Abstract
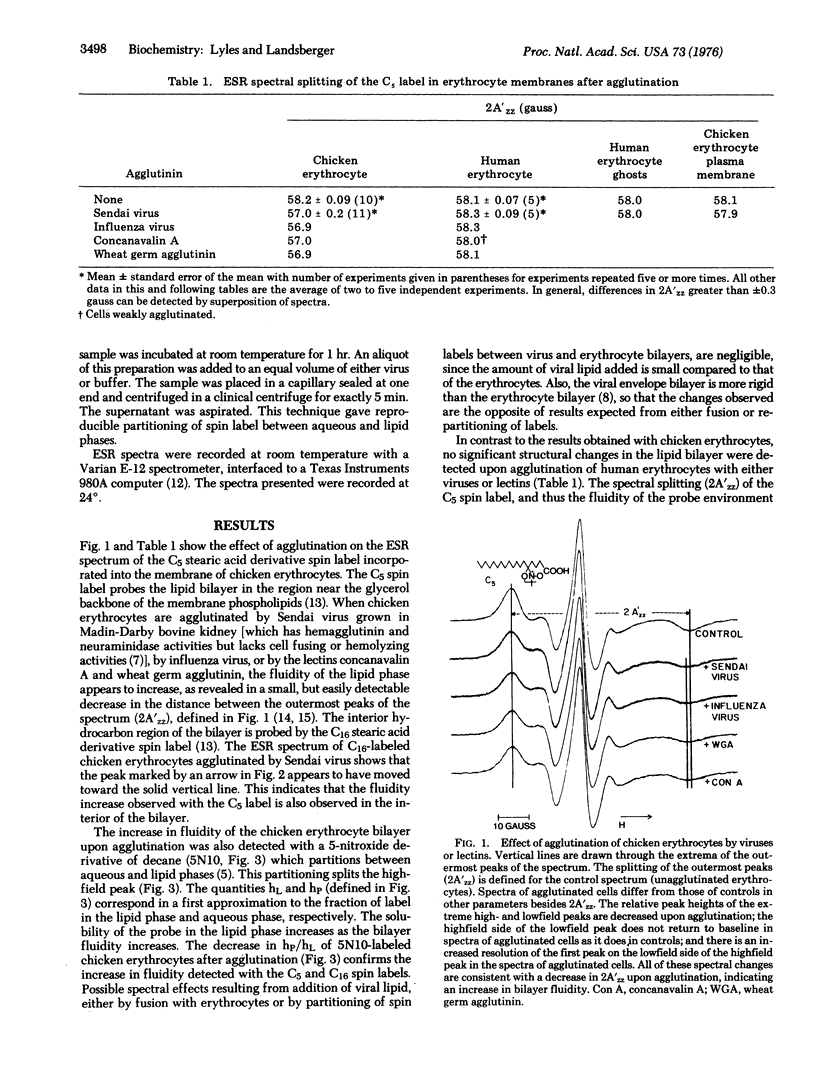
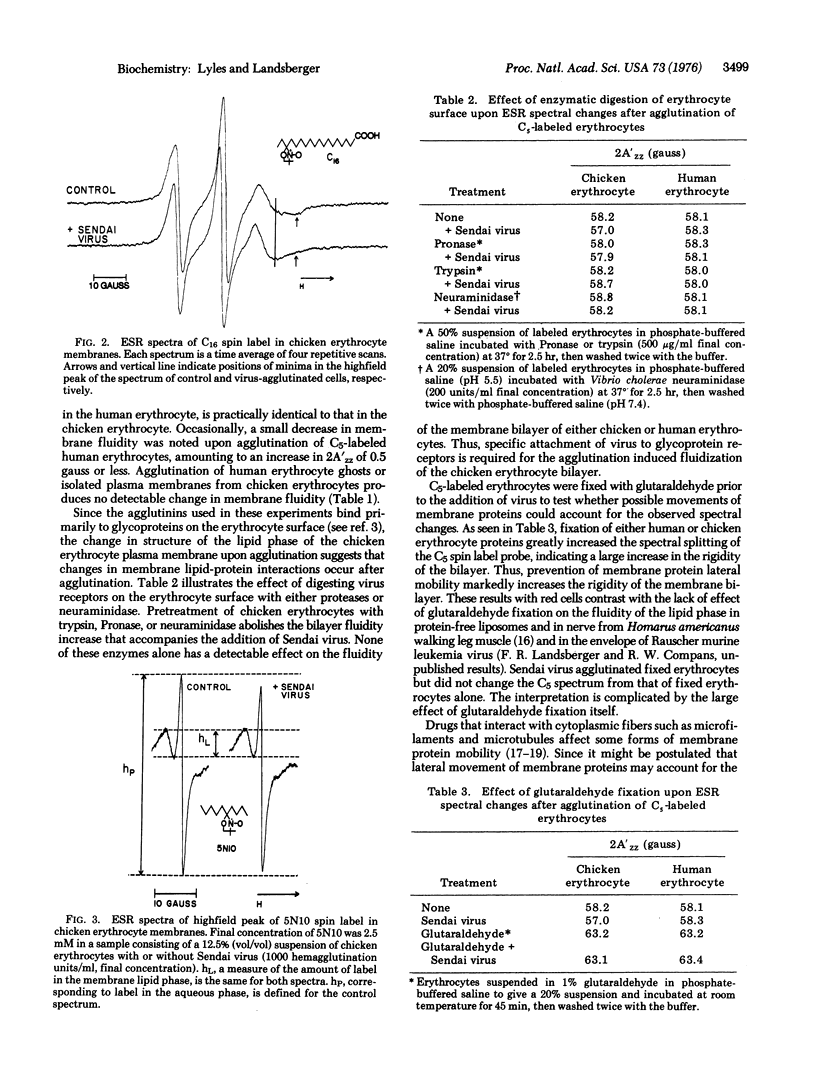
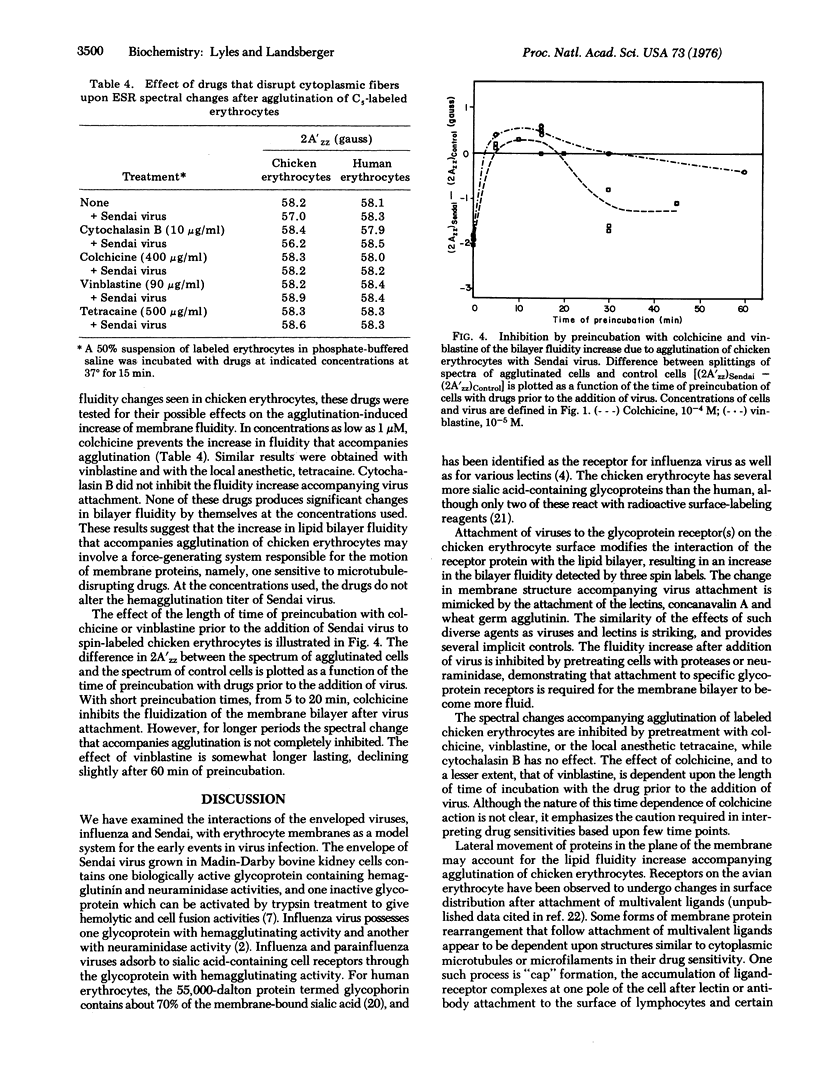
Techniques of spin-label electron spin resonance have been used to prove changes in the structure of the lipid phase of erythrocyte membrane after agglutination by viruses and lectins. When chicken erythrocytes are agglutinated by Sendai and influenza viruses and by the lectins concanavalin A and wheat germ agglutinin, the membrane lipid phase becomes more fluid, as detected by three different lipophilic spin-laveled probes. Colchicine, vinblastine, and tetracaine inhibit the fluidization of chicken erythrocyte membrane by Sendai virus, whereas cytochalasin B has no effect. The effect of colchicine was time dependent, the initial inhibition decreasing with longer preincubation times. Extensive treatment of erythrocytes with proteases or neuraminidase, while not altering the bilayer structure, abolishes the effect of Sendai virus on the erythrocyte membrane, suggesting that a change in the interaction of the receptor protein with the lipid phase occurs upon virus attachment. Glutaraldehyde fixation increased the structural rigidity of the chicken erythrocyte membrane and inhibited the effect of viral agglutination. No change in bilayer structure was observed upon agglutination of human erythrocytes or the isolated plasma membranes of either human or chicken erythrocytes. This result is consistent with the drug sensitivity of the effects of agglutination upon chicken erythrocytes, since human erythrocytes and isolated membranes lack microtubule-like structures.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchet J. P. Chicken erythrocyte membranes: comparison of nuclear and plasma membranes from adults and embryos. Exp Cell Res. 1974 Mar 15;84(1):159–166. doi: 10.1016/0014-4827(74)90392-9. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- De Petris S. Inhibition and reversal of capping by cytochalasin B, vinblastine and colchicine. Nature. 1974 Jul 5;250(461):54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Weiss A. Antigen cap formation in cultured fibroblasts: a reflection of membrane fluidity and of cell motility. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2456–2459. doi: 10.1073/pnas.69.9.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT W., WITEBSKY F. OBSERVATIONS ON THE ULTRASTRUCTURE OF NUCLEATED ERYTHROCYTES AND THROMBOCYTES, WITH PARTICULAR REFERENCE TO THE STRUCTURAL BASIS OF THEIR DISCOIDAL SHAPE. Z Zellforsch Mikrosk Anat. 1964 May 29;62:785–806. doi: 10.1007/BF00342184. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jackson R. C. The exterior surface of the chicken erythrocyte. J Biol Chem. 1975 Jan 25;250(2):617–622. [PubMed] [Google Scholar]
- Jost P., Brooks U. J., Griffith O. H. Fluidity of phospholipid bilayers and membranes after exposure to osmium tetroxide and gluteraldehyde. J Mol Biol. 1973 May 15;76(2):313–318. doi: 10.1016/0022-2836(73)90394-x. [DOI] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger F. R., Paxton J., Lenard J. A study of intact human erythrocytes and their ghosts using stearic acid spin labels. Biochim Biophys Acta. 1972 Apr 14;266(1):1–6. doi: 10.1016/0005-2736(72)90113-7. [DOI] [PubMed] [Google Scholar]
- Lenard J., Tsai D. K., Compans R. W., Landsberger F. R. Observations on the membrane organization of standard and incomplete influenza grown in MDBK cells. Virology. 1976 Jun;71(2):389–394. doi: 10.1016/0042-6822(76)90366-4. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Borysenko J. Z., Karnovsky M. J. Factors affecting the redistribution of surface-bound concanavalin A on human polymorphonuclear leukocytes. J Cell Biol. 1974 Aug;62(2):351–365. doi: 10.1083/jcb.62.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Unanue E. R., Karnovsky M. J. Inhibition of surface capping of macromolecules by local anaesthetics and tranquillisers. Nature. 1974 Jul 5;250(461):56–57. doi: 10.1038/250056a0. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena T. E., Borysenko J. Z., Karnovsky M. J., Berlin R. D. Effects of colchicine, cytochalasin B, and 2-deoxyglucose on the topographical organization of surface-bound concanavalin A in normal and transformed fibroblasts. J Cell Biol. 1974 Apr;61(1):70–82. doi: 10.1083/jcb.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]


