Abstract
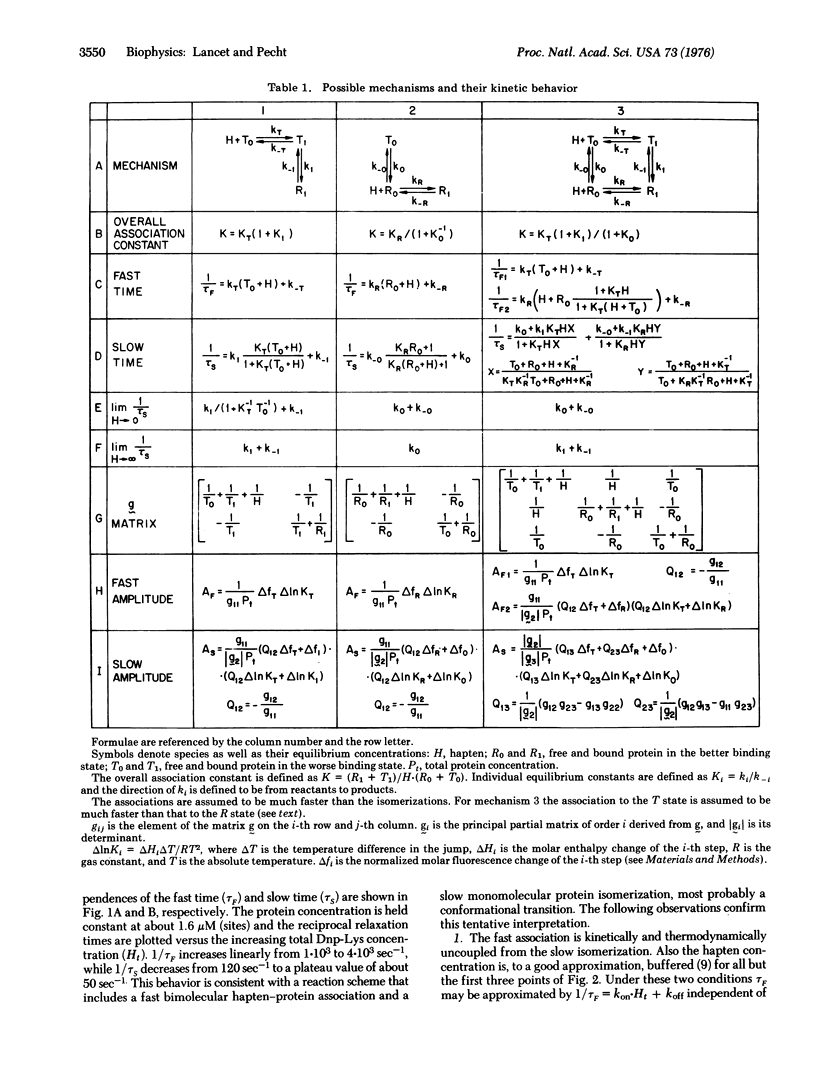
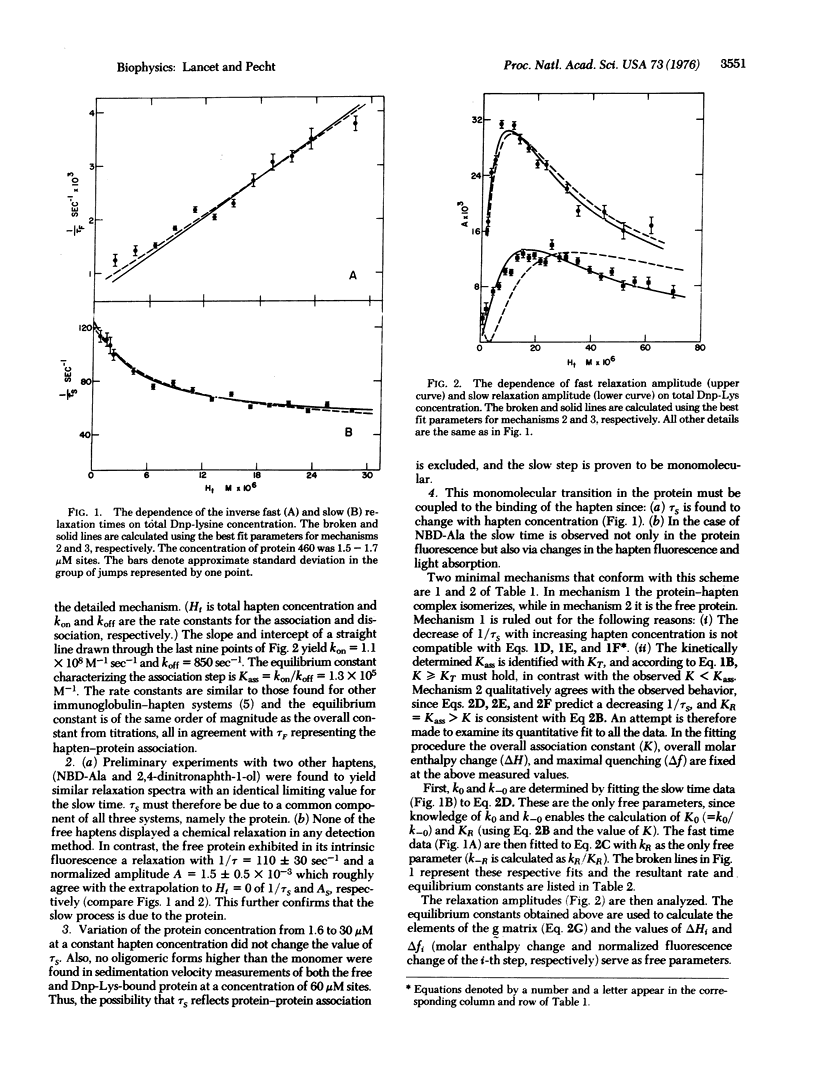
The kinetics of hapten binding to the homogeneous immunoglobulin A secreted by the murine plasmacytoma MOPC 460 was investigated by the chemical relaxation method. Two distinct relaxation times were observed in the binding equilibrium with three different haptens. A detailed concentration dependence analysis of relaxation times and amplitudes was performed with the hapten epsilon-N(2,4-dinitrophenyl)-lysine (Dnp-Lys). The results support a mechanism in which two interconvertible conformational states of the protein bind the hapten with different association constants. Hapten binding shifts the equilibrium towards the better binding state. These observations form kinetic evidence for a conformational transition induced in the immunoglobulin by ligand binding to its antigen binding site, and are in line with the allosteric hypothesis for the initiation of physiological functions by antigen-antibody association.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. C., Koshland M. E. Activation of antibody Fc function by antigen-induced conformational changes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5111–5115. doi: 10.1073/pnas.72.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese A., Sehon A. H. Kinetics of antibody--hapten reactions. Contemp Top Mol Immunol. 1975;4:23–54. [PubMed] [Google Scholar]
- Ghosh P. B., Whitehouse M. W. 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole: a new fluorigenic reagent for amino acids and other amines. Biochem J. 1968 Jun;108(1):155–156. doi: 10.1042/bj1080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Haselkorn D., Friedman S., Givol D., Pecht I. Kinetic mapping of the antibody combining site by chemical relaxation spectrometry. Biochemistry. 1974 May 7;13(10):2210–2222. doi: 10.1021/bi00707a030. [DOI] [PubMed] [Google Scholar]
- Jaffe B. M., Simms E. S., Eisen H. N. Specificity and structure of the myeloma protein produced by mouse plasmacytoma MOPC-460. Biochemistry. 1971 Apr 27;10(9):1693–1699. doi: 10.1021/bi00785a029. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Barisas B. G., Sturtevant J. M. Thermodynamics of hapten binding to MOPC 315 and MOPC 460 mouse myeloma proteins. Biochemistry. 1974 Jan 15;13(2):390–396. doi: 10.1021/bi00699a026. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Eigen M., Bittman R., Voigt B. The binding of nicotinamide-adenine dinucleotide to yeast d-glyceraldehyde-3-phosphate dehydrogenase: temperature-jump relaxation studies on the mechanism of an allosteric enzyme. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1661–1667. doi: 10.1073/pnas.56.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169–207. doi: 10.1016/s0065-2776(08)60310-7. [DOI] [PubMed] [Google Scholar]
- Pilz I., Kratky O., Licht A., Sela M. Shape and volume of fragments Fab' and (Fab')2 of anti-poly(D-alanyl) antibodies in the presence and absence of tetra-D-alanine as determined by small-angle x-ray scattering. Biochemistry. 1975 Mar 25;14(6):1326–1333. doi: 10.1021/bi00677a035. [DOI] [PubMed] [Google Scholar]
- Rosenstein R. W., Musson R. A., Armstrong M. K., Konigsberg W. H., Richards F. F. Contact regions for dinitrophenyl and menadione haptens in an immunoglobulin binding more than one antigen. Proc Natl Acad Sci U S A. 1972 Apr;69(4):877–881. doi: 10.1073/pnas.69.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Givol D., Hochman J., Pecht I. Antigen-induced conformational changes in antibodies and their Fab fragments studied by circular polarization of fluorescence. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2775–2779. doi: 10.1073/pnas.72.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]


