Abstract
Objective
To evaluate whether Nrf2-deficiency impacts insulin resistance and lipid accumulation in liver and white adipose tissue.
Design and Methods
Lepob/ob mice (OB) targeted with Nrf2 deletion (OB-Nrf2KO) were generated. Pathogenesis of obesity and Type-2 Diabetes were measured in C57BL/6J, Nrf2KO, OB, and OB-Nrf2KO mice. Hepatic lipid content, lipid clearance and VLDL secretion were determined between OB and OB-Nrf2KO mice.
Results
OB-Nrf2KO mice exhibited decreased white adipose tissue mass, and decreased adipogenic and lipogenic gene expression compared to OB mice. Nrf2-deficiency prolonged hyperglycemia in response to glucose challenge, which was paralleled by reduced insulin-stimulated Akt phosphorylation. In OB mice, Nrf2-deficiency decreased hepatic lipid accumulation, decreased Peroxisome proliferator-activated receptor γ expression and NADPH content, and enhanced VLDL secretion. However, this observation was opposite in lean mice. Additionally, OB-Nrf2KO mice exhibited increased plasma triglyceride content, decreased HDL-cholesterol content, and enhanced Apolipoprotein B expression, suggesting Nrf2-deficiency caused dyslipidemia in these mice.
Conclusions
Nrf2-deficiency in Lepob/ob mice reduced white adipose tissue mass, prevented hepatic lipid accumulation, but induced insulin resistance and dyslipidemia. The current study indicates a dual role of Nrf2 during metabolic dysregulation — increasing lipid accumulation in liver and white adipose tissue, but preventing lipid accumulation in obese mice.
Keywords: Nrf2, VLDL, insulin resistance, metabolic syndrome, obesity, NAFLD, NADPH, Warburg Effect, Keap1
Introduction
Obesity increases risk of developing metabolic syndrome, which encompasses insulin resistance (IR), Type 2 Diabetes (T2D) and Non-alcoholic fatty liver disease (NAFLD). During the past 20 years, there has been a marked increase of obesity in US, with more than 33% of adults and almost 17% of youths reported as obese (BMI≥30 kg/m2) (1). Obesity is an enlargement of adipose tissue to store excess energy in the form of triglycerides (TG). Multiple transcription regulators, including CCAAT/enhancer-binding proteins (Cebp) α, Cebpβ and Peroxisome proliferator-activated receptor (Ppar) γ have been reported involved in inducing adipogenic programming (2). Activation of Pparγ2 in fibroblasts is known to induce adipogenesis and stimulate adipocyte differentiation (3). Maintenance of normal adipogenesis and having adequate mature adipocytes is important for lipid storage, energy homeostasis, and whole-body insulin sensitivity. Impaired adipogenesis is associated with IR (4). Failure of adipocytes to differentiate caused IR and T2D (5). Dyslipidemia in IR is characterized by increased plasma VLDL-triglycerides, and increased hepatic Apolipoprotein B (ApoB) expression (6). Impaired lipid clearance in familial combined hyperlipidemia has been associated with higher serum TG, cholesterol, and ApoB content than present in normal patients (7).
Nuclear Factor E2-related factor 2 (Nrf2) function in adipocyte differentiation, lipid metabolism, IR and dyslipidemia has been examined, but many gaps in knowledge still remain. Nrf2-null mice have been described as resistant to high-fat diet (HFD)-induced obesity and hepatic steatosis, in association with suppressed adipogenesis, as well as, decreased expression of Pparγ (8), Srebp1c, Fatty acid synthase (Fas), and Stearoyl-CoA desaturase-1 (Scd1), and Fibroblast growth factor 21 (9). Constitutive activation of Nrf2 via Kelch-like ECH-associated protein 1-knockdown (Keap1-KD), predisposes mice to fatty liver with long-term HFD challenge (10) or with leptin-deficiency (11). In contrast, Nrf2 activation by Keap1-KD in fibroblasts suppressed adipogenesis (12) and treatment with the Nrf2 activator, CDDO-imidazolide (CDDO-Im) reduced HFD-induced adipose expansion concomitant with decreased hepatic lipid accumulation and expression of genes encoding fatty acid synthesis enzyme (13), indicating a protective function of Nrf2 activation against obesity. Thus, a clear role for Nrf2 activation and impact on metabolic disease has yet to emerge.
The purpose of this study was to explore whether Nrf2 impacts lipid metabolism in a model of obesity-induced dyslipidemia and IR, targeted deletion of Nrf2 in Lepob/ob background. The data herein illustrates that whole body Nrf2-deficiency modulates lipid metabolism in WAT and liver, as well as, glucose metabolism in Lepob/ob mice.
Material and Methods
Mouse breeding
C57BL/6J and Lepob/+ (C57BL/6J background) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Nrf2-null mice (Nrf2−/−, Nrf2KO) (14) were bred with Lepob/+ mice to create compound heterozygotes — Lepob/+;Nrf2+/−, then bred with Nrf2KO mice to produce Lepob/+;Nrf2−/− mice. Male and female Lepob/+;Nrf2−/− mice were crossed to obtain homozygous with Nrf2/leptin-deficiency (OB-Nrf2KO) and Nrf2KO mice. Wild type (WT) and Lepob/ob (OB) mice were produced by crossing male and female Lepob/+ mice. Male age-matched littermates were used and maintained under a 12h light/dark cycle freely access to water and diet (LM485, Harlan Laboratories, Madison, WI).
Food intake
Mice (8-week-old) of the four experimental genotypes were housed individually. Food intake for each mouse was measured every day for at least one week.
Glucose and insulin tolerance tests (GTT and ITT)
Mice (8-week-old) were fasted (16h for GTT, 6h for ITT). A single injection of glucose (2 g/kg) or insulin (1 U/kg) was administered ip. Blood glucose was determined at 0, 15, 30, 60, and 120 mins post glucose or insulin administration.
Pyruvate tolerance test
Male Nrf2KO (24-week-old) or Keap1-KD (16-week-old) mice (15) fasted overnight were administered 10% sodium pyruvate in PBS (2 g/kg, ip). Blood glucose was determined at 0, 15, 30, 60, and 120 mins after.
Acute insulin treatment
Mice (15-week-old) fasted overnight were anesthetized (ketamine, 150 mg/kg and xylazine, 5 mg/kg) and administered insulin (5 U/kg, ip) (Sigma-Aldrich, St. Louis, MO). Gastrocnemius and epididymal WAT were collected 5 mins after injection and immediately stored in liquid nitrogen. Samples were collected from 10:00 to 11:00am.
Histopathology
Formalin-fixed WAT and liver sections were embedded in paraffin; sections were cut and stained with hematoxylin/eosin. Four fields from each WAT section were analyzed to obtain the mean cell-area (n=4 per genotype). For Oil Red O staining, frozen sections were fixed and incubated with Oil Red O solution for 15 min, then counterstained with hematoxylin and mounted in glycerin jelly.
Glucose and insulin measurement
Serum glucose levels were analyzed using a glucose assay kit (Cayman Chemical Company, Ann Arbor, MI). Serum insulin was measured using a rat/mouse insulin ELISA kit (Millipore, Billerica, MA).
TG, and FFA, cholesterol, HDL-cholesterol, and LDL-cholesterol measurement
Serum TG, cholesterol, HDL- and LDL-cholesterol content was measured with reagent kits (Pointe Scientific, Ann Arbor, MI). FFA concentration was measured with a reagent kit (Wako Chemicals USA, Richmond, VA). The hepatic TG, FFA and cholesterol content was normalized with tissue weight.
VLDL secretion measurement
Mice (8-week-old) fasted 5h were injected with Tyloxapol via tail vein (Triton WR-1339, Sigma-Aldrich) (0.5 g/kg) in 0.9% NaCl solution containing 10% Triton-X. Blood samples were collected at 0, 60, and 120 min after injection and TG content was measured.
Exogenous lipid clearance measurement
Mice (8-week-old) fasted overnight were administered olive oil via oral gavage (15 ml/kg, Sigma-Aldrich). Blood samples were collected at 0, 1, 3, and 6h after oil administration. Serum was extracted from whole blood and TG content was measured.
Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) assay
Hepatic NADPH content was measured using a commercial kit (Biovision, Milpitas, CA). The hepatic NADPH content was normalized with tissue weight.
RNA isolation and quantitative real-time PCR
Total RNA isolation was performed using TRIzol reagent (Life Technologies, Grand Island, NY) and converted to cDNA. Relative mRNA levels were quantified by SYBR green chemistry using a LightCycler 480 system. The sequences of primers used are listed in Table S1.
Western blot analysis
Proteins (40 μg) were electrophoretically transferred to PVDF membrane, and then incubated with primary antibodies against Akt, Akt-S473 (Cell Signaling Technology, Danvers, MA), and Glut4 (Sigma-Aldrich) overnight at 4 °C. Chemiluminescence using ECL detection kit (GE Healthcare, Piscataway, NJ) was detected on autoradiography film.
Statistical Analysis
Quantitative data were presented as average ± SE. Statistic differences were determined by a one-way ANOVA followed by a Duncan’s Multiple Range post hoc test or two different genotypes were calculated by one-tailed Student’s t-test. All statistical tests with P<0.05 were considered significant.
Results
Adipose tissue mass is decreased in OB-Nrf2KO mice, but is increased in Nrf2KO mice
BW was similar between WT and Nrf2KO littermates, but BW of OB-Nrf2KO was 8% lower than OB mice at 8-weeks (Figure 1A). Food consumption was similar between WT and Nrf2KO or OB and OB-Nrf2KO mice (Figure 1B). The total WAT mass was 38% higher in Nrf2KO than WT mice, but decreased by 22% in OB-Nrf2KO than OB mice (Figure 1C). Epididymal mass was similar between Nrf2KO and WT mice, but 26% lower in OB-Nrf2KO than OB mice (Figure. 1D). Visceral fat was increased in Nrf2KO mice (by 65%), but decreased in OB-Nrf2KO mice (by 21%) (Figure S1A). Perirenal fat mass also tended to be higher in Nrf2KO than WT mice (P=0.077) (Figure S1B). Relative liver weight was 15% and 32% lower in Nrf2KO and OB-Nrf2KO than WT and OB mice, respectively (Figure 1E). Relative heart and kidney weight (Figure. 1F, 1G) were less in obese than lean mice, but similar between WT and Nrf2KO mice, or between OB and OB-Nrf2KO mice.
Figure 1. Adipose tissue mass is decreased in OB-Nrf2KO mice, but is increased in Nrf2KO mice.
(A) Body Weight (BW) of WT, Nrf2KO, OB, and OB-Nrf2KO mice at 4-, 8- and 12-weeks (n=5 to 9 per group). (B) Food intake from male mice at 12-weeks (n=5 to 7 per group). (C) Total fat and (D) epididymal fat in WT, Nrf2KO, OB, and OB-Nrf2KO mice at 12-weeks. Ratios of liver (E), heart (F), and kidney weight (G) to BW from WT, Nrf2KO, OB, and OB-Nrf2KO mice at 12-weeks (n=6 per group). $, P<0.05, Nrf2KO compared with WT mice; #, P<0.05, OB compared with WT mice; *, P<0.05, OB-Nrf2KO compared with OB mice.
Adipocyte cell size is decreased in OB-Nrf2KO mice, but is increased in Nrf2KO mice
Histomorphometric analysis of WAT revealed that Nrf2KO mice have bigger adipocytes than WT mice, but OB-Nrf2KO mice had smaller adipocytes than OB mice (Figure 2A, 2B). Adipocyte differentiation, or adipogenesis, is a critical determinant for the amount of WAT and is regulated by sequential expression of genes, such as Cebp, Pparγ, and Lipoprotein lipase (Lpl) (16). Nrf2 and (NAD(P)H:quinone oxidoreductase 1 (Nqo1), Glutamate-cysteine ligase catalytic subunit (Gclc), and Heme oxygenase-1 (Ho1)), Nrf2-target genes were decreased in Nrf2KO and OB-Nrf2KO mice (Figure S2). Pparγ and Fabp4 were induced in Nrf2KO mice (Figure. 1). However, the expression of most genes associated with adipocyte differentiation, including Cebpα, Cebpβ, Pparγ, and Lpl, was down-regulated in OB-Nrf2KO compared to OB mice (Figure 2C). Srebp1c was down-regulated in OB-Nrf2KO mice, along with decreased expression of Acetyl-CoA carboxylase (Acc)-1, Scd1, and Fas (Figure 2D, 2E). Furthermore, Pparγ was positively correlated with Nrf2 expression from human study, which is consistent with the study by Pi. et al (8), confirming the previous observation from animal work (Table S2).
Figure 2. Adipocyte cell size is decreased in OB-Nrf2KO mice, but is increased in Nrf2KO mice.
(A) Representative images of hematoxylin and eosin staining of epididymal pad from 12-week-old male mice (n=4 per group, magnification: 100×, scale bar =100 μm). (B) Distribution of adipocyte diameter in epididymal pad from 12-week-old mice (n=4 per group). Nrf2-deficiency down-regulated gene expression related with adipogenesis (C) and lipogenesis (D) in OB-Nrf2KO mice (n=4 to 6 per group). The relative mRNA levels were measured by quantitative real-time PCR and normalized with Rpl13a levels. (E) Immunoblot analysis of Acetyl-CoA Carboxylase (Acc-1), Stearoyl-Coenzyme A Desaturase 1 (Scd-1), Fatty Acid Synthase (Fas) in epididymal WAT from 12-week-old mice. $, P<0.05, Nrf2KO compared with WT mice; #, P<0.05, OB compared with WT mice; *, P<0.05, OB-Nrf2KO compared with OB mice.
Nrf2-deficiency impairs glucose tolerance and induces IR
Nrf2KO and OB-Nrf2KO mice had similar non-fasting blood glucose levels compared to WT and OB mice. Serum TG content was increased in OB-Nrf2KO mice (143% higher than OB mice). FFA content was similar between WT and Nrf2KO or between OB and OB-Nrf2KO mice. Insulin levels were similar between WT and Nrf2KO mice, and 16% higher in OB-Nrf2KO than OB mice. Total cholesterol content was reduced in Nrf2KO and OB-Nrf2KO compared to WT and OB mice (by 16% and 26%, respectively); accompanied by a lower HDL- and LDL-cholesterol content in OB-Nrf2KO than OB mice, suggesting a disturbance in lipid metabolism in OB-Nrf2KO mice (Table 1).
Table 1.
Serum metabolic parameters in WT, Nrf2KO, OB and OB-Nrf2KO mice at 12-weeks
| Parameters | Unit | WT | Nrf2KO | OB | OB-Nrf2KO |
|---|---|---|---|---|---|
| Glucose | mg/dl | 175.52±8.60 | 171.57±4.45 | 270.05±27.00# | 236.36±27.56 |
| TG | mg/dl | 79.20±8.55 | 78.83±4.92 | 55.52±3.61# | 134.94±11.43 * |
| FFA | μmol/ml | 0.89±0.06 | 0.83±0.07 | 1.14±0.06# | 1.13±0.11 |
| Insulin | ng/ml | 1.47±0.26 | 1.36±0.13 | 47.92±3.62# | 55.76±1.77* |
| Cholesterol | mg/dl | 122.56±5.08 | 103.46±6.81$ | 181.52±6.21# | 133.86±10.13* |
| HDL-cholesterol | mg/dl | 27.02±0.82 | 25.62±1.65 | 42.09±0.90# | 29.07±2.58* |
| LDL-cholesterol | mg/dl | 14.25±0.88 | 7.28±0.51$ | 79.62±6.00# | 34.79±5.03* |
Serum glucose, TG and FFA, insulin, cholesterol, HDL-cholesterol, LDL-cholesterol levels were measured in mice at 12-weeks. Data were represented as average ±S.E. (n=5 to 7 per group).
P<0.05, Nrf2KO compared with WT mice;
P<0.05, OB compared with WT mice;
P<0.05, OB-Nrf2KO compared with OB mice.
Nrf2KO and OB-Nrf2KO mice exhibited increased glucose levels and significant higher AUCglucose upon acute glucose challenge (24% and 50% higher than WT and OB mice, respectively) (Figure 3A–3D). No difference for ITT was observed between WT and Nrf2KO mice (Figure 3E, 3F). Compared to OB mice, OB-Nrf2KO had lower glucose levels after 6h-fasting, and exhibited enhanced glucose levels upon insulin administration (120 min), suggesting Nrf2 deficiency may induce resistance of glucose removal and IR in Lepob/ob mice (Figure 3G, 3H).
Figure 3. Nrf2-deficiency impairs glucose tolerance and induces IR.
Glucose tolerance test from WT and Nrf2KO (A, B) or OB and OB-Nrf2KO (C, D) mice at 8-weeks (n=5 per group). Insulin tolerance test from WT and Nrf2KO (E, F) or OB and OB-Nrf2KO (G, H) mice at 8-weeks (n=5 to 6 per group). The glucose content has been normalized to the average basal content. Immunoblot analysis of Ser473-phospholylated-Akt (p-Akt), total-Akt (Akt), glucose transporter (Glut4) in skeletal muscle from WT and Nrf2KO (I) or OB and OB-Nrf2KO mice (J) at basal level. Immunoblot analysis of Ser473-phospholylated-Akt (p-Akt), total-Akt (Akt) in response to insulin (5 U/kg) in skeletal muscle (gastrocnemius) (K). GAPDH was used as loading control. *, P<0.05, OB-Nrf2KO compared with OB mice.
p-Akt and Glut4 protein levels were determined in skeletal muscle. Nrf2-deficiency decreased p-Akt and Glut4 expression in lean mice (Figure 3I). Under basal conditions, p-Akt and Glut4 were similar between OB and OB-Nrf2KO mice (Figure 3J). However, after insulin challenge, more p-Akt was decreased in OB-Nrf2KO than OB mice (Figure 3K). This demonstrated Nrf2-deficiency impaired Akt activation in response to insulin, which could explain impaired insulin signaling and induction IR in Lepob/ob mice.
Nrf2-deficiency impairs insulin signaling in WAT
In unchallenged tissue, p-Akt levels were similar between OB and OB-Nrf2KO mice (Figure 4A). However, upon insulin challenge, p-Akt levels were decreased in OB-Nrf2KO compared to OB mice (Figure 4B). Genes related to insulin signaling, such as Insr were decreased, along with a slight decrease of Glut4 and Insulin receptor substrate (Irs)-1 expression in OB-Nrf2KO mice (Figure 4C).
Figure 4. Nrf2-deficiency impairs insulin signaling in WAT.
Immunoblot analysis of Ser473-phospholylated-Akt (p-Akt), total Akt (Akt) at basal level (A) or in response to insulin (5 U/kg) (B) in white adipose tissue from OB and OB-Nrf2KO mice at 12-weeks. GAPDH was used as loading control. (C) Real-time PCR was performed in white adipose tissue. The mRNA levels have been normalized with Rpl13a levels. *, P<0.05, OB-Nrf2KO compared with OB mice.
Nrf2-deficiency lessens hepatic steatosis in Lepob/ob mice
Large vacuoles were observed in liver sections of OB mice, but not in OB-Nrf2KO mice, suggesting decreased hepatic triglyceride content (Figure 5A). The results were further confirmed by Oil red O staining (Figure 5B) and lipid extract quantification (29%, 9% and 35% lower than OB mice for TG, FFA, and cholesterol content, respectively) (Figure 5C–5E). In contrast, neutral lipid staining near the central vein was observed in Nrf2KO mice, but not in WT mice (Figure 5B), and further evidence from lipid quantification demonstrated increased lipid content in Nrf2KO mice (46% of TG and 29% of cholesterol higher than WT mice) (Figure 5C, 5E), suggesting Nrf2-deficiency promotes steatosis in lean mice.
Figure 5. Nrf2-deficiency lessens hepatic steatosis in Lepob/ob mice.
(A) Representative images of hematoxylin and eosin staining from WT, Nrf2KO, OB, and OB-Nrf2KO mice at 12-weeks. Magnification: 200×. Scale bar=50 μm (n=4 per group). (B) Representative images of Oil Red O staining at 12-weeks. Magnification: 200×. Scale bar=100 μm. Hepatic (C) triglycerides (TG), (D) free fatty acid (FFA), and (E) cholesterol were measured to quantify hepatic lipid content. The triglycerides, free fatty acid, and cholesterol content were normalized to tissue weight (n=5 to 6 per group). $, P<0.05, Nrf2KO compared with WT mice; #, P<0.05, OB compared with WT mice; *, P<0.05, OB-Nrf2KO compared with OB mice.
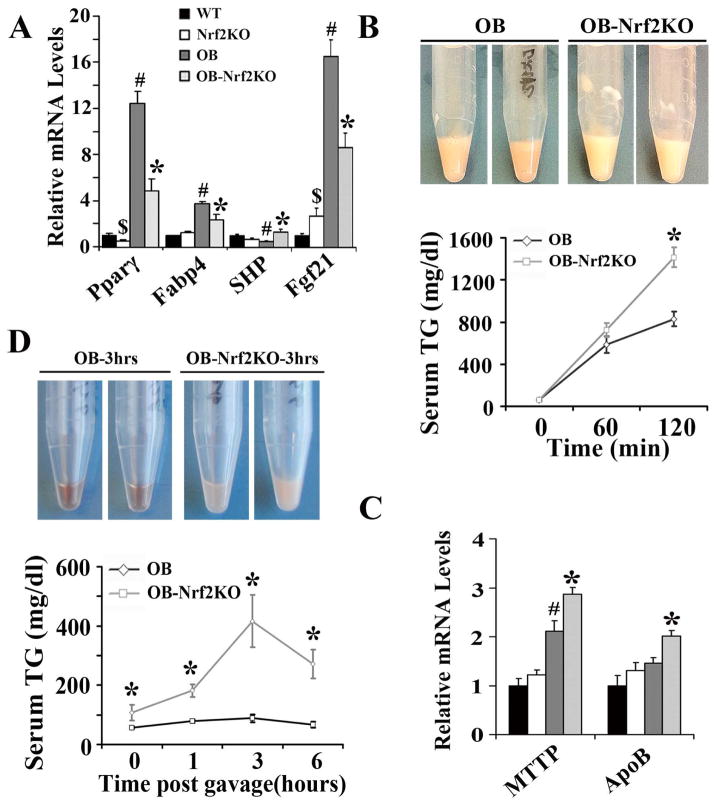
Nrf2, Nqo1, and Gclc were reduced in Nrf2KO and OB-Nrf2KO mice (Figure S2). Pparγ and Fabp4 were decreased in OB-Nrf2KO mice (Figure 6A). Nrf2-deficiency reduced SHP slightly in lean mice, but induced the expression in obese mice. Fgf21 was increased in Nrf2KO mice, but this increase was attenuated in OB-Nrf2KO mice. Nrf2KO and OB-Nrf2KO mice showed marked induction of Srebp1c, in coordination with downstreams (Acc1, Acc2, and Fas), despite lower hepatic lipid content in OB-Nrf2KO mice (Figure S3).
Figure 6. Nrf2-deficiency increases VLDL secretion, but impairs lipid clearance in OB-Nrf2KO mice.
(A) Hepatic mRNA levels were measured by quantitative real-time PCR; (B) Nrf2-deficiency enhanced VLDL secretion in OB-Nrf2KO compared with OB mice (n=4 to 7 per group). (C) Nrf2-deficiency increased MTTP and ApoB mRNA levels in liver. The relative mRNA levels were measured by quantitative real-time PCR and normalized with 36b4 levels. (D) Nrf2-deficiency impaired exogenous lipid clearance rate in OB-Nrf2KO mice (n=7 per group). $, P<0.05, Nrf2KO compared with WT mice; #, P<0.05, OB compared with WT mice; *, P<0.05, OB-Nrf2KO compared with OB mice.
Nrf2-deficiency increases VLDL secretion, but impairs lipid clearance in OB-Nrf2KO mice
After administration of WR-1339, an inhibitor of lipoprotein lipase, OB-Nrf2KO mice exhibited higher serum TG/lipids than OB mice, suggesting Nrf2-deficiency may enhance VLDL secretion (Figure 6B). Additionally, hepatic MTTP and ApoB were markedly induced in OB-Nrf2KO mice, which are responsible for lipoprotein assembly and VLDL secretion, further suggesting enhanced VLDL secretion in OB-Nrf2KO mice (Figure 6C). When mice were challenged with a high amount of exogenous lipid, OB-Nrf2KO mice exhibited higher serum TG content than OB mice, suggesting a lower lipid clearance rate in OB-Nrf2KO mice (Figure 6D). The current data suggest that Nrf2-deficiency enhanced the assembly and secretion of VLDL, but impaired serum lipid clearance, which in combination could explain the observed decrease in hepatic lipid accumulation and increase in serum TG levels in OB-Nrf2KO mice (Table 1).
Nrf2-deficiency reduces hepatic NADPH content and glucose production
OB-Nrf2KO mice had decreased serum glucose and cholesterol levels. It was hypothesized that Nrf2 might participate in pathways that support glucose and co-factor production. NADPH is a necessary reducing equivalent needed for the synthesis of triglycerides and cholesterol in liver. Hepatic NADPH content was decreased by 9% and 11% in Nrf2KO and OB-Nrf2KO in comparison to WT and OB mice, respectively (Figure 7A). Malic enzyme 1 (Me1), encoding one of the key synthetic enzymes for NADPH generation, was decreased in Nrf2KO and OB-Nrf2KO mice (Figure 7B). Additionally, Nrf2KO mice exhibited decreased glucose levels and decreased AUCPTT following intraperitoneal injection of pyruvate, suggesting impaired glucose generation initiated by Nrf2-deficiency (Figure 7C). Moreover, Keap1-KD mice exhibited increased glucose levels and increased AUCPTT after pyruvate administration, further indicating that increased Nrf2 activity can increase the gluconeogenesis process and glucose generation from a non-glucose substrate (Figure 7D).
Figure 7. Nrf2-deficiency reduces hepatic NADPH content and glucose production.
(A) NADPH content and (B) Malic enzyme 1 (Me1) mRNA levels were down-regulated in OB-Nrf2KO mice (n=5 to 6 per group). The relative mRNA levels were measured by quantitative real-time PCR and normalized with 36b4 levels. $, P<0.05, Nrf2KO compared with WT mice; #, P<0.05, OB compared with WT mice; *, P<0.05, OB-Nrf2KO compared with OB mice. (C) Pyruvate tolerance test (2 g/kg) was performed on WT and Nrf2KO mice (n=3 to 4 per group) (left) and AUC was determined (right). $, P<0.05, Nrf2KO compared with WT mice. (D) Pyruvate tolerance test (2 g/kg) was performed on WT and Keap1-KD mice (n=5 per group) (left) and AUC was determined (right). &, P<0.05, Keap1-KD compared with WT mice. Proposed model of Nrf2 function in regulating lipid metabolism in (E) white adipose tissue and (F) liver.
Discussion
The work herein reports that Nrf2-deficiency decreased WAT mass and hepatic lipid accumulation in OB-Nrf2KO mice, which was associated with enhanced VLDL secretion and impaired NADPH generation. Our work confirms the work by Xue et al. and provides additional mechanistic insight into these observations (17). It is consistent with observations in which Nrf2KO mice were resistant to HFD-feeding (8) and increased WAT mass in Keap1-KD mice after prolonged HFD-feeding (10).
Our observation that Nrf2-deficiency attenuates hepatic lipid accumulation in OB-Nrf2KO mice also supports others published work (Table S3), in which Nrf2-deficieny results in decreased hepatic lipids upon HFD-feeding (17, 18, 19). Nrf2-deficiency prevented HFD-induced obesity via repressing Fgf21 expression (9). In the current study, Nrf2-deficiency induced Fgf21 expression in lean mice, but the induction was repressed in OB-Nrf2KO mice, suggesting Nrf2 might regulate Fgf21 transcription levels by leptin-dependent mechanism. Nrf2 deficiency also increased hepatic VLDL secretion, which may overcome the effect of de novo lipogenesis to reduce hepatic lipids in Lepob/ob mice. MTTP was induced, and Pparγ and Fabp4 were reduced, supporting the notion of decreased lipid influx and increased efflux in OB-Nrf2KO mice. Last, lipid synthetic enzymes, such as Acc, Fas, and Scd-1, require reduced NADPH to function at the enzymatic level and carry out the conversion from acetyl-CoA to cholesterol or TG (20). Decreased hepatic lipid content could be explained through alteration of reducing equivalent, which was reduced in Nrf2KO and OB-Nrf2KO mice. Limited NADPH content in leptin-deficient mice may be of significant consequence because although lipogenic gene expression is increased, there is significantly reduced cofactor present to drive the subsequent lipid synthesis reactions. Reduced NADPH observed in Nrf2KO mice may be of lesser consequence, because in lean mice the systemic lipid load is low, with adequate NADPH to sustain lipid production.
Nrf2-deficiency also induced dyslipidemia in Lepob/ob mice, consistent with previous observations in obesity models (Tables S3, S4). Reduced HDL-cholesterol is an additional characteristic of atherogenic dyslipidemia associated with abdominal obesity (21). HDL-cholesterol content was decreased in OB-Nrf2KO mice, accompanied by a high concentration of plasma TG. MTTP and ApoB are essential for the assembly and secretion of VLDL to blood. Obesity associated with plasma lipid abnormalities was due to overproduction of VLDL-ApoB particles and decreased catabolism of ApoB containing particles (22). Moreover, overexpression of mouse ApoB increased plasma TG content (23). We reported that MTTP and ApoB expression was increased in OB-Nrf2KO mice and promoted VLDL/TG secretion, which is consistent with a previous report (24). Additionally, the lipid clearance was impaired in OB-Nrf2KO mice, which attenuated lipid removal from blood and eventually increased plasma TG content, as well as induced dyslimidemia in OB-Nrf2KO mice. Together, these data suggest that Nrf2 play an important role in regulating plasma lipid content. It can be concluded that enhanced Nrf2 activity reduced plasma lipid content under basal conditions (25, 26, 27). In contrast, Nrf2-deficiency increased serum lipid content at the basal level, displaying a trend of increased lipid content by Nrf2-deficiency (17, 29).
Nrf2-deficiency impaired glucose tolerance and induced IR in Lepob/ob mice, consistent with work by Xue et al. (17) and Aleksunes et al. (30). This was further supported by decreased Akt phosphorylation in skeletal muscle after insulin challenge and suppressed Insr expression. However, these results do not entirely agree with Chartoumpekis et al. (9) and Zhang et al. (31), whose data demonstrated that Nrf2-deficiency decreased the glucose content upon acute glucose challenge, which improved glucose tolerance. The possible reason for the inconsistency was that the glucose content of Nrf2KO mice at time zero for the test was lower than the control mice for these two studies, which may affect the data analysis of the later experimental points. Normalization of the glucose content with baseline may be helpful to obtain unbiased conclusions.
Nrf2KO mice displayed impaired gluconeogenesis, exhibited by the decreased glucose generation upon pyruvate perfusion. We observed the opposite observation in Keap1-KD mice. Fasting decreases plasma glucose levels, but increases pyruvate content, this increases NAD+/NADH ratio and gluconeogenesis. Nrf2KO mice displayed a lower NAD+/NADH ratio than control group (32), which will reduce the process of gluconeogenesis and glucose generation from pyruvate.
An opposite effect of lipid accumulation was observed when comparing WT with Nrf2KO mice. On standard chow diet, enhanced Nrf2 activity via genetically (27, 33) or pharmacologically (13, 34) lessened hepatic lipid accumulation and suppressed lipogenic gene expression, suggesting Nrf2 plays negative roles in hepatic lipid modulation (Table S4). Enhanced Nrf2 activity via Keap1-KD inhibited lipogenic gene expression, such as Fas, Acc1, and finally reduced hepatic lipid accumulation. Moreover, Nrf2-deficiency increased lipogenic gene expression (Srebp1c and Scd1) and free-fatty acid accumulation in liver (19), also confirming a negative role of Nrf2 function in lipid modulation.
It is hard to come to a “big picture” conclusion regarding Nrf2 activation and metabolic disease because conflicting reports exist (Tables S3, S4). There were not dramatic differences between Nrf2-expressing and -deficient mice fed standard chow. This is perhaps due to housing conditions and lack of use of littermate congenic controls. Consistently among studies, Nrf2KO mice appeared to have higher serum lipids. Our observations were supported by others, indicating that Nrf2-deficient mice have a slight increase in hepatic lipids.
A relevant, intriguing question to the field is whether Nrf2 should be targeted to alleviate metabolic-related symptoms/disease. First, the change in body weight observed was slight and may not be substantial enough to develop as a therapeutic target to treat obesity. Second, the decreased body weight likely comes at the expense of glucose tolerance, which is also observed by others (Tables S3, S4). When Nrf2 expression is knocked out in obese mice, there is an observed increase of hepatic lipids, but this is at the expense of impaired response to glucose challenge and an altered lipid profile. So, it might pose metabolic risks to modulate Nrf2 activation in multiple metabolic tissues, as might occur with a drug that targets Nrf2 activation.
The current study provides in vivo evidence that Nrf2 participates in the conversion of pyruvate to glucose and drives NAPDH generation. This has been observed in Keap1-KD mice (35). This has implications for cancer and lipid synthesis, as the pentose phosphate pathway is implicated in the “Warburg Effect”. For the Warburg effect, excess glucose is diverted through the pentose phosphate shunt (PPS) to create nucleotides. Fatty acids are critical for new membrane production and are synthesized from citrate in the cytosol through the action of ATP-citrate lyase (ACL) to generate acetyl-CoA. This process requires NADPH reducing equivalents, which is generated through the actions of malic enzyme. Nrf2 has recently been implicated in cancer research to participate in the Warburg Effect (36). As obesity and cancer are co-morbid risk factors (37), this observation is highly significant and provides an interesting link between Nrf2 and perhaps obesity-induced cancer.
Overall, the data illustrate that Nrf2-deficiency modestly decreased adipose tissue expandability, but appreciably decreased hepatic steatosis in leptin-deficient mice, which is consistent with other observations (9, 17). Nrf2-deficiency impaired response to acute glucose and insulin administration, suggesting IR existing in OB-Nrf2KO mice. The current data point to a complex role for Nrf2 in regulating lipid and glucose homeostasis and leptin interactions should be considered when evaluating Nrf2 function.
Supplementary Material
What is already known about this subject?
Nrf2-deficiency impaired glucose tolerance and induced insulin resistance
Nrf2-deficiency induced dyslipidemia in Lepob/ob mice
Nrf2-deficiency reduced white adipose tissue mass and decreased Pparγ and Glut4 expression in Lepob/ob mice
Enhanced Nrf2 activity via Keap1-KD increased hepatic lipid accumulation with high-fat diet challenge and in Lepob/ob mice
What this study adds?
Nrf2-deficiency decreased hepatic lipid accumulation in Lepob/ob mice
Nrf2-deficiency enhanced VLDL/TG secretion, accompanied by enhanced MTTP and ApoB expression in Lepob/ob mice
Nrf2-deficiency impaired exogenous lipid clearance in Lepob/ob mice
Nrf2-deficiency decreased hepatic NADPH content and Malic enzyme-1 mRNA levels
Nrf2-deficiency decreased glucose synthesis from pyruvate
Acknowledgments
This work was supported by National Institute of Health [5R01ES016042 and 5K22ES013782] awarded to AS, and by Rhode Island IDeA Network of Biomedical Research Excellence Award [P20RR016457-10] from the National Center for Research Resources. JX was also supported by the National Science Foundation of China [81341102], and Fundamental Research Funds of Northeastern University [N130220001]. We thank Dr. Curtis D. Klaassen (University of Kansas Medical School) for generously sharing Nrf2KO and Keap1-KD mice.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, 2009–2010. NCHS Data Brief. 2012 [PubMed] [Google Scholar]
- 2.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes & development. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 3.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, et al. Evidence of impaired adipogenesis in insulin resistance. Biochemical and biophysical research communications. 2004;317:1045–1051. doi: 10.1016/j.bbrc.2004.03.152. [DOI] [PubMed] [Google Scholar]
- 5.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nature genetics. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends in endocrinology and metabolism: TEM. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabezas MC, de Bruin TW, Jansen H, Kock LA, Kortlandt W, Erkelens DW. Impaired chylomicron remnant clearance in familial combined hyperlipidemia. Arterioscler Thromb. 1993;13:804–814. doi: 10.1161/01.atv.13.6.804. [DOI] [PubMed] [Google Scholar]
- 8.Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, et al. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. The Journal of biological chemistry. 2010;285:9292–9300. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP, et al. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes. 2011;60:2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.More VR, Xu J, Shimpi PC, Belgrave C, Luyendyk JP, Yamamoto M, et al. Keap1 knockdown increases markers of metabolic syndrome after long-term high fat diet feeding. Free radical biology & medicine. 2013;61C:85–94. doi: 10.1016/j.freeradbiomed.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208–3218. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, et al. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Molecular and cellular biology. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, et al. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. European journal of pharmacology. 2009;620:138–144. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 16.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes & development. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 17.Xue P, Hou Y, Chen Y, Yang B, Fu J, Zheng H, et al. Adipose Deficiency of Nrf2 in ob/ob Mice Results in Severe Metabolic Syndrome. Diabetes. 2012 doi: 10.2337/db12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Tabbi-Anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1211–1221. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu KC, Liu J, Klaassen CD. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicology and applied pharmacology. 2012;262:321–329. doi: 10.1016/j.taap.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of clinical investigation. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascot A, Lemieux I, Prud’homme D, Tremblay A, Nadeau A, Couillard C, et al. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. Journal of lipid research. 2001;42:2007–2014. [PubMed] [Google Scholar]
- 22.Chan DC, Watts GF, Redgrave TG, Mori TA, Barrett PH. Apolipoprotein B-100 kinetics in visceral obesity: associations with plasma apolipoprotein C-III concentration. Metabolism: clinical and experimental. 2002;51:1041–1046. doi: 10.1053/meta.2002.33339. [DOI] [PubMed] [Google Scholar]
- 23.McCormick SP, Ng JK, Veniant M, Boren J, Pierotti V, Flynn LM, et al. Transgenic mice that overexpress mouse apolipoprotein B. Evidence that the DNA sequences controlling intestinal expression of the apolipoprotein B gene are distant from the structural gene. The Journal of biological chemistry. 1996;271:11963–11970. doi: 10.1074/jbc.271.20.11963. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Newberry EP, Norris JY, Xie Y, Luo J, Kennedy SM, et al. ApoB100 is required for increased VLDL-triglyceride secretion by microsomal triglyceride transfer protein in ob/ob mice. Journal of lipid research. 2008;49:2013–2022. doi: 10.1194/jlr.M800240-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, et al. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia. 2011;54:922–934. doi: 10.1007/s00125-010-2001-8. [DOI] [PubMed] [Google Scholar]
- 26.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Molecular and cellular biology. 2013;33:2996–3010. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Donepudi AC, Moscovitz JE, Slitt AL. Keap1-Knockdown Decreases Fasting-Induced Fatty Liver via Altered Lipid Metabolism and Decreased Fatty Acid Mobilization from Adipose Tissue. PloS one. 2013;8:e79841. doi: 10.1371/journal.pone.0079841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Cui Y, Li C, Zhang Y, Xu S, Li X, et al. Nrf2 deletion causes “benign” simple steatosis to develop into nonalcoholic steatohepatitis in mice fed a high-fat diet. Lipids Health Dis. 2013;12:165. doi: 10.1186/1476-511X-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. The Journal of pharmacology and experimental therapeutics. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 30.Aleksunes LM, Reisman SA, Yeager RL, Goedken MJ, Klaassen CD. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. The Journal of pharmacology and experimental therapeutics. 2010;333:140–151. doi: 10.1124/jpet.109.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang YK, Wu KC, Liu J, Klaassen CD. Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicology and applied pharmacology. 2012;264:305–314. doi: 10.1016/j.taap.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulkarni SR, Donepudi AC, Xu J, Wei W, Cheng QC, Driscoll MV, et al. Fasting induces nuclear factor E2-related factor 2 and ATP-binding Cassette transporters via protein kinase A and Sirtuin-1 in mouse and human. Antioxidants & redox signaling. 2014;20:15–30. doi: 10.1089/ars.2012.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada K, Warabi E, Sugimoto H, Horie M, Tokushige K, Ueda T, et al. Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J Gastroenterol. 2012;47:924–935. doi: 10.1007/s00535-012-0552-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochimica et biophysica acta. 2014;1840:209–218. doi: 10.1016/j.bbagen.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A, Happel C, Manna SK, Acquaah-Mensah G, Carrerero J, Kumar S, et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. The Journal of clinical investigation. 2013;123:2921–2934. doi: 10.1172/JCI66353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.