This phase I/II study in advanced renal cell carcinoma demonstrated that lenalidomide plus sunitinib produced unacceptable toxicity at the protocol-defined maximum tolerated dose, precluding further development. Correlative studies demonstrated modulation of the immune cell repertoire, including alteration in B cell and T cell subsets.
Keywords: sunitinib, lenalidomide, metastatic renal cell carcinoma, phase I/II, maximum tolerated dose
Abstract
Background
This phase I/II study was conducted to determine the maximum tolerated dose (MTD), safety, and efficacy of lenalidomide plus sunitinib in metastatic renal cell carcinoma (RCC) patients.
Patients and methods
Patients with histologically confirmed, metastatic RCC were treated with 10 mg/day lenalidomide plus 37.5 mg/day sunitinib, orally in 21-day cycles. Doses were escalated to determine the MTD in phase I, with additional patients planned at this dose in phase II. Primary end points were MTD and response rate.
Results
Sixteen patients received a median of 2, 3, and 5 cycles in cohort 1 [lenalidomide 10 mg (days 1–21) and sunitinib 37.5 mg (days 1–21)], cohort 2 [lenalidomide 10 mg (days 1–21) and sunitinib 37.5 mg (days 1–14)], and cohort 3 [lenalidomide 15 mg (days 1–21) and sunitinib 37.5 mg (days 1–14)], respectively. Median treatment durations were 41, 63, and 97 days for lenalidomide; and 41, 57, and 97.5 days for sunitinib. The MTD was found to be continuous dosing of lenalidomide 10 mg/day plus sunitinib 37.5 mg/day for 14 of 21 days. Dose-limiting toxicities included neutropenia, leukopenia, thrombocytopenia, asthenia, atrial fibrillation, and increased transaminases. The most frequent grade 3–4 treatment-emergent adverse events were hematologic, including neutropenia and leukopenia. One patient achieved partial response, and seven had stable disease of which three were confirmed at subsequent tumor assessments. B cells and several T-cell subsets were modulated versus baseline.
Conclusion
The dose schedules of lenalidomide and sunitinib evaluated in this study were not well tolerated; cumulative toxicity precluded enrollment at the MTD.
introduction
Factors regulating angiogenesis, such as vascular endothelial growth factor (VEGF), play an important role in the pathogenesis of renal cell carcinoma (RCC) [1].
Sunitinib inhibits cellular signaling by targeting multiple receptor tyrosine kinases, including VEGF receptors, KIT, and Fms-like tyrosine kinase 3 [2]. The inhibition of these targets leads to reduced tumor vascularization, cancer cell death, and tumor shrinkage. Sunitinib has demonstrated efficacy and tolerability in the treatment of RCC [3], and is a first-line treatment option in metastatic RCC patients [4]. It is hypothesized that the judicious use of novel combination therapies can enhance treatment effect and delay or preclude the development of treatment resistance [5].
Lenalidomide is a pleiotropic pathway modifier that has demonstrated antiangiogenic and antitumor activity with a favorable safety profile in advanced RCC [6, 7]. In a phase II study of single-agent lenalidomide at 25 mg daily in patients with refractory metastatic RCC, the overall response rate was 10% and median overall survival ≥17 months [7]. In vitro, both lenalidomide and sunitinib significantly inhibited tube formation in endothelial cells and outgrowth of a rat aortic-ring model by creating an inhospitable microenvironment [8]. Combining these two agents led to additive inhibition of angiogenesis [9].
This phase I/II study aimed to determine the maximum tolerated dose (MTD) of lenalidomide in combination with sunitinib, and to evaluate the safety and efficacy of this combination in patients with advanced or metastatic RCC. To maximize the combined antiangiogenic, immunomodulatory, and tumor microenvironment effects of the combination, lenalidomide and sunitinib were administered continuously. To minimize toxicity, the sunitinib starting dose was 37.5 mg; lenalidomide was dose escalated until the MTD was reached.
patients and methods.
This study (ClinicalTrials.gov identifier, NCT00975806) was approved by the institutional review boards of the participating sites, and was conducted in accordance with the Declaration of Helsinki and all applicable regulatory guidelines. The primary objective in phase I of the study was identification of the MTD of lenalidomide administered in combination with sunitinib. Secondary and exploratory objectives included evaluation of safety, tolerability, antitumor activity, plasma pharmacokinetics, and assessment of biomarkers. All patients signed an informed consent form before inclusion.
study population
Patients aged ≥18 years with histologically confirmed unresectable or metastatic RCC were eligible to participate. RCC of any histological subtype was allowed in phase I, whereas the phase II portion would include patients with clear-cell component RCC only. Documented clinical or radiographic evidence of unresectable or metastatic disease was mandatory. Patients were required to have an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of ≤1 and to be chemotherapy-naive. In phase I, patients were allowed prior treatment with tyrosine kinase inhibitors or bevacizumab, as long as treatment was completed 14 or 28 days before study entry, respectively. Furthermore, patients were required to have no active brain metastases and adequate organ function defined as: absolute neutrophil count ≥1.5 × 103/mm3; hemoglobin ≥9 g/dl; platelet count ≥100 × 103/µl; serum creatinine ≤2.5 mg/dl; aspartate transaminase and alanine transaminase ≤3× ECOG PS (ULN) or ≤5× ULN in patients with liver metastases; and total bilirubin ≤2.0 mg/dl. Any patient with, or with a history of, uncontrolled hypertension (blood pressure >160/90 mmHg), venous thromboembolism, arterial thrombotic events, unstable angina, myocardial infarction, clinically significant peripheral artery disease, congestive heart failure, ≤50% left ventricular ejection fraction, or peripheral neuropathy was excluded.
study design and treatment schedule
Dose escalation followed a standard ‘3 + 3’ dose escalation design [9]. Initially, lenalidomide and sunitinib were both administered orally, on a continuous once-daily schedule for each 21-day cycle. The starting dose of lenalidomide was 10 mg/day, with potential for escalation to 15, 20, and 25 mg/day. If the initial dose level was deemed intolerable, the protocol allowed the same planned escalation of lenalidomide in combination with sunitinib administered on an intermittent schedule: days 1–14 of each 21-day cycle. Treatment would continue until documented tumor progression, unacceptable toxicity, death, or treatment discontinuation for any other reason.
The MTD was defined as the highest dose level at which ≤1 of 6 patients experienced a dose-limiting toxicity (DLT) during the first cycle of therapy. DLTs were defined as: any drug-related grade 3–4 nonhematologic adverse event (AE) occurring in cycle 1 and lasting ≥14 days; febrile neutropenia; grade 4 neutropenia lasting for ≥7 days; or grade 4 thrombocytopenia. Toxicities that did not meet the above criteria, but resulted in the inability to continue treatment at the same dose beyond cycle 2 were considered ‘functional DLTs’.
If the MTD was exceeded at any dose level, up to six patients were to be enrolled into the level immediately below the dose level that exceeded the MTD. Once the MTD had been established, up to 50 additional patients were to be enrolled into phase II of the study at the MTD dose level.
biomarker analysis
Biomarkers were analyzed based on the biomarker evaluable (BE) population. The BE population included all patients who received one or more doses of study drug. Blood samples were analyzed by ICON, a central immunoflow cytometry laboratory, using FCS Express analysis software (DeNovo Software Clinical Edition version 3.00.0601; Los Angeles, CA). Circulating endothelial cells were processed using the CellSearch® Circulating Endothelial Kit (Veridex LLC; Raritan, NJ).
As systemic corticosteroids are known to abrogate the immunomodulatory effects of lenalidomide on T cells and NK cells [10], all descriptive and inferential biomarker analyses were carried out both for the total BE population and for the group of patients who did not receive concomitant treatment with systemic corticosteroids. The biomarker data were pooled for analysis because of the small number of patients enrolled in the phase I portion before study termination. Due to the decision to terminate the study prematurely, the exploratory analyses of pharmacokinetics and cytokines/immune markers were not carried out.
statistical analysis
There was no hypothesis testing for the phase I portion of the study. The statistical analyses were descriptive and exploratory. Data from all patients receiving one or more doses of study drug were included in the safety analyses. AEs were classified using the Medical Dictionary for Regulatory Activities classification system. AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0 [11]. The sample size of 50 patients for phase II would have ≥80% power to claim the response rate is >20% at the one-sided 2.5% level when the true response rate is at 40%. Tumor response analysis would be carried out after all patients completed six or more cycles of therapy or discontinued the study.
Biomarker values collected during the study were compared with the baseline value using the paired t-test. The method of Benjamini and Hochberg [12] was used to control the false discovery rate (FDR).
results
baseline characteristics and treatment
A total of 16 patients were enrolled with a median age of 58 years (range 35–78 years). Of these patients, 50% were male and 75% had an ECOG PS score of 0 (supplementary Table S1, available at Annals of Oncology online). None of the patients received prior radiation therapy. Patients were enrolled into three cohorts, and received a median of 2.0, 3.0, and 5.0 cycles in cohorts 1, 2, and 3, respectively. Median durations of treatment were 41, 63, and 97 days for lenalidomide; and 41, 57, and 97.5 days for sunitinib.
dose-limiting toxicities
The number of patients enrolled and incidences of DLTs per cohort are presented in supplementary Table S2, available at Annals of Oncology online. The initial cohort of continuous daily dosing of both lenalidomide 10 mg and sunitinib 37.5 mg on days 1–21 of each 21-day cycle, exceeded the MTD as two patients experienced DLTs. The protocol allowed for dose escalation to proceed with intermittent dosing of sunitinib (days 1–14 of each 21-day cycle); three patients were enrolled into cohort 2 (lenalidomide 10 mg on days 1–21 plus sunitinib 37.5 mg on days 1–14 of each 21-day cycle) with no DLTs. Cohort 3 (lenalidomide 15 mg on days 1–21 plus sunitinib 37.5 mg on days 1–14 of each 21-day cycle) also exceeded the MTD, with three of the four patients enrolled experiencing DLTs. Therefore, enrollment into cohort 2 was expanded with four additional patients (one of the three patients enrolled as per protocol discontinued before completing cycle 1 and was replaced), none of whom experienced DLTs.
Of the 11 occurrences of DLT, only one (grade 3 asthenia cohort 1) met the protocol-specified criteria for a DLT. All others were considered to be functional DLTs. The MTD was determined to be 10 mg lenalidomide on days 1–21 in combination with 37.5 mg sunitinib given on days 1–14 of each 21-day cycle.
safety
All 16 patients were assessable for safety analysis and experienced one or more treatment-emergent AEs (TEAEs) that were attributed by the investigator to lenalidomide and/or sunitinib. The TEAEs most commonly attributed to both study drugs were fatigue (87.5%), dysgeusia (56.3%), neutropenia (50%), and diarrhea (50% for lenalidomide and 62.5% for sunitinib).
At least one grade 3 AE was reported by 60%, 57%, and 25% of patients in cohorts 1, 2, and 3, respectively (Table 1). At least one grade 4 AE was reported by 40%, 14%, and 75% of patients in cohorts 1, 2, and 3, respectively. One patient (cohort 2) experienced a grade 5 AE of general physical health deterioration resulting in death due to disease progression.
Table 1.
Highest-grade TEAEs occurring in two or more patients (safety population), regardless of relationship to study drug
| n (%) | Cohort 1 (N = 5) |
Cohort 2 (N = 7) |
Cohort 3 (N = 4) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | Total | G1 | G2 | G3 | G4 | Total | G1 | G2 | G3 | G4 | Total | |
| Patients with ≥1 TEAE | 0 | 0 | 3 (60) | 2 (40) | 5 (100) | 1 (14) | 0 | 4 (57) | 1 (14) | 7 (100) | 0 | 0 | 1 (25) | 3 (75) | 4 (100) |
| Hematologic | |||||||||||||||
| Neutropenia/neutrophil count decreased | 0 | 1 (20) | 1 (20) | 1 (20) | 3 (60) | 0 | 0 | 3 (43) | 1 (14) | 4 (57) | 0 | 0 | 1 (25) | 3 (75) | 4 (100) |
| Leukopenia | 0 | 0 | 2 (40) | 0 | 2 (40) | 0 | 0 | 1 (14) | 0 | 1 (14) | 0 | 0 | 3 (75) | 0 | 3 (75) |
| Anemia | 2 (40) | 0 | 1 (20) | 0 | 3 (60) | 1 (14) | 1 (14) | 1 (14) | 0 | 3 (43) | 1 (25) | 0 | 0 | 0 | 1 (25) |
| Thrombocytopenia | 1 (20) | 0 | 0 | 1 (20) | 2 (40) | 0 | 1 (14) | 1 (14) | 0 | 2 (29) | 1 (25) | 0 | 0 | 0 | 1 (25) |
| Nonhematologic | |||||||||||||||
| Vomiting | 2 (40) | 0 | 1 (20) | 0 | 3 (60) | 0 | 2 (29) | 1 (14) | 0 | 3 (43) | 2 (50) | 0 | 0 | 0 | 2 (50) |
| Nausea | 2 (40) | 1 (20) | 1 (20) | 0 | 4 (80) | 1 (14) | 2 (29) | 1 (14) | 0 | 4 (57) | 2 (50) | 1 (25) | 0 | 0 | 3 (75) |
| Dehydration | 1 (20) | 0 | 2 (40) | 0 | 3 (60) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperkalemia | 0 | 0 | 1 (20) | 0 | 1 (20) | 0 | 0 | 1 (14) | 0 | 1 (14) | 1 (25) | 0 | 0 | 0 | 1 (25) |
| Hyperuricemia | 0 | 0 | 0 | 1 (20) | 1 (20) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (25) |
G, grade.
Nine deaths occurred during the study: four each in cohorts 1 and 2; and one in cohort 3. All deaths were due to disease progression. A 36-year-old male patient enrolled in cohort 1, died due to cardiorespiratory arrest with progressive disease 349 days after the first, and 161 days after the last dose of the study drug. With the exception of the patient with a grade 5 AE of general physical health deterioration resulting in death due to disease progression mentioned above, all deaths occurred after discontinuation and >30 days after the last dose of either study drug.
Nine patients experienced one or more serious AEs: three in cohort 1; four in cohort 2; and two in cohort 3. The majority of these AEs were grade 3, and the frequency was similar across the three dosing cohorts. Two patients in each cohort experienced TEAEs that led to treatment discontinuation of both lenalidomide and sunitinib. Neutropenia was the most common TEAE that led to discontinuation of both study drugs (one patient in each cohort).
Fifteen patients had TEAEs that led to dose reductions and/or interruptions of lenalidomide, and 13 patients had TEAEs that led to dose reductions and/or interruptions of sunitinib. The majority of TEAEs leading to dose reductions occurred in cycles 1 or 2; the most common of these were neutropenia (75% for lenalidomide and 69% for sunitinib) and leukopenia (25% for lenalidomide and 19% for sunitinib).
All patients in each cohort required one or more dose reductions and/or interruptions of lenalidomide and sunitinib. However, patients in cohorts 1 and 3 required more frequent dose reductions and/or interruptions than patients in cohort 2, suggesting that daily administration of sunitinib and a 15-mg lenalidomide dose led to more dose reductions and interruptions.
The cumulative frequency and severity of toxicities, as well as the need for frequent dosing-modifications, at each dose level were notably higher than expected during all cycles and at all dose levels of study phase I. A consensus decision was made by the investigators and sponsor not to initiate phase II due to the inability to deliver an optimal long-term phase II dose and schedule with this combination. The study was terminated when all phase I patients had discontinued treatment and a 28-day follow-up had been completed. Seven patients discontinued due to disease progression, five due to AEs (grade 3 neutropenia in three patients, grade 3 elevated transaminases in one patient, and grade 3 pancreatitis in one patient), one withdrew consent, one experienced ECOG PS deterioration resulting in death due to disease progression, and two discontinued for other reasons [one investigator decision after poor tolerance due to laboratory toxicities (including dose-limiting neutropenia) and one indeterminate brain lesion on magnetic resonance imaging]. Two patients stayed on study for >6 months (7 and 11 months).
efficacy
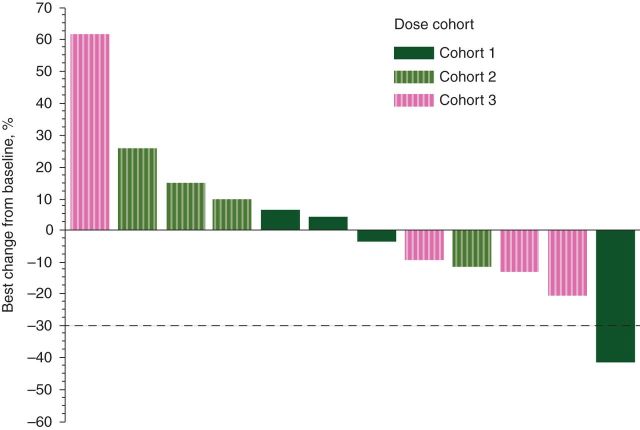
The percentage of tumor burden reduction is shown in Figure 1. One patient (cohort 1) achieved a Response Evaluation Criteria in Solid Tumors (RECIST) defined [13] as confirmed partial response for a duration of >6 months (through to cycle 10). No tumor assessments were carried out for this patient at discontinuation. Seven patients had a RECIST-defined stable disease, of which three were confirmed at subsequent tumor assessments.
Figure 1.
Best percentage change from baseline in total length of target lesion. Figure shows the results for 12 patients who had both baseline and postbaseline total length of target lesion values.
biomarker analysis
At both time points (day 1 of cycles 2 and 4), five cell subsets were decreased compared with baseline, both in terms of absolute and percentage values: B cells; effector T-helper cells; naïve T-helper cells; total naïve T-cytotoxic/suppressor cells; and total naïve T-helper cells (supplementary Figure S1, available at Annals of Oncology online). The analysis was also conducted excluding three patients who received concomitant treatment with systemic corticosteroids. In this subgroup analysis, only one subset (all B cells) was modulated with FDR ≤0.2 and decreased compared with baseline at both time points, in terms of both absolute and percentage count (data not shown).
Median levels of circulating endothelial cells per 4 ml blood were 59 (range 11–201) at baseline, 30 (range 11–133) at day 1 of cycle 2, and 58 (range 7–83) at day 1 of cycle 4. Due to the small sample size and the fact that 3 of the 16 BE patients had no baseline values, data on circulating endothelial cells were not further evaluated.
discussion
The present study demonstrated that treatment with lenalidomide in combination with sunitinib was not feasible in patients with metastatic RCC. The MTD was determined to be 10 mg/day of lenalidomide for 21 days of each 21-day cycle in combination with 37.5 mg/day sunitinib for days 1–14 of each 21-day cycle. However, the frequency and severity of toxicities, and the need for frequent dose modifications observed were notably higher than anticipated and were evident during all cycles across all dose levels. The study was therefore terminated early.
Our study enrolled a total of 16 patients; of which 50% were female and only 37.5% showed pulmonary lesions at baseline. Based on clinical practice, we would have expected a lower female enrollment and a higher percentage of baseline pulmonary lesions; this observed discrepancy is probably due to the low number of enrolled patients in the study.
With only one patient achieving a PR, the response rate is lower than expected for patients treated with sunitinib. This is probably due to the limited exposure of these patients to sunitinib as a result of the frequency and severity of toxicities.
Overlapping nonhematologic and hematologic toxicities associated with both lenalidomide and sunitinib precluded the ability to administer these two drugs in combination [3, 6, 7, 14]. Fatigue, nausea, diarrhea, dysgeusia, vomiting, and neutropenia were common. The most frequent grade 3–4 TEAEs were neutropenia and leukopenia, and these were the most common cause for dose modification. The rates of AEs observed were much higher than expected with sunitinib monotherapy in RCC [5], suggesting that the addition of lenalidomide to sunitinib exacerbated the incidence of hematologic toxicities.
Despite the high rates of neutropenia, there were no cases of neutropenic fever and no neutropenia-associated infections; infections in general were rare during the study. Deep vein thrombosis, a known toxicity associated with lenalidomide, occurred in one patient; whereas pulmonary embolisms did not occur in this study. Six patients (37.5%) received low-dose aspirin (81 mg) prophylaxis while on trial. There were also no incidences of hand–foot syndrome (HFS) in the present study. HFS has been shown to occur frequently with sunitinib treatment in several studies [3], most commonly near the end of a 4-week treatment period at 50 mg/day. Its absence in our study is most likely due to the lower sunitinib dose used (37.5 mg/day compared with the standard 50 mg/day) and the relatively short exposure time.
Both lenalidomide and sunitinib are known to affect levels of circulating endothelial cells. In a novel xenograft mouse model of human blastic NK-cell lymphoma/blastic plasmacytoid dendritic cell neoplasm, treatment with lenalidomide resulted in a significant decrease in the number of circulating endothelial cells and circulating progenitor cells [15]. The number of circulating endothelial cells is known to increase in patients with RCC who are treated with sunitinib [16], and is thought to result from targeting of immature tumor vessels. Unfortunately, interpretation of the analysis of circulating endothelial cells in this study was limited by the fact that only 13 of the 16 patients had a baseline value, 2 patients had one or more on-study values but no baseline value, and 1 patient had no circulating endothelial cell data.
CD45+ T cells, B cells, and NK cells are known targets of the immunomodulatory activity of lenalidomide, which co-stimulates cytotoxic T cells and T-helper cells, inhibits regulatory T cells, stimulates antibody production by B cells, and enhances NK-cell immunity via induction of interleukin-2 transcription and secretion in T cells [17]. In this study, five cell subsets were modulated compared with baseline: B cells decreased; effector T-helper cells decreased; naïve T-helper cells decreased; total naïve T-cytotoxic/suppressor cells decreased; and total naïve T-helper cells decreased. Most of these findings seem to be inconsistent with the known immunomodulatory activity of lenalidomide monotherapy and contrary to an effective antitumor immune response. Due to the small sample size, there was insufficient power to correlate the observed changes in CD45+ immune populations and circulating endothelial cells with clinical responses. Thus, the significance of these immune cell changes is currently unknown.
Combined targeted agents in RCC have been investigated by several groups, and toxicity has been a common occurrence [18, 19]. Even where phase I studies have identified ‘tolerable’ dosing regimens, dose reductions—usually of each drug—have been mandated, and have still resulted in excessive toxicity relative to monotherapy. The most common observation has been that typically mild adverse effects associated with one drug are exacerbated by the addition of the second drug [20]. The challenge of investigating combination therapies in RCC is also illustrated by the phase III INTORACT trial and the phase II RECORD-2 trial, which have shown no advantage of bevacizumab combined with temsirolimus or everolimus over bevacizumab and interferon in patients with metastatic RCC [21, 22].
In conclusion, the dose schedules of lenalidomide and sunitinib evaluated in this study were not well tolerated in patients with metastatic RCC. The protocol-defined MTD of this combination incorporated doses of each agent below those recommended as monotherapy. Even at these lower doses, cumulative toxicity resulted in treatment modifications during later cycles, limiting the ability to deliver adequately dosed treatment. Based on these findings and the limited efficacy, planned enrollment to the phase II portion was halted, and further investigation of this combination in patients with RCC is unwarranted.
funding
This work was supported (no grant numbers apply) by Celgene Corporation, Summit, NJ, USA. BRi receives institutional research funding from Pfizer. JAG received institutional research funding from Celgene and Pfizer.
disclosure
BRi serves as a paid consultant to Pfizer. JAG is a paid consultant for Pfizer. SL, AF, RB, and UJ are Celgene employees and stockholders. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Medical writing services were provided by Kim Grootscholten, of Excerpta Medica BV, funded by Celgene Corporation. The authors are fully responsible for content and editorial decisions for this manuscript.
references
- 1.Turner KJ, Moore JW, Jones A, et al. Expression of hypoxia-inducible factors in human renal cancer: relationship to angiogenesis and to the von Hippel-Lindau gene mutation. Cancer Res. 2002;62:2957–2961. [PubMed] [Google Scholar]
- 2.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer v2.2014. http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. (March 2014, date last accessed) [DOI] [PubMed]
- 5.Cho DC, Atkins MB. Future directions in renal cell carcinoma: 2011 and beyond. Hematol Oncol Clin North Am. 2011;25:917–935. doi: 10.1016/j.hoc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Dreicer R, Rini BI, et al. Phase II study of lenalidomide in patients with metastatic renal cell carcinoma. Cancer. 2006;107:2609–2616. doi: 10.1002/cncr.22290. [DOI] [PubMed] [Google Scholar]
- 7.Amato RJ, Hernandez-McClain J, Saxena S, Khan M. Lenalidomide therapy for metastatic renal cell carcinoma. Am J Clin Oncol. 2008;31:244–249. doi: 10.1097/COC.0b013e31815e451f. [DOI] [PubMed] [Google Scholar]
- 8.Blansfield JA, Caragacianu D, Alexander HR, III, et al. Combining agents that target the tumor microenvironment improves the efficacy of anticancer therapy. Clin Cancer Res. 2008;14:270–280. doi: 10.1158/1078-0432.CCR-07-1562. [DOI] [PubMed] [Google Scholar]
- 9.Schneiderman MA. Mouse to man: statistical problems in bringing a drug to clinical trial. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; 1967. pp. 855–866. [Google Scholar]
- 10.Gandhi AK, Kang J, Capone L, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. (3 March 2014, date last accessed)
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Patel PH, Kondagunta GV, Schwartz L, et al. Phase II trial of lenalidomide in patients with metastatic renal cell carcinoma. Invest New Drugs. 2008;26:273–276. doi: 10.1007/s10637-007-9107-y. [DOI] [PubMed] [Google Scholar]
- 15.Agliano A, Martin-Padura I, Marighetti P, et al. Therapeutic effect of lenalidomide in a novel xenograft mouse model of human blastic NK cell lymphoma/blastic plasmacytoid dendritic cell neoplasm. Clin Cancer Res. 2011;17:6163–6173. doi: 10.1158/1078-0432.CCR-11-0212. [DOI] [PubMed] [Google Scholar]
- 16.Vroling L, van der Veldt AA, de Haas RR, et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis. 2009;12:69–79. doi: 10.1007/s10456-009-9133-9. [DOI] [PubMed] [Google Scholar]
- 17.Tageja N. Lenalidomide—current understanding of mechanistic properties. Anticancer Agents Med Chem. 2011;11:315–326. doi: 10.2174/187152011795347487. [DOI] [PubMed] [Google Scholar]
- 18.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina AM, Feldman DR, Voss MH, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1868–1876. doi: 10.1002/cncr.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaelson MD. Combination of targeted agents in metastatic renal cell carcinoma: a path forward or a dead-end street? Cancer. 2012;118:1744–1746. doi: 10.1002/cncr.26427. [DOI] [PubMed] [Google Scholar]
- 21.Rini BI, Bellmunt J, Clancy J, et al. Randomized phase IIIb trial of temsirolimus and bevacizumab versus interferon and bevacizumab in metastatic renal cell carcinoma: results from INTORACT. Ann Oncol. 2012;23(Suppl 9) doi: 10.1200/JCO.2013.50.5305. abstr LBA21_PR. [DOI] [PubMed] [Google Scholar]
- 22.Ravaud A, Barrios C, Anak Ö, et al. Randomized phase II study of first-line everolimus (EVE) + bevacizumab (BEV) versus interferon alfa-2a (IFN) + BEV in patients (pts) with metastatic renal cell carcinoma (MRCC): RECORD-2. Ann Oncol. 2012;23(Suppl 9) doi: 10.1093/annonc/mdv170. abstr 783O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.