Abstract
Objective
Findings on weight training and waist circumference (WC) change are controversial. This study examined prospectively whether weight training, moderate-to-vigorous aerobic activity (MVAA), and replacement of one activity for another were associated with favorable changes in WC and body weight (BW).
Methods
Physical activity, WC, and BW were reported in 1996 and 2008 in a cohort of 10,500 healthy U.S. men in the Health Professionals Follow-up Study. We used multiple linear regression models (partition/substitution) to assess these associations.
Results
After adjusting for potential confounders, we observed a significant inverse dose-response relationship between weight training and WC change (P-trend<0.001). Less age-associated WC increase was seen with a 20 min/day activity increase; this benefit was significantly stronger for weight training (-0.67cm, 95%CI -0.93, -0.41) than for MVAA (-0.33cm, 95%CI -0.40, -0.27), other activities (-0.16cm, 95%CI -0.28, -0.03), or TV watching (0.08cm, 95%CI 0.05, 0.12). Substituting 20 min/day of weight training for any other discretionary activity had the strongest inverse association with WC change. MVAA had the strongest inverse association with BW change (-0.23kg, 95%CI -0.29, -0.17).
Conclusions
Among various activities, weight training had the strongest association with less WC increase. Studies on frequency /volume of weight training and WC change are warranted.
Keywords: weight training, aerobic activity, waist circumference, body weight
Introduction
Muscle mass loss is common during aging (especially among sedentary individuals after the age of fifty (1) and during intentional weight loss, which may lead to impairments in muscle strength and limitations in physical function (2, 3). Therefore, it becomes important to know how different physical activity (PA) types are associated with changes in waist circumference (WC) and body weight (BW), especially when one activity replaces another per given time. Ideally, older adults are recommended to engage in PAs that achieve the most favorable changes in body composition, such as loss of fat mass while preserving lean body mass. Randomized controlled trials on the effect of aerobic vs. resistance training on WC (4, 5) or visceral fat (6, 7) have been conflicting; while one meta-analysis (6) and two recent trials (5, 7) showed aerobic exercise (e.g., bike; elliptical machine; walking) to be more effective than resistance training (e.g., sets of machine exercises), another trial showed weight training to be not effective on decreasing abdominal fat (WC) in midlife women with a wide BMI range between 20 and 35 kg/m2 (4). Other trials showed weight training to be effective in reducing visceral fat in overweight and obese premenopausal women (8) and in sedentary men and women with type 2 diabetes (9). While most of these studies focused on one specific population type (overweight, obese, or with type 2 diabetes) and were of short duration, evaluating this association over longer follow-up periods and on a larger sample becomes crucial. We believe this large cohort study coupled with an understanding of the underlying physiology of the results would help better design future randomized trials.
Therefore, we prospectively examined in a large sample of healthy men from the Health Professionals Follow-Up Study whether moderate-to-vigorous aerobic activity (MVAA), weight training, and replacement of one activity for another were associated with favorable changes in WC and BW over 12 years.
Methods and Procedures
Study Population
The Health Professionals Follow-up Study (HPFS) is an ongoing prospective study of 51,529 male health professionals, including dentists, veterinarians, pharmacists, optometrists, osteopaths, and podiatrists, aged 40-75 years upon enrollment in 1986. Participants have been followed through mailed biennial questionnaires about their medical history, lifestyle, and health-related behaviors including PA. Dietary intake was assessed every four years starting 1986 using a 131-item food frequency questionnaire (FFQ). Dietary information was updated with subsequent similar FFQs mailed every 4 years thereafter until 2010. This study was approved by the Institutional Review Board of the Harvard School of Public Health, Boston, Massachusetts.
Assessment of weight training, MVAAs, and TV viewing
In 1996 and 2008, participants were asked to report the average time spent per week in the previous year in each of walking, jogging (slower than 10 minutes/mile), running (10 minutes/mile or faster), bicycling, lap swimming, tennis, squash/racquetball, calisthenics, rowing, stair/ski machine, weightlifting/ weight machine, heavy outdoor work (e.g. digging, chopping), and TV watching. For each activity, men chose one of the 13 duration categories that ranged from none to 40+ hours/week. Men also reported their normal walking pace: easy (<2 miles per hour [mph]), normal, average (2-2.9 mph), brisk (3-3.9 mph), very brisk, striding (≥4 mph). Moreover, men were asked to report the average number of flights of stairs they climbed daily. Stair climbing (min/d) was then estimated. We classified the different PAs into 4 types as previously described(10): 1) MVAA (≥ 3 metabolic equivalent tasks) that are performed repetitively producing dynamic contractions of large muscle groups (11); these included brisk and very brisk walking paces, jogging, running, bicycling, lap swimming, tennis, squash/racquetball, calisthenics, rowing, and stair/ski machine; 2) Other activities which included unstructured physical activities of at least moderate intensity such as heavy outdoor work and stair climbing; 3) weight training; and 4) Slow and average walking paces that we controlled for in our models. The PA questionnaire has been validated in a random representative sample of the HPFS participants (n=280) in 1991 (12). Correlations between four 1-week diaries and the 1992 questionnaire were 0.65 for total activity and 0.79 for weight training. Reproducibility of weight training from two questionnaires was 0.50. Additionally, to reflect long-term PA, the pulse rate was used before and after a step test among a subset of participants. Spearman correlations between vigorous activity and pulse rate were -0.45 (before stepping) and -0.41 (after stepping). These associations provide further support for our questionnaire validity (12). Because TV watching has been associated with obesity (13), we included change in hours of TV watching in our models.
Assessment of covariates
Because some diet components have been observed to be predictive of weight gain (14, 15), they were included in the analysis. Using a validated FFQ, we assessed intakes of sugar-sweetened beverages, energy-adjusted trans-fats, dietary fiber, alcohol, percent energy of protein intake, and glycemic load in 1994 and 2006. Values in both 1994 and 2006 were included in the model to account for changes in these covariates. Smoking status, antidepressant use (both in 1996 and 2008), sleep duration (hours/day) (in 2000 and 2008) and baseline age were also included. In a validation study of the FFQ vs. 2 diet records (14-day average) conducted among 127 participants of the HPFS, the calorie-adjusted Pearson correlation coefficient for protein intake was 0.38 and the de-attenuated coefficient was 0.44 (16).
Exclusion of participants
Because WC (our primary outcome) was first assessed in 1996, we used 1996 as baseline. For this investigation, among those who reported their BW, WC, and walking pace in 1996 or 2008 (n=39,111), participants could not participate in the study if they died before the 1996 follow-up cycle (n=4,190), had implausible energy intakes at baseline (>4200 or <800 kcal/d) (n=1,203), and were disabled or otherwise unable to walk in 2008 (n=373). Outliers (defined as values outside the range of the mean value ± 3×SD) for the main exposure (PA) and for both outcomes (WC and BW) were excluded in 1996 and 2008 (n=712). Further exclusions through 2008 included reporting myocardial infarction (n=3,711), stroke (n=1,143), angina (n=3,112), coronary artery bypass grafting (n=1,852), diabetes (n=3,518), or cancer (n=8,797) because the development of these diseases could lead to changes in WC and PA levels. Hence, 10,500 remained in the analysis.
Outcome definitions
The primary outcome was 12-year WC change (cm) and the secondary outcome was 12-year BW change (kg) from 1996 to 2008. Specific instructions for WC measurements were written on the front page of the questionnaires and a simple tape measure was enclosed. A drawing was also depicted to help standardize the proper location of the WC. The reproducibility and validity of the self-reported measures of WC and weight were evaluated by comparing them with technician-assessed measurements taken 6 months apart in a subset of the cohort participants (n=123 men). The age-strata of these men were selected to reflect the age distribution of the entire cohort. The self-reported measures were highly correlated with the average of 2 technician measurements (weight: r=0.97; WC; r=0.95) (17). Self-reported height has been previously reported as highly valid (18).
Statistical Analysis
Waist circumference (WC) change (cm) from 1996 to 2008 was modeled as WC in 2008 as the outcome and baseline WC as a covariate; this is mathematically equivalent to assessing WC change as the outcome (19). To shed light on the association between weight training and WC change, we first modeled weight training in categories based on the distribution of our sample (0, >0-10, >10-25, >25 min/day) in an age-adjusted model. In a multivariate model 1, we additionally controlled for total average alcohol intake, sugar-sweetened beverage intake, percent energy of trans-fat, energy-adjusted fibers, percent energy of protein intake (all in 1994; 2006), smoking, anti-depressant intake (1996; 2008), sleep duration (2000; 2008), and all other activities such as TV watching, MVAA, other activities (1996; 2008), and weight training (1996). In a multivariate model 2, we additionally adjusted for BW (kg) (1996, 2008) to assess the relation with the change in central adiposity independent of BW change. Tests for linear trends across categories of weight training were performed by using Wald's test (1 df) of an ordinal term that represented median values of these categories. The different PA types were then modeled continuously using different multiple linear regression models (partition and substitution) to assess the associations between 12-year changes in the different activities and 12-year WC change. More details on the time substitution models can be found elsewhere (19, 20). Briefly, in the partition model, the regression coefficient for each activity type reflects the addition of a certain activity time (here, 20 min/day) on top of other activities in the model. The substitution model includes each of the activities and their sum with one activity being dropped out. The coefficients for a certain activity represents the consequence of substituting that activity for the one that was dropped out, by the same amount of time.
To examine the joint association of weight training and MVAA on WC change, we constructed a joint variable of weight training (3 categories: none, >0-25; >25 min/day) and MVAA (3 categories closely representing adherence to current recommendations of 150 minutes/week: none, >0-≤25 min/day; >25 min/day).
Similar analytical models were replicated for BW change (kg) from 1996 to 2008 as an outcome. In the first multivariate model, we adjusted for the same covariates as in the WC change model except for WC and in addition to baseline height (m) and weight (kg). In the second multivariate model, we additionally adjusted for WC change to assess the relation with BW change (or muscle mass) independent of WC change.
We conducted a sensitivity analysis where we did not exclude participants who developed cardiovascular diseases (CVD) or diabetes between 1996 and 2008, but we controlled for these 2 variables of developing CVD (yes/no) or diabetes (yes/no) in our multivariate models (n=12,965). SAS version 9.3 (SAS, Cary, NC, USA) was used for all analyses, and a p value <0.05 was considered statistically significant.
Results
In this cohort of 10,500 men [mean age (SD)=58 (7) years], as compared with those who did not engage in any weight training activity, men who engaged in ≥25 min/day of weight training had a smaller WC and a lower body mass index (BMI), spent fewer hours watching TV, consumed less trans-fat, more fiber, engaged in more MVAA, had a higher glycemic load diet, had a better diet quality as reflected by the higher alternative healthy eating index (21), and smoked more (Table 1). The mean (SD) time spent in total PA at baseline was 48 (49) min/day and the median was 34 min/day. The mean (SD) WC increase over 12 years was 3.11 (6.61)cm. In this cohort of men, weight training contributed to 7% of the total minutes of PA reported daily. Study participants spent most of their reported discretionary time watching TV (61%) and less time engaging in MVAA (25%), weight training (3%), or other activities (11%).
Table 1. Age-standardized baseline participant characteristics, a by daily levels of weight training among 10,500 US health professionals.
| Weight training (1996), minutes per day (min/d) | ||||
|---|---|---|---|---|
| None | >0-10 | > 10-25 | >25 | |
| Median value, min/d | 0 | 4 | 13 | 43 |
| N | 7,518 | 1,784 | 1,001 | 197 |
| Age, years | 58.5 (7.5) | 56.6 (6.7) | 56.8 (6.7) | 56.0 (6.5) |
| Waist, cm | 96.9(9.1) | 94.7(8.5) | 93.3(8.2) | 92.3(9.7) |
| Body Mass Index at 1991, kg/m2 | 25.7(3.1) | 25.2(3.0) | 25.0(2.9) | 25.1(3.0) |
| TV watching, min/d | 83.8(70.9) | 74.8(64.4) | 80.1(65.0) | 76.1(66.4) |
| Slow walking, min/d | 11.4(27.3) | 9.8(26.3) | 8.6(23.6) | 7.8(19.3) |
| Moderate-to-vigorous aerobic activity b, min/d | 26.5(36.9) | 38.0(35.4) | 52.1(42.6) | 58.5(56.1) |
| Weight training, min/d, | 0.0(0.0) | 5.1(2.9) | 17.0(4.3) | 47.0(13.0) |
| Other activities c, min/d | 14.1(29.8) | 9.3(20.7) | 10.6(19.9) | 12.7(20.9) |
| Energy intake, kcals/day | 2055(624) | 2048(591) | 2010(590) | 2012(591) |
| Alcohol intake, grams/day | 11.4(14.7) | 11.7(13.8) | 11.2(13.0) | 10.2(14.9) |
| Sugar-sweetened beverage intake d, servings/d | 0.4(0.7) | 0.4(0.6) | 0.3(0.5) | 0.5(0.9) |
| Trans-fat percent energy | 1.4(0.6) | 1.2(0.5) | 1.1(0.6) | 1.1(0.6) |
| Fiber e, grams/day | 21.5(7.0) | 23.3(7.2) | 24.6(7.7) | 25.2(8.4) |
| Protein, percent energy | 17.3(3.0) | 17.4(2.8) | 17.5(3.0) | 17.6(3.6) |
| Glycemic load | 134(26) | 139(25) | 141(28) | 145(29) |
| Alternative healthy eating index 2010f | 52.0(10.2) | 55.4(10.0) | 57.0(9.9) | 56.9(11.2) |
| Current smoking (%) | 5 | 3 | 3 | 8 |
| Antidepressant use (%) | 4 | 3 | 4 | 3 |
| Sleep (2000), hours/d | 7.2(0.9) | 7.1(0.8) | 7.1(0.8) | 7.1(0.8) |
Values are presented as means ± SD (all such values) except otherwise indicated.
Moderate-to-vigorous aerobic activity included brisk/ very brisk walking; jogging, running, bicycling, lap swimming, tennis, squash or racquetball, calisthenics, rowing, and stair or ski machine.
Other activities included, heavy outdoor work (e.g. digging, chopping) and stair climbing.
Sugar-sweetened beverages include sugar-sweetened carbonated beverages, punch, fruit drinks, lemonade, or ice tea.
Adjusted for total energy intake.
This index is a new measure of diet quality that incorporates current scientific evidence on diet and health. In this analysis, higher indexes indicate better diet quality.
In table 2, an inverse dose-response relationship was apparent between weight training and 12-year WC change even after controlling for potential confounders (model 1) and for BW change (model 2) (both P-trend<0.001). The different PA types in addition to TV watching were shown using 2 statistical models: partition model (Table 3) and isotemporal substitution model (Table 4). In the partition model (model a), total time was partitioned among time spent watching TV and engaging in different PA types. There was less age-associated WC increase with a 20 min/day activity increase; this benefit was significantly stronger for weight training (-0.67 cm, 95%CI=-0.93, -0.41) than for MVAA (-0.33 cm, 95%CI=-0.40, -0.27), other activities (-0.16 cm, 95%CI=-0.28, -0.03), or TV watching (0.08 cm, 95%CI=0.05, 0.12) (Table 3). Therefore, for the same increased activity time, different activity types were associated with different degrees of WC change. All of the substitution models (b through e, Table 4) consistently suggested that weight training had the strongest association with less WC increase. Substituting 20 min/day of weight training was associated with less WC increase if it replaced 20 min/day of TV watching (-0.76 cm, 95%CI=-1.02, -0.50) (model b), MVAA (-0.34 cm, 95%CI=-0.62, -0.07) (model c), or other activities (-0.52 cm, 95%CI=-0.80, -0.23) (model e).
Table 2. Categories of Weight training, and 12-year relative waist change (cm) or body weight change (kg) from 1996-2008 among 10,500 men in the Health Professional Follow-up Study.
| Minutes/day weight training | |||||
|---|---|---|---|---|---|
| None | >0-10 | > 10-25 | >25 | p trend | |
| Median time (minutes/day) | 0 | 4.21 | 21.4 | 42.9 | |
| Waist circumference change (1996-2008) (cm) | |||||
| Age-adjusted model a | --- | -1.05 (-1.36, -0.75) | -1.33 (-1.69, -0.97) | -2.11 (-2.92, -1.30) | <0.001 |
| Multivariable model 1b | --- | -0.69 (-1.03, -0.36) | -0.85 (-1.25, -0.45) | -1.70 (-2.54, -0.86) | <0.001 |
| Multivariable model 2c | --- | -0.33 (-0.59, -0.07) | -0.95 (-1.26, -0.64) | -1.39 (-2.04, -0.73) | 0.02 |
| Body weight change (1996-2008) (kg) | |||||
| Age-adjusted model d | --- | -0.59 (-0.86, -0.31) | -0.49 (-0.81, -0.17) | -0.71 (-1.43, 0.01) | <0.01 |
| Multivariable model 3 e | --- | -0.47 (-0.77, -0.18) | -0.29 (-0.64, 0.06) | -0.45 (-1.20, 0.30) | 0.17 |
| Multivariable model 4f | --- | -0.14 (-0.37, 0.10) | 0.33 (0.04, 0.62) | 0.56 (-0.05, 1.16) | <0.01 |
For the age-adjusted model, it was adjusted for baseline (1996) age (years) and waist circumference (continuous, cm). Data on weight training was presented in 2008 without adjustment for PA at baseline (1996).
Multivariate model 1 is adjusted for the same variables in the age-adjusted model in addition to total average alcohol intake (1994, 2006), (0; >0-<2.5; 2.5-5; >5-10; >10 g/day; missing); sugar-sweetened beverage intake (1994, 2006), (0; >0-0.5; >0.5-1; >1 servings/day); percent energy of trans-fat (1994, 2006), (quintiles); energy-adjusted fibers (1994, 2006) (quintiles g/day), energy-adjusted glycemic load (1994, 2006) (quintiles); smoking (1996, 2008) (never; past; nonsmoker, unknown past history; current; missing), anti-depressant intake (1996, 2008) (never; current; past), percent energy of protein intake, (1994, 2006) (quintiles), sleep duration (2000, 2008) (continuous, hours/day), in addition to slow walking, TV watching, moderate-to-vigorous aerobic activity, and other activities (all in 1996, 2008), and weight training (1996) (all categorical, min/day).
Multivariate model 2 is adjusted for the same variables in multivariate model 1 in addition to weight (1996, 2008) (continuous, kg).
For the body weight change outcome, the age-adjusted model was adjusted for baseline (1996) age (years) and body weight (continuous, kg). Data on weight training was presented in 2008 without adjustment for PA at baseline (1996).
Model 3 is adjusted for the same covariates in model 1 except for waist circumference and in addition to baseline height (m) and weight (kg).
Same as model 3 in addition to waist circumference (1996, 2008) (continuous, cm).
Table 3. Different discretionary activities, per 20 min/day increase, a and 12-year relative change in waist circumference (cm) from 1996-2008 among 10,500 men in the Health Professionals Follow-up Study.
| Variable | TV watching | Moderate-to-vigorous aerobic activity b | Weight training | Other activities b |
|---|---|---|---|---|
| Waist circumference change (1996-2008) (cm) | ||||
| β0 (95%CI) | β1 (95%CI) | β2 (95%CI) | β3 (95%CI) | |
| Crude model c | 0.08 (0.05, 0.12) | -0.33 (-0.39, -0.27) | -0.66 (-0.89, -0.42) | -0.14 (-0.26, -0.03) |
| Multivariate-adjusted model (a) d | 0.08 (0.05, 0.12) | -0.33 (-0.40, -0.27) | -0.67 (-0.93, -0.41) | -0.16 (-0.28, -0.03) |
Each regression coefficient (95% CI) represents a comparison of waist circumference change (in cm) for every 20 min/day increase in the predictor variable, not restricting total PA time, nor controlling the displacement of other activity time.
Moderate-to-vigorous aerobic activity included brisk/ very brisk walking; jogging, running, bicycling, lap swimming, tennis, squash or racquetball, calisthenics, rowing, and stair or ski machine; other activities included heavy outdoor work (e.g. digging, chopping), and stair climbing.
The crude model was adjusted for baseline (1996) age (years) and waist circumference (continuous, cm), and included 4 main exposure mutually adjusted for each other: TV watching, moderate-to-vigorous physical activity, weight training, and other activities (all continuous, min/day) (all in 2008)
The multivariate model (also called partition model) was adjusted for baseline (1996) age (years), waist circumference (continuous, cm), total average alcohol intake (1994, 2006); sugar-sweetened beverage intake (1994, 2006); percent energy of trans-fat (1994, 2006); energy-adjusted fibers (1994, 2006) (g/day), energy-adjusted glycemic load (1994, 2006); smoking (1996, 2008), anti-depressant intake (1996, 2008), percent energy of protein intake, (1994, 2006), sleep duration (2000, 2008), slow walking (1996, 2008), (min/day, continuous), in addition to TV watching, moderate-to-vigorous aerobic activity, weight training, and other activities (all in 1996) (all continuous, min/day).
Table 4. Isotemporal substitution a of activities, per 20 min/day increase, and 12-year relative waist circumference change (cm) from 1996-2008 among 10,500 men in the Health Professional Follow-up Study.
| Analysis Methods | TV watching | Moderate-to-vigorous aerobic exercise | Weight training | Other activities | Total discretionary Time b |
|---|---|---|---|---|---|
|
| |||||
| β0 (95%CI) | β1 (95%CI) | β2 (95%CI) | β3 (95%CI) | β4 (95%CI) | |
|
|
|||||
| Substitution of a 20-min/day of an activity to replace a 30 min/day of TV watching c | |||||
| Substitution model (b) | Dropped | -0.42 (-0.50, -0.34) | -0.76 (-1.02, -0.50) | -0.24 (-0.37, -0.11) | 0.08 (0.05, 0.12) |
|
|
|||||
| Substitution of a 20-min/day of an activity to replace a 30 min/day of moderate-to-vigorous aerobic exercise c | |||||
| Substitution model (c) | 0.41 (0.33, 0.49) | Dropped | -0.34 (-0.62, -0.07) | 0.17 (0.03, 0.31) | -0.33 (-0.39, -0.26) |
|
|
|||||
| Substitution of a 20-min/day of an activity to replace a 30 min/day of weight training c | |||||
| Substitution model (d) | 0.75 (0.49, 1.01) | 0.34 (0.07, 0.62) | Dropped | 0.52 (0.23, 0.80) | -0.67 (-0.93, -0.41) |
|
|
|||||
| Substitution of a 20-min/day of an activity to replace a 30 min/day of other activities c | |||||
| Substitution model (e) | 0.24 (0.11, 0.37) | -0.17 (-0.31, -0.03) | -0.52 (-0.80, -0.23) | Dropped | -0.15 (-0.28, -0.03) |
Each regression coefficient (95% CI) represents a comparison of waist circumference (in cm) for every 20 min/day increase in the predictor variable replacing 20 min/day of the activity that is dropped out of the model.
Total discretionary time” includes total discretionary physical activity and TV watching
All models are adjusted for baseline (1996) age (years) and waist circumference (continuous, cm), total average alcohol intake (1994, 2006); sugar-sweetened beverage intake (1994, 2006); percent energy of trans-fat (1994, 2006); energy-adjusted fibers (1994, 2006), energy-adjusted glycemic load (1994, 2006); smoking (1996, 2008), anti-depressant intake (1996, 2008), percent energy of protein intake, (1994, 2006), sleep duration (2000, 2008), slow walking (1996, 2008) (min/day, continuous); in addition to TV watching, moderate-to-vigorous aerobic activity, weight training, and other activities (all in 1996) (all continuous, min/day). The corresponding physical activity would be dropped in 1996 if it was dropped outside the model in 2008.
We examined the joint association of weight training and MVAA on WC change (P-for-interaction<0.001) (Figure 1). Among men who did no MVAA at all or who did not adhere to the current recommendations for MVAA of >25 min/day, men who engaged in some level of weight training had less increase in WC over 12 years as compared to men who did not do any weight training. Although the combination of MVAA to weight training appeared more beneficial for men who engaged in >0-25 min/day of weight training, no additional benefits on WC change were seen among men who engaged in >25 min/day of weight training upon adding MVAA to their routine. Weight training appeared to be beneficial for WC change regardless of adhering to the MVAA guidelines.
Figure 1.
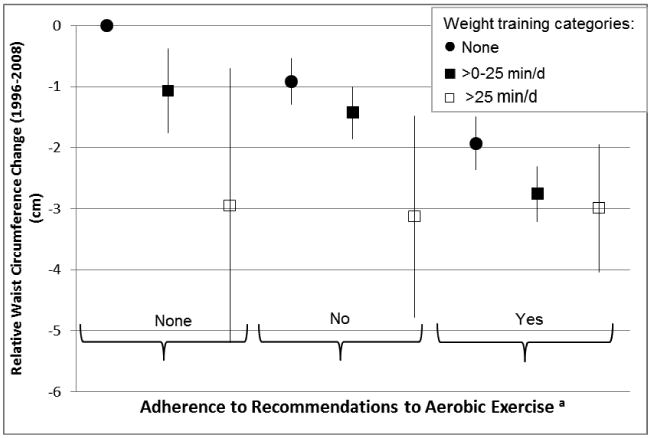
Joint association b of weight training and moderate-to-vigorous aerobic exercise and 12-year relative waist circumference change (cm) from 1996-2008 among 10,500 men in the Health Professional Follow-up Study. Going from left to right, n= 2,701 (reference); 373; 31; 2,009; 1,523; 59; 1,727; 1,909; and 162. Data are estimates of regression coefficients with 95%CIs (vertical line) from multivariate regression models adjusted for baseline (1996) age (years) and waist circumference (continuous, cm), total average alcohol intake (1994, 2006); sugar-sweetened beverage intake (1994, 2006); percent energy of trans-fat (1994, 2006); energy-adjusted fibers (1994, 2006), energy-adjusted glycemic load (1994, 2006); smoking (1996, 2008), anti-depressant intake (1996, 2008), percent energy of protein intake, (1994, 2006), sleep duration (2000, 2008), in addition to slow walking, other activities, and TV watching (all in 1996, 2008) (all categorical, min/day).
a Adherence to the recommendation of MVAA is at least 25 min/day (closest to the 150 minutes per week recommendation).
b P-for-interaction between weight training in 2008 & moderate-to-vigorous aerobic exercise in 2008 <0.001.
Analyses of the BW change (kg) (1996-2008) as an outcome lead to different findings. There was no dose-response relationship between weight training and BW change (P-trend=0.17, Table 2) in the first multivariate model. However, after further controlling for WC change, the dose-response relationship became positive (P-trend<0.01) suggesting an increase in BW, which is most likely due to gain in muscle mass associated with increased weight training (Table 2). The partition multivariate model1 showed that while a 20-min increase in MVAA was significantly associated with less BW gain, a 20 min/day increase in weight training was not significantly associated with less weight gain (Supplemental table 1). However, after further controlling for WC change, for the same comparison, these associations became non-significant for MVAA or even associated with more BW gain for weight training.
The association between resistance training and changes in WC was attenuated but remained significant in the sensitivity analysis where we included participants who developed CVD or diabetes during follow-up. For the main multivariate partition model and after adjusting for BW change, the regression coefficients were as such: (0.05 cm, 95%CI=-0.03, 0.08) for TV watching; (-0.21 cm, 95%CI=-0.26, -0.16) for MVAA; (-0.63 cm, 95%CI=-0.82, -0.45) for weight training; and (-0.19 cm, 95%CI=-0.28, -0.10) for other activities.
Discussion
In this large cohort of men, we found a significant inverse dose-response relationship between weight training and WC change. For similar increased activity time, while weight training had the strongest inverse association with WC change, MVAA had the strongest inverse association with BW change.
Aging is often associated with sarcopenia, the loss of skeletal muscle mass, especially if not accompanied by weight training; hence, relying on anthropometric measures that only reflect BW (which reflects both adipose and lean body mass) is misleading and insufficient for the study of healthy aging. Weight training has been shown to decrease body fat mass in older men with type 2 diabetes (22) and obese women (23), and to increase muscle mass and function especially among older people to counteract sarcopenia (24); however, a recent meta-262 analysis showed that aerobic exercise was more effective than resistance training to reduce visceral adipose tissue (6). Another meta-analysis confirmed the positive influence of weight training on lean body mass in aging adults, with higher volume interventions (# sets/session) resulting in greater increase in lean body mass (25). Furthermore, body fatness based on BMI can be overestimated among individuals who regularly engage in weight training. WC, on the other hand, reflects central adiposity, and could be a better alternative measure than BW to assess the effect of weight training on central adiposity. In these models, weight training had the strongest inverse association with WC change, even more than MVAA, and was associated with no changes in BW or even modest weight gain after controlling for WC change. This could be explained by the fact that weight training increases muscle mass (26). This leads to an increase in BW, while it also decreases fat mass (27) in the long run owing to the increased metabolic rate and total energy expenditure (26), which is mostly reflected in less central adiposity or WC gain. Our results also suggested that combining weight training and MVAA lead to the most optimal results in WC change; however, even men who did not adhere to the MVAA recommendations still benefited if they engaged in some level of weight training.
While weight loss could be the goal for many people to improve overall health and could be achieved by engaging in a cardiovascular activity, not only does it entail fat loss, but also muscle mass loss. This could be disadvantageous, mainly in elderly and also some athletes, whose goal is to spare muscle mass while reducing fat mass. This concomitant fat loss and muscle gain has been shown to prevent and treat many chronic diseases including obesity, diabetes, heart disease, and osteoporosis (28, 29); hence, in the light of our results of weight training on WC change and on MVAA on BW change, it would be ideal to combine MVAA (e.g. jogging/running) with a weight training activity type to achieve this beneficial body composition change. Notably, one should not neglect the advantages of aerobic PA on preventing different chronic diseases such as obesity (30), type 2 diabetes (31), and stroke among others. Concordant with the present observations, a randomized controlled trial on the effect of strength and/or endurance training for 21 weeks in older men (40-65 years) showed that while both training methods decreased body fat, only the strength training group had increased lean mass (33). The authors concluded that combined training was of greater value than each alone to optimize body composition among older men.
Although the relationship between PA and WC or BW change seems to depend on total energy expenditure where activity intensity could compensate for activity duration as shown in our previous findings (30), the relationship between weight training per se and these outcomes seems to be more complex and to require a longer follow-up to observe favorable changes. According to the coding scheme of physical activities by rate of energy expenditure (34), the corresponding intensities are 8-9 METs for jogging, 10-11 METS for running, 3 METs for light weight lifting, and 6 METs for vigorous weight lifting. Despite the fact that greater energy is spent engaging in 20 min/day of MVAA than weight training, our data suggested that engaging in weight training over the long term (as engaged in among participants in this cohort) would lead to more favorable WC change than in MVAA. In other words, to use time efficiently and obtain a better WC change, weight training appears to be the best choice among other activities, despite the non-significant change in BW; however, it is unknown, if total PA energy expenditure is controlled for in this comparison. Our findings could be explained by the effect of intense weight training exercise on greater Excess Post-exercise Oxygen Consumption (EPOC) as compared with aerobic training (35). This difference would lead to an extended (up to 48 hours) higher energy expenditure at rest time, not only in between exercise sets, but also after exercise (36). Another potential explanation is the shift in substrate utilization from carbohydrates (mostly used during unaerobic training such as weight training) to lipid oxidation (mostly used during aerobic training) due to an induced-training adaptation involving increased mitochondrial content in the muscle (37), so that short-term aerobic responses could result from long-term anaerobic training.
Strengths include the large sample and the long follow-up (12 years). Repeated measurements on a wide variety of potential confounding variables allowed for better control of these variables. Finally, we assessed a change in the exposure in relation to a change in the outcome, which is a useful way for reducing –but not totally eliminating--reverse causation bias.
Our study participants were mostly white, which limits the generalizability to women and men of other ethnic background. However, we believe the benefits of strength straining could also apply to elderly women as reported elsewhere (25). In addition, these findings may not be generalizable to men with chronic diseases as we only included healthy participants; however, a sensitivity analysis showed similar results when we included participants who developed CVD or diabetes during follow-up. Also, we did not have data on body composition to further elucidate our findings but our variables on WC and BW have been previously validated. Further, we were not able to explore the importance of the volume or frequency of reported weight training as only weekly non-specific weight training was reported. Weight training intensity was not assessed, which might have independent effects on WC.
Our findings suggest that while long-term weight training is associated with less WC increase, MVAA is associated with less BW gain in healthy men. Further studies are needed among women, older men, and other ethnic groups to compare the frequency, volume, and intensity of weight training on WC change.
Supplementary Material
Supplemental Table 1: Different discretionary activities, per 20 min/day increase, a and 12-year relative change in body weight (kg) from 1996-2008 among 10,500 men in the Health Professionals Follow-up Study
(1) What is already known about this subject?
There is sarcopenia (loss of muscle mass) associated with aging accompanied with an age-associated waist circumference (WC) increase.
Anthropometric measures such as body weight (BW) or body mass index (BMI) among older adults (which reflects both adipose and lean body mass) are misleading and insufficient for the study of healthy aging.
The results of weight training and WC change are mixed.
(2) What does this study add?
Despite the mixed results of weight training on WC, long-term weight training was the most associated with less WC increase as compared to aerobic activities among healthy men.
Substituting 20 min/day of weight training for any other discretionary activity had the strongest inverse association with WC change.
While weight training is associated with less WC increase, moderate to vigorous aerobic exercise is associated with less BW gain in healthy men
Acknowledgments
RM, ER and FH collected data. RM, FH, WW, and EG provided statistical expertise. RM analyzed the data and wrote the first draft. All authors contributed to results' interpretation, manuscript's revision and approval.
Funding: The study was supported by National Institutes of Health grants CA55075, DK58845, P30 DK46200, UM1 CA166752. The funding sources were not involved in data collection, data analysis, manuscript writing and publication
Footnotes
Conflict of interest: None
References
- 1.Melton LJ, 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 2.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz KH, Jensen MD, Kugler KC, Jeffery RW, Leon AS. Strength training for obesity prevention in midlife women. Int J Obes Relat Metab Disord. 2003;27:326–333. doi: 10.1038/sj.ijo.0802198. [DOI] [PubMed] [Google Scholar]
- 5.Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 2012;113:1831–1837. doi: 10.1152/japplphysiol.01370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68–91. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Deldin AR, White D, Kim Y, Libman I, Rivera-Vega M, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305:E1222–1229. doi: 10.1152/ajpendo.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, Jensen MD. Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am J Clin Nutr. 2007;86:566–572. doi: 10.1093/ajcn/86.3.566. [DOI] [PubMed] [Google Scholar]
- 9.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grontved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med. 2012;172:1306–1312. doi: 10.1001/archinternmed.2012.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. Physical activity guidelines Advisory Committee Report. Wahington, DC: 2008. Available from: http://www.health.gov/paguidelines/guidelines/chapter5.aspx. [Google Scholar]
- 12.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. Jama. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 14.Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz G, Willett W, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr. 2003;78:719–727. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 15.Kruger J, Blanck HM, Gillespie C. Dietary practices, dining out behavior, and physical activity correlates of weight loss maintenance. Prev Chronic Dis. 2008;5:A11. [PMC free article] [PubMed] [Google Scholar]
- 16.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of a expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115:223–230. doi: 10.1093/oxfordjournals.aje.a113294. [DOI] [PubMed] [Google Scholar]
- 19.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170:519–527. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekary RA, Lucas M, Pan A, Okereke OI, Willett WC, Hu FB, et al. Isotemporal Substitution Analysis for Physical Activity, Television Watching, and Risk of Depression. Am J Epidemiol. 2013 doi: 10.1093/aje/kws590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibanez J, Izquierdo M, Arguelles I, Forga L, Larrion JL, Garcia-Unciti M, et al. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662–667. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- 23.Ibanez J, Izquierdo M, Martinez-Labari C, Ortega F, Grijalba A, Forga L, et al. Resistance training improves cardiovascular risk factors in obese women despite a significative decrease in serum adiponectin levels. Obesity (Silver Spring) 2010;18:535–541. doi: 10.1038/oby.2009.277. [DOI] [PubMed] [Google Scholar]
- 24.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 25.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly JE, Jakicic JM, Pronk N, Smith BK, Kirk EP, Jacobsen DJ, et al. Is resistance training effective for weight management? Evidence-based Preventive Medicine. 2003;I:1–9. [Google Scholar]
- 27.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol (1985) 2000;89:977–984. doi: 10.1152/jappl.2000.89.3.977. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 29.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 30.Mekary RA, Feskanich D, Malspeis S, Hu FB, Willett WC, Field AE. Physical activity patterns and prevention of weight gain in premenopausal women. Int J Obes (Lond) 2009;33:1039–1047. doi: 10.1038/ijo.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 32.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, et al. Physical activity and risk of stroke in women. Jama. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 33.Sillanpaa E, Hakkinen A, Nyman K, Mattila M, Cheng S, Karavirta L, et al. Body composition and fitness during strength and/or endurance training in older men. Med Sci Sports Exerc. 2008;40:950–958. doi: 10.1249/MSS.0b013e318165c854. [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 35.Thornton MK, Potteiger JA. Effects of resistance exercise bouts of different intensities but equal work on EPOC. Med Sci Sports Exerc. 2002;34:715–722. doi: 10.1097/00005768-200204000-00024. [DOI] [PubMed] [Google Scholar]
- 36.Borsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003;33:1037–1060. doi: 10.2165/00007256-200333140-00002. [DOI] [PubMed] [Google Scholar]
- 37.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Different discretionary activities, per 20 min/day increase, a and 12-year relative change in body weight (kg) from 1996-2008 among 10,500 men in the Health Professionals Follow-up Study


