotes the establishment and maintenance of cellular senescence during aging. Mitochondria are damaged over time leading to perturbation of mitochondrial homeostasis. Loss of proper mitochondrial homeostasis can promote cellular senescence through (1) excessive ROS production (orange), (2) impaired mitochondrial dynamics (brown), (3) electron transport chain defect (blue), (4) bioenergetics imbalance and increased AMPK activity (red), (5) decreased mitochondrial NAD+ and altered metabolism (green), and (6) mitochondrial calcium accumulation (purple). These mitochondrial signals trigger p53/p21 and/or p16/pRb pathways and ultimately lead to cellular senescence, which subsequently promotes age-related phenotypes, such as loss of tissue regeneration and function.
In this review, we summarize the mechanisms involved in the contribution of mitochondria to senescence. In addition to mitochondrial-derived ROS production, we also discuss the role of other mitochondrial effectors, such as impaired mitochondrial dynamics, defective ETC, imbalanced bioenergetics, altered redox state, altered metabolism, and dysregulated calcium homeostasis, in establishing permanent growth arrest (Fig. 1).
Mitochondrial free radical theory of aging and cellular senescence
The free radical theory of aging has been adapted to the study of cellular senescence. Many studies show that ROS can induce cellular senescence. Indeed, hydrogen peroxide (H2O2), which is considered as the major ROS within the cell, is a potent inducer of cellular senescence in many cell types (Ben-Porath & Weinberg, 2005). While exogenous treatment with H2O2 can promote cellular senescence, endogenous ROS (such as superoxides and hydroxyl radicals) is also implicated in the establishment and maintenance of the irreversible growth arrest. Excessive production of ROS is associated with the implementation of replicative senescence, oncogene-induced senescence, and p16INK4A-induced senescence (Colavitti & Finkel, 2005; Passos et al., 2007, 2010; Lu & Finkel, 2008; Moiseeva et al., 2009; Imai et al., 2014). A positive feedback loop of mitochondrial damage, ROS production, and DNA damage response by the activation of p53/p21CIP1/WAF1 pathway is required for the establishment of the growth arrest phenotype during cellular senescence (Macip et al., 2002; Passos et al., 2010; Luo et al., 2011). The steady increase in ROS production by this positive feedback loop is shown to replenish short-lived DNA damage foci and maintain an ongoing DNA damage response, which are thought to be both necessary and sufficient to establish and maintain cell cycle arrest during the early development of the senescence phenotype (Passos et al., 2010). However, this loop is no longer required to maintain the growth arrest phenotype at time points later than 9 days after initiation of senescence, suggesting that ROS production is dispensable once the senescent phenotype is fully established.
Aside from the p53/p21CIP1/WAF1 pathway, the p16INK4A/Rb pathway can also promote a ROS-dependent positive feedback loop, which reinforces the irreversible cell cycle arrest in senescent cells, partly through the downregulation of large tumor suppressor kinase 1 (LATS1), a kinase required for cytokinesis (Takahashi et al., 2006). Moreover, decreasing ROS levels by treatment with the mitochondria-targeted antioxidant MitoQ delays replicative senescence (Saretzki et al., 2003).
While several studies implicate the role of ROS during cellular senescence, others also suggest that mitochondrial ROS generation may not necessarily be the primary cause of cellular senescence. One study using an empirical mathematical model (stochastic step model of replicative senescence) suggests that increased mitochondrial ROS production in replicative senescent cells is a consequence of the senescence phenotype rather than the reverse (Lawless et al., 2012). Another report shows that over-expression of the mitochondrial localized antioxidant superoxide dismutase 2 (SOD2) and the mitochondrial targeted catalase are not sufficient to inhibit the senescence phenotype in hyperoxia-induced senescent cells (Klimova et al., 2009). Because ROS are produced by mitochondrial and nonmitochondrial enzymes during hyperoxia (70% O2), the inability of mitochondrial antioxidants to reverse growth arrest in hyperoxia-induced senescence suggests that cytosolic ROS may be sufficient to induce growth arrest. Hence, the mechanisms involved in linking mitochondrial ROS and cellular senescence still need to be further studied.
Because mitochondria influence many cellular processes, accumulation of mitochondrial oxidative damage, as proposed in the free radical theory of aging, may be an oversimplification of the signaling mechanisms involved in the establishment of cellular senescence. It is also possible that mitochondrial ROS can act as signaling molecules to trigger cellular senescence, independent of mitochondrial oxidative damage, although this hypothesis still needs to be proven. It is then necessary to go beyond the free radical theory and examine other mitochondrial effectors that may be involved in the irreversible cell growth arrest.
Mitochondrial dynamics and cellular senescence
Mitochondria are known to be highly dynamic organelles. They are able to divide and combine through the process of fission and fusion, allowing them to adjust their size, shape, and organization inside the cell (Chan, 2006). Mitochondrial dynamics are regulated during cell division, apoptosis, autophagy, mitochondrial biogenesis, and mtDNA integrity maintenance (Detmer & Chan, 2007) and are implicated in aging (Seo et al., 2010). In mammalian cells, dynamin 1-like (DNM1L or DRP1) and fission 1 (FIS1) are involved in the fission process, while optic atrophy 1 (OPA1) and mitofusin 1 & 2 (MFN1 and MFN2) participate in the fusion process.
Altering mitochondrial dynamics can cause mitochondrial defects (Detmer & Chan, 2007; Seo et al., 2010), and in some cases, the implementation of cellular senescence (Jendrach et al., 2005; Yoon et al., 2006; Lee et al., 2007; Mai et al., 2010; Park et al., 2010; Hara et al., 2013). Maintenance of elongated mitochondria by blocking the fission process through FIS1 depletion leads to the establishment of senescence (Lee et al., 2007). Depletion of membrane-associated ring finger C3HC4 5 (MARCH5), a mitochondrial E3 ubiquitin ligase, which blocks DRP1 activity and elongates mitochondria, also induces senescence (Park et al., 2010). These data suggest that senescent cells are typically associated with an overall shift toward more fusion events, resulting in the presence of abnormally enlarged mitochondria.
While studies correlate elongated mitochondria with the establishment of cellular senescence, it is still unclear how mitochondrial fusion contributes to the permanent cell growth arrest phenotype or whether mitochondrial fusion is merely a response to cellular stress. Some studies show that prolonged elongated mitochondria result in higher production of intracellular ROS and lower activity of mitochondrial respiration, which then ultimately leads to cellular senescence (Yoon et al., 2006). However, others suggest that mitochondrial fusion may protect a cell from excessive mitochondrial stress by maintaining a functional population of mitochondria within a cell (Chen et al., 2005). Mitochondrial fusion, in response to cellular stress, allows mitochondria to possess more cristae, stimulate more ATP synthase activity, maintain ATP production, and escape autophagic degradation (Gomes et al., 2011). Moreover, increasing mitochondrial fusion also prevents mitochondrial membrane depolarization, inhibits cytochrome c release, and promotes resistance to apoptosis (Frank et al., 2001; Karbowski et al., 2002; Beckenridge et al., 2003; Brooks et al., 2009). Hence, mitochondrial fusion can also provide a way for defective mitochondria to restore their essential components and regain their cellular function. Whether this pro-survival response after mitochondrial stress predisposes cells to senesce instead of apoptose remains to be determined.
There are still many remaining questions regarding the role of mitochondrial dynamics and cellular senescence. It is still unknown whether changes in mitochondrial morphology play a significant role in establishing senescence or whether these changes are merely a consequence of the process. Nonetheless, current data do suggest that changes in mitochondrial dynamics can promote cellular senescence (Fig. 1). Elucidating the consequences of prolonged elongated mitochondria on cell signaling and cellular function may help determine the mechanisms involved in cell growth arrest following mitochondrial stress.
Mitochondrial electron transport chain and cellular senescence
In addition to altered mitochondrial dynamics, damage to the mitochondrial ETC is also a form of mitochondrial stress, shown to cause cellular senescence (Fig. 1). Indeed, pharmacological inhibition and genetic loss of function of the ETC can lead to premature senescence. Inhibition of complex I by rotenone or of complex II by 2-thenoyltrifluoroacetone (TFFA) induces cellular senescence (Yoon et al., 2003; Moiseeva et al., 2009). Similarly, knockdown of the mitochondrial Rieske iron-sulfur polypeptide (RISP), which is involved in the transport of electrons to complex III, also drives senescence in human fibroblasts (Moiseeva et al., 2009). Inhibition of mitochondrial complex III by antimycin A also promotes a cell proliferation arrest and premature senescence, as evident by upregulation of the cyclin-dependent kinase inhibitors p16INK4A and p21CIP1/WAF1 (Stöckl et al., 2006).
The Mitochondrial ETC requires a proton gradient across the mitochondrial membrane to function (Saraste, 1999). Hence, mitochondrial depolarization stalls the mitochondrial ETC and promotes mitochondrial defect. Interestingly, loss of this proton gradient by uncouplers such as carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) also induces cellular senescence in human fibroblasts (Stöckl et al., 2007), further supporting the consequence of defective mitochondrial ETC to establish cellular senescence.
While several evidences show that inhibition of the ETC leads to cellular senescence, the specific signaling pathway linking the ETC defect and growth arrest is still unclear. One speculation is that impaired ETC can increase ROS production and promote mitochondrial damage, which then results in cellular senescence. However, this hypothesis still needs to be critically tested.
Studies suggest that there is an age-dependent decrease in the ETC. In vivo, such as those in flies (McCarroll et al., 2004; Ferguson et al., 2005), worms (McCarroll et al., 2004), and monkeys (Kayo et al., 2001), commonly through a downregulation of genes involved in the ETC function. Agents such as mitochondrial oxidative damage, mitochondrial DNA mutation, and environmental factors can all damage the ETC (Li et al., 1995; Vermulst et al., 2008; Krutmann & Schroeder, 2009), but which of these factors is the primary driver of ETC defects in vivo remains debatable. Further research needs to be performed to identify the specific pathway most relevant during the aging process.
Mitochondrial bioenergetic balance and cellular senescence
The mitochondrial ETC produces ATP as an important source of cellular energy during aerobic respiration. Defects in the ETC lead to a drop in ATP production and can result in the induction of cellular senescence (Fig. 1). Indeed, inhibition of ATP synthesis triggers senescence, as observed by upregulation of p16INK4A and p21CIP1/WAF1 expression (Stöckl et al., 2006). Decrease in ATP production can also increase AMP (or ADP) to ATP ratio, creating a bioenergetic imbalance within the cell. Interestingly, some reports do show an increased AMP to ATP ratio during cellular senescence (Wang et al., 2003; Zwerschke et al., 2003). Elevation of AMP to ATP ratios by depleting ATP levels or by addition of exogenous AMP promotes cellular growth arrest and senescence features (Zwerschke et al., 2003).
Increased AMP (or ADP) to ATP ratios stimulate AMP-activated protein kinase (AMPK), which is known to be a central mediator of cellular metabolism in eukaryotes (Mihaylova & Shaw, 2011). AMPK activation induces cell cycle arrest in many cells, including mouse embryonic cells (MEFs), human fibroblasts, human cancer cells, and fly eye cells (Jones et al., 2005; Rattan et al., 2005; Owusu-Ansah et al., 2008; Humbert et al., 2010; Mandal et al., 2010; Hou et al., 2011; Peyton et al., 2012). Multiple distinct AMPK-related mechanisms have been described in establishing and maintaining cellular senescence (Fig. 1). One mechanism involves an AMPK-dependent pathway and the other an AMPK-related protein kinase 5 (ARK5 or NUAK1)-dependent pathway. Persistent activation of AMPK increases p53 expression and phosphorylation, upregulates p21CIP1/WAF1 and p27 expression (Peyton et al., 2012), and promotes a p53-dependent senescence (Jones et al., 2005; Jiang et al., 2013). Activated AMPK also induces cell cycle arrest by downregulating pro-proliferation genes, such as cyclin A, cyclin B1, and cyclin E (Wang et al., 2002, 2003; Mandal et al., 2010; Peyton et al., 2012). AMPK also inhibits the RNA-stabilizing factor human antigen R (HuR), which destabilizes p16INK4A, leading to increased p16INK4A expression and ultimately to senescence (Wang et al., 2002, 2003). AMPK activation reduces retinoblastoma protein phosphorylation (Peyton et al., 2012), leading to the inhibition of cell proliferation. Furthermore, activation of the AMPK-related protein ARK5 promotes senescence either through a p53/p21CIP1/WAF1-dependent pathway (Hou et al., 2011) or through a p53-independent LATS1-dependent pathway (Humbert et al., 2010).
AMPK activity is highly increased in oncogene-induced senescent cells (Moiseeva et al., 2009). In contrast, inactivation of the AMPK pathway is known to promote cancer (Bardeesy et al., 2002; Huang et al., 2008b; Shackelford & Shaw, 2009; Zhou et al., 2009), further supporting the role of AMPK in establishing growth arrest and tumor suppression. Hence, studies emphasizing the impact of mitochondrial bioenergetic balance and subsequent AMPK activation may provide insights into the mechanisms involved in establishing cellular senescence and their contribution to aging and age-related phenotypes.
Mitochondrial metabolites and cellular senescence
Protein complexes in the mitochondrial ETC produce important cofactors and metabolites necessary for cellular function. Complex I of the ETC oxidizes the reduced form of nicotinamide adenine dinucleotide (NADH) into NAD+, which is a cofactor of many intracellular enzymes. Interestingly, depletion of NAD+ is implicated in cellular senescence (Fig. 1). Indeed, impaired NAD+ salvage pathway induces premature senescence, while activation of the NAD+ salvage pathway extends cellular replicative lifespan (van der Veer et al., 2007; Borradaile & Pickering, 2009; Ho et al., 2009). Furthermore, depletion of cytosolic malate dehydrogenase (MDH1), a key component in the malate–aspartate shuttle, which transfers reducing equivalents of NADH across the inner mitochondrial membrane, results in a decrease in NAD+/NADH ratio, AMPK activation, and cellular senescence (Lee et al., 2012). Increased NAD+/NADH ratios seem to limit oxidative stress by enhancing aerobic glycolysis, which supports proliferation while limiting ROS production (Borradaile & Pickering, 2009).
NAD+ is required for many enzymatic reactions, such as those involved in glycolysis, the tricarboxylic acid (TCA) cycle, DNA repair, and protein acetylation. For example, NAD+ is essential for the activities of poly-ADP ribose polymerases (PARPs), which are important for DNA repair and for the activities of sirtuins, which constitute a class of protein deacetylases implicated in aging and longevity (Longo & Kennedy, 2006; Haigis & Sinclair, 2010). PARPs and sirtuins are known to play roles in cellular senescence. PARPs prevent cellular senescence by promoting repair of DNA strand breaks in response to genotoxic stress (Efimova et al., 2010). Sirtuin 1 antagonizes senescence by deacetylating p53 in MEFs (Langley et al., 2002), activating ERK/S6K1 signaling pathway in human fibroblasts (Huang et al., 2008a), and preventing LKB1 dependent-AMPK activation in porcine endothelial cells (Zu et al., 2010). Another NAD+-dependent protein implicated in senescence is malic enzyme, which converts malate into pyruvate. Depletion of the mitochondrial NAD(P)+-dependent malic enzyme (ME2) triggers p53-dependent senescence by increasing ROS level and activating AMPK (Jiang et al., 2013). Taken together, these data indicate that mitochondrial depletion of NAD+ levels and subsequent decreased activity of NAD+-dependent enzymes can ultimately lead to cellular senescence.
Besides NAD+, other metabolites are also produced in the mitochondria. Several metabolic intermediates of the TCA cycle can influence cellular function and potentially contribute to aging and age-related phenotypes (Salminen et al., 2014). Senescent cells are associated with altered metabolism, such as decreased aerobic glycolysis, increased alanine production, decreased overall ribonucleotide triphosphate content (Wang et al., 2003; Zwerschke et al., 2003), reduced lipid synthesis, and enhanced fatty acid oxidation (Quijano et al., 2012), but whether these changes are causes or consequences of cellular senescence remains unclear. One study suggests that increasing pyruvate consumption and cellular respiration by overexpression of the mitochondrial enzyme pyruvate dehydrogenase (PDH) enhances BRAFV600E oncogene-induced senescence (Kaplon et al., 2013). Another study reveals that senescence-associated telomere dysfunction is sufficient to perturb mitochondrial function, through a p53-dependent downregulation of the mitochondrial master regulators such as peroxisome proliferator-activated receptor gamma, coactivator one alpha and beta (Sahin et al., 2011).
While several reports implicate the role of mitochondrial metabolites in establishing senescence, the particular mechanism on how these cofactors can promote cell cycle arrest remains elusive. Because studies regarding the role of the different metabolites on cellular senescence are limited, understanding the metabolic signature of senescent cells and whether this altered metabolic profile contributes to permanent cell cycle arrest are important avenues to explore in future.
Mitochondrial calcium homeostasis and cellular senescence
As mentioned above, mitochondria require a proton gradient across their membrane for the ETC to function. Disrupting this mitochondrial membrane potential can result in decreased ATP production and cellular senescence. Mitochondria import calcium to maintain ETC function and intracellular calcium homeostasis (Gunter et al., 2004), but as a consequence of increased uptake, this also depolarizes the mitochondria, decreases overall ATP production (Nguyen & Jafri, 2005), elevates cytosolic NADH, and reduces sirtuin activity (Marcu et al., 2014), which can all lead to cellular senescence (Fig. 1). Indeed, accumulation of mitochondrial calcium is implicated in oncogene-induced senescence and replicative senescence (Bolinches-Amorós et al., 2014; Wiel et al., 2014, 2014). Consistently, loss of functional calcium channels, such as mitochondrial calcium uniporter (MCU), prevents calcium uptake by the mitochondria, resulting in the escape from oncogene-induced senescence (Wiel et al., 2014).
While the accumulation of calcium in the mitochondria disrupts mitochondrial membrane potential, mitochondria do maintain low levels of calcium and act as intracellular calcium reservoirs (Butow & Avadhani, 2004). In response to stress, mitochondria can release these calcium ions and trigger a retrograde response, which signals to the nucleus and activates specific nuclear transcription factors (Butow & Avadhani, 2004). One of these transcription factors, cAMP-responsive element binding protein 1 (CREB), upregulates p21CIP1/WAF1 expression and acts as a potent inhibitor of cell proliferation (Arnould et al., 2002). While the retrograde response is an attractive mechanism for mitochondrial stress-induced senescence, this hypothesis needs to be further confirmed. Understanding the contribution of calcium homeostasis to permanent cell cycle arrest may potentially offer new perspectives in linking mitochondrial stress and cellular senescence.
Conclusion
In this review, we report the different mechanisms by which mitochondria contribute to the implementation of cellular senescence. We propose a model where the mitochondrion acts as a key player in promoting and establishing permanent growth arrest. We suggest that perturbation of mitochondrial homeostasis triggers cellular senescence, which can ultimately lead to age-associated pathologies (Fig. 1). Multiple mitochondrial factors, such as excessive mitochondrial ROS production, aberrant mitochondrial dynamics, defective electron transport chain, imbalanced bioenergetics, activated AMPK, decreased NAD+ levels, altered metabolism, and dysregulated mitochondrial calcium homeostasis, contribute to the establishment of irreversible growth arrest (Fig. 1). All of these different mitochondrial signaling pathways can regulate each other, but how these factors cooperate to promote cellular senescence, and whether these pathways are conserved in all senescent cells still remains unclear. Nonetheless, studying these different factors can provide new insights into the mechanisms involved in mitochondrial dysfunction-associated senescence. Because both mitochondrial defects and cellular senescence accumulate with age, linking the pathways involved in these two phenomena may help us understand the biology of aging, providing new potential targets to treat age-related diseases.
Acknowledgments
We thank Pierre-Yves Desprez for critically reading the manuscript and Fabien Mongelard for insightful discussions.
Funding
Part of this work was supported by National Institute of Health, K99-AG041221 (Velarde).
Conflict of interest
None declared.
References
- Arnould T, Vankoningsloo S, Renard P, Houbion A, Ninane N, Demazy C, Remacle J, Raes M. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002;21:53–63. doi: 10.1093/emboj/21.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, Van de Sluis B, Kirkland JL, Van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Beckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Bolinches-Amorós A, Mollá B, Pla-Martín D, Palau F, González-Cabo P. Mitochondrial dysfunction induced by frataxin deficiency is associated with cellular senescence and abnormal calcium metabolism. Front. Cell Neurosci. 2014;8:124. doi: 10.3389/fncel.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol. Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, D'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Develop. Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- Coppé J-P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez P-Y, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe J-P, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. PNAS. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE, Chakraborty C, Andrade JCB, Crawley C, Sutton HG, Kron SJ, Weichselbaum RR. Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res. 2010;70:6277–6282. doi: 10.1158/0008-5472.CAN-09-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 2005;390(Pt 2):501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Freund A, Laberge R-M, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kamiya N, Odaka M, Morikawa T, Kaneko Y, Nakayama K, Kuwano K. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;305:L737–L746. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Ho C, Van der Veer E, Akawi O, Pickering JG. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 2009;583:3081–3085. doi: 10.1016/j.febslet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Hou X, Liu J-E, Liu W, Liu C-Y, Liu Z-Y, Sun Z-Y. A new role of NUAK1: directly phosphorylating p53 and regulating cell proliferation. Oncogene. 2011;30:2933–2942. doi: 10.1038/onc.2011.19. [DOI] [PubMed] [Google Scholar]
- Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, Zhang Z, Tong T. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS ONE. 2008a;3:e1710. doi: 10.1371/journal.pone.0001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, Mcburnie W, Fleming S, Alessi DR. Important role of the LKB1–AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 2008b;412:211. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- Humbert N, Navaratnam N, Augert A, Da Costa M, Martien S, Wang J, Martinez D, Abbadie C, Carling D, De Launoit Y, Gil J, Bernard D. Regulation of ploidy and senescence by the AMPK-related kinase NUAK1. EMBO J. 2010;29:376–386. doi: 10.1038/emboj.2009.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Takahashi A, Hanyu A, Hori S, Sato S, Naka K, Hirao A, Ohtani N, Hara E. Crosstalk between the Rb pathway and AKT signaling forms a quiescence-senescence switch. Cell Rep. 2014;7:194–207. doi: 10.1016/j.celrep.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Jendrach M, Pohl S, Vöth M, Kowald A, Hammerstein P, Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech. Ageing Dev. 2005;126:813–821. doi: 10.1016/j.mad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–693. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, Van der Burg SH, Verdegaal EME, Cascante M, Shlomi T, Gottlieb E, Peeper DS. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc. Natl Acad. Sci. USA. 2001;98:5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc. Natl Acad. Sci. USA. 2002;99:16162–16167. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimova TA, Bell EL, Shroff EH, Weinberg FD, Snyder CM, Dimri GP, Schumacker PT, Budinger GRS, Chandel NS. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J. 2009;23:783–794. doi: 10.1096/fj.08-114256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez P-Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. PNAS. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutmann J, Schroeder P. Role of mitochondria in photoaging of human skin: the defective powerhouse model. J. Investig. Dermatol. Symp. Proc. 2009;14:44–49. doi: 10.1038/jidsymp.2009.1. [DOI] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000;113(Pt 20):3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- Langley E, Pearson M, Faretta M, Bauer U-M, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless C, Jurk D, Gillespie CS, Shanley D, Saretzki G, Von Zglinicki T, Passos JF. A stochastic step model of replicative senescence explains ROS production rate in ageing cell populations. PLoS ONE. 2012;7:e32117. doi: 10.1371/journal.pone.0032117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J. Biol. Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Lee SM, Dho SH, Ju SK, Maeng JS, Kim JY, Kwon KS. Cytosolic malate dehydrogenase regulates senescence in human fibroblasts. Biogerontology. 2012;13:525–536. doi: 10.1007/s10522-012-9397-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Lu T, Finkel T. Free radicals and senescence. Exp. Cell Res. 2008;314:1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zou P, Zou J, Wang J, Zhou D, Liu L. Autophagy regulates ROS-induced cellular senescence via p21 in a p38 MAPKα dependent manner. Exp. Gerontol. 2011;46:860–867. doi: 10.1016/j.exger.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macip S, Igarashi M, Fang L, Chen A, Pan Z-Q, Lee SW, Aaronson SA. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 2002;21:2180–2188. doi: 10.1093/emboj/21.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 2010;123:917–926. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- Mandal S, Freije WA, Guptan P, Banerjee U. Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J. Cell Biol. 2010;188:473–479. doi: 10.1083/jcb.200912024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu R, Wiczer BM, Neeley CK, Hawkins BJ. Mitochondrial matrix Ca2+ accumulation regulates cytosolic NAD+/NADH metabolism, protein acetylation and sirtuins expression. Mol. Cell. Biol. 2014;34:2890–2902. doi: 10.1128/MCB.00068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN, Kenyon C, Bargmann CI, Li H. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat. Genet. 2004;36:197–204. doi: 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O, Bourdeau V, Roux A, Deschenes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol. 2009;29:4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Nguyen M-HT, Jafri MS. Mitochondrial calcium signaling and energy metabolism. Ann. N. Y. Acad. Sci. 2005;1047:127–137. doi: 10.1196/annals.1341.012. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J. Cell Sci. 2010;123:619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TBL, Von Zglinicki T. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TBL, Von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton KJ, Liu X, Yu Y, Yates B, Durante W. Activation of AMP-activated protein kinase inhibits the proliferation of human endothelial cells. J. Pharmacol. Exp. Ther. 2012;342:827–834. doi: 10.1124/jpet.112.194712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano C, Cao L, Fergusson MM, Romero H, Liu J, Gutkind S, Rovira II, Mohney RP, Karoly ED, Finkel T. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11:1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox. Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan R, Giri S, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J. Biol. Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppe J-P, Patil CK, Hoeijmakers WAM, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Hiltunen M, Kauppinen A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: Novel insights into mitochondrial regulation of aging process. Cell. Signal. 2014;26:1598–1603. doi: 10.1016/j.cellsig.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Saraste M. Oxidative phosphorylation at the fin de siècle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Murphy MP, Von Zglinicki T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell. 2003;2:141–143. doi: 10.1046/j.1474-9728.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Seo AY, Joseph A-M, Dutta D, Hwang JCY, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J. Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckl P, Hütter E, Zwerschke W, Jansen-Dürr P. Sustained inhibition of oxidative phosphorylation impairs cell proliferation and induces premature senescence in human fibroblasts. Exp. Gerontol. 2006;41:674–682. doi: 10.1016/j.exger.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Stöckl P, Zankl C, Hütter E, Unterluggauer H, Laun P, Heeren G, Bogengruber E, Herndler-Brandstetter D, Breitenbach M, Jansen-Dürr P. Partial uncoupling of oxidative phosphorylation induces premature senescence in human fibroblasts and yeast mother cells. Free Radic. Biol. Med. 2007;43:947–958. doi: 10.1016/j.freeradbiomed.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc. Natl Acad. Sci. USA. 2012;109:5785–5790. doi: 10.1073/pnas.1116158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Flynn JM, Day NU, Melov S, Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY) 2012;4:3–12. doi: 10.18632/aging.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat. Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Wang W, Fan J, Yang X, Fürer-Galban S, de Silanes IL, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol. Cell. Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang X, de Silanes IL, Carling D, Gorospe M. Increased AMP: ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J. Biol. Chem. 2003;278:27016–27023. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- Wiel C, Lallet-Daher H, Gitenay D, Gras B, Le Calvé B, Augert A, Ferrand M, Prevarskaya N, Simonnet H, Vindrieux D, Bernard D. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat. Commun. 2014;5:3792. doi: 10.1038/ncomms4792. [DOI] [PubMed] [Google Scholar]
- Yee C, Yang W, Hekimi S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell. 2014;157:897–909. doi: 10.1016/j.cell.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Byun HO, Cho H, Kim B-K, Yoon G. Complex II defect via down-regulation of iron-sulfur subunit induces mitochondrial dysfunction and cell cycle delay in iron chelation-induced senescence-associated growth arrest. J. Biol. Chem. 2003;278:51577–51586. doi: 10.1074/jbc.M308489200. [DOI] [PubMed] [Google Scholar]
- Yoon Y-S, Yoon D-S, Lim IK, Yoon S-H, Chung H-Y, Rojo M, Malka F, Jou M-J, Martinou J-C, Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J. Cell. Physiol. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- Zhou J, Huang W, Tao R, Ibaragi S, Lan F, Ido Y, Wu X, Alekseyev YO, Lenburg ME, Hu GF. Luo Z. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene. 2009;28:1993–2002. doi: 10.1038/onc.2009.63. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Y, Liu L, Lee MYK, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Stockl P, Hutter E, Eigenbrodt E, Jansen-Durr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 2003;376:403–411. doi: 10.1042/BJ20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]