Abstract
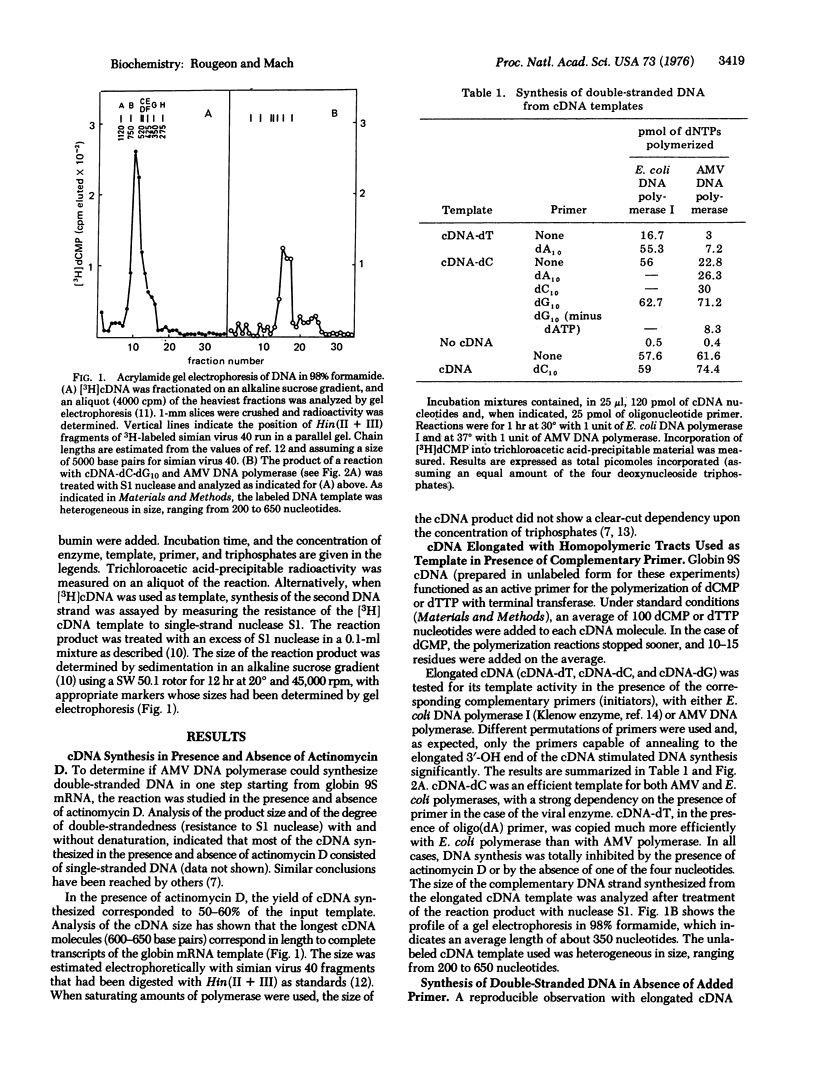
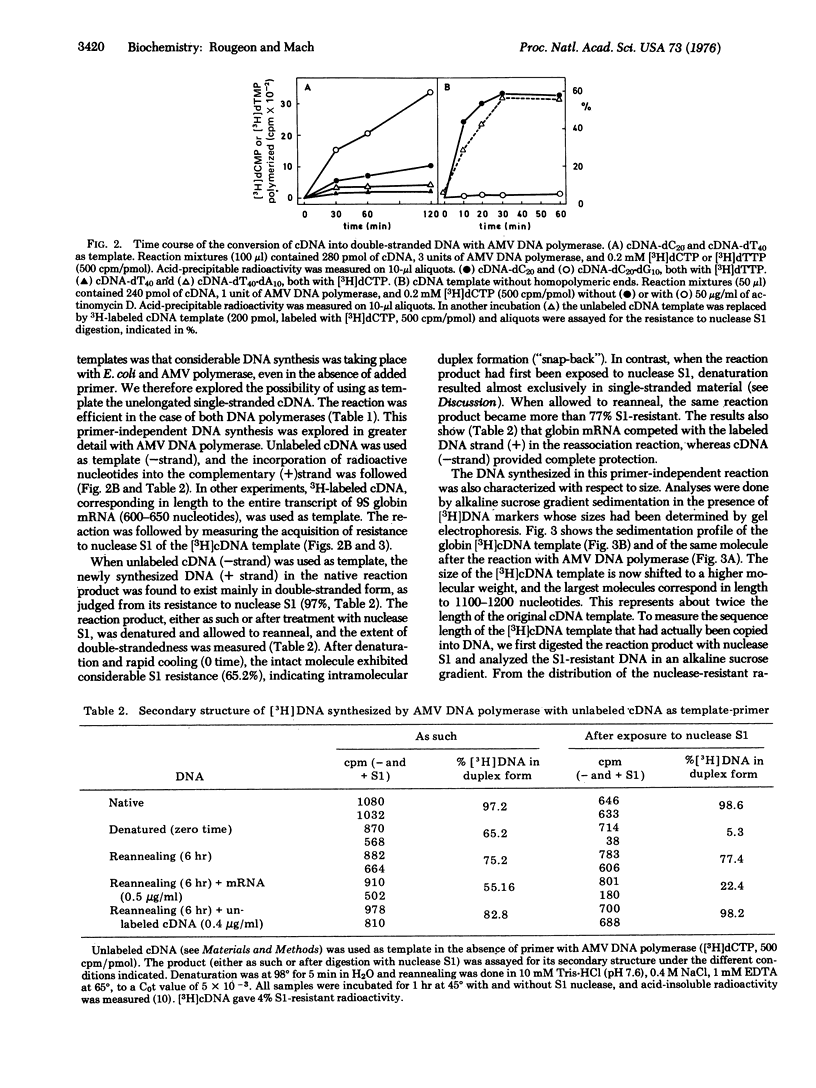
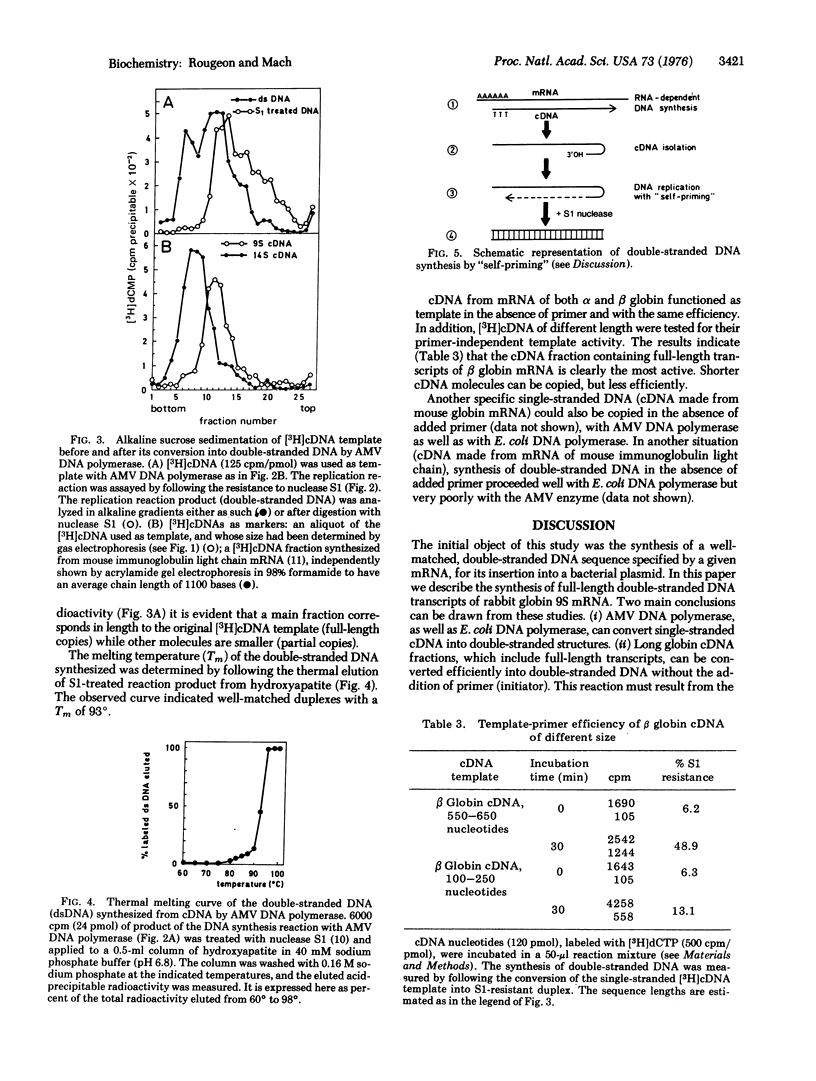
Two approaches have been explored for the synthesis of double-stranded DNA from single-stranded DNA template complementary to rabbit 9S globin mRNA (cDNA). (i) cDNA was elongated with dCMP or dTMP homopolymeric tracts using terminal deoxynucleotidyltransferase (EC 2.7.7.31; nucleosidetriphosphate:DNA deoxynucleotidylexotransferase). cDNA-dC, in the presence of an oligo(dG)10 primer, was an efficient template with either DNA polymerase of Escherichia coli (EC 2.7.7.7; deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase) or RNA-directed DNA polymerase of avian myeloblastosis virus. cDNA-dT [ with an oligo(dA)10 primer] functioned as template only with E. coli polymerase. (ii) cDNA, without homopolymeric tails, was also efficiently copied in the absence of oligonucleotide primer, by DNA polymerase of avian myeloblastosis virus or of E. coli. The product of the reaction consisted of long hairpin molecules which could be converted into DNA duplex (melting temperature, 93 degrees) by digestion with single-strand nuclease S1. The data indicate that a loop structure on the 3' end of cDNA allowed DNA synthesis to take place by a "self-priming" mechanism. Some of the double-stranded DNA synthesized corresponded to the entire sequence of the 9S mRNA template. The synthesis of full-length double-stranded DNA from mouse globin mRNA and immunoglobulin light chain mRNA is also discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digglemann H., Faust C. H., Jr, Mach B. Enzymatic synthesis of DNA complementary to purified 14S messenger RNA of immunoglobulin light chain. Proc Natl Acad Sci U S A. 1973 Mar;70(3):693–696. doi: 10.1073/pnas.70.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The initial step of in vitro synthesis of deoxyribonucleic acid by the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1971 Sep 25;246(18):5684–5687. [PubMed] [Google Scholar]
- Farace M. G., Aellen M. F., Briand P. A., Faust C. H., Vassalli P., Mach B. No detectable reiteration of genes coding for mouse MOPC 41 immunoglobulin light-chain mRNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):727–731. doi: 10.1073/pnas.73.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B. TRANSITIONS OF DNA HOMOPOLYMERS. J Mol Biol. 1964 Sep;9:624–637. doi: 10.1016/s0022-2836(64)80171-6. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K., Patkar S. A. Proteolytic cleavage fo native DNA polymerase into two different catalytic fragments. Influence of assay condtions on the change of exonuclease activity and polymerase activity accompanying cleavage. Eur J Biochem. 1971 Oct 14;22(3):371–381. doi: 10.1111/j.1432-1033.1971.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Brun G., Chapeville F. Comparative studies on the template-initiator requirements of the chick-embryo DNA polymerase I and the avian-myeloblastosis-virus DNA polymerase. Eur J Biochem. 1974 Jan 16;41(2):253–261. doi: 10.1111/j.1432-1033.1974.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Flügel R. M., Larson J. E., Schendel P. F., Sweet R. W. Comparison of some reactions catalyzed by deoxyribonucleic acid polymerase from avian myeloblastosis virus, Escherichia coli, and Micrococcus luteus. Biochemistry. 1972 Feb 15;11(4):621–629. doi: 10.1021/bi00754a025. [DOI] [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]


