Abstract
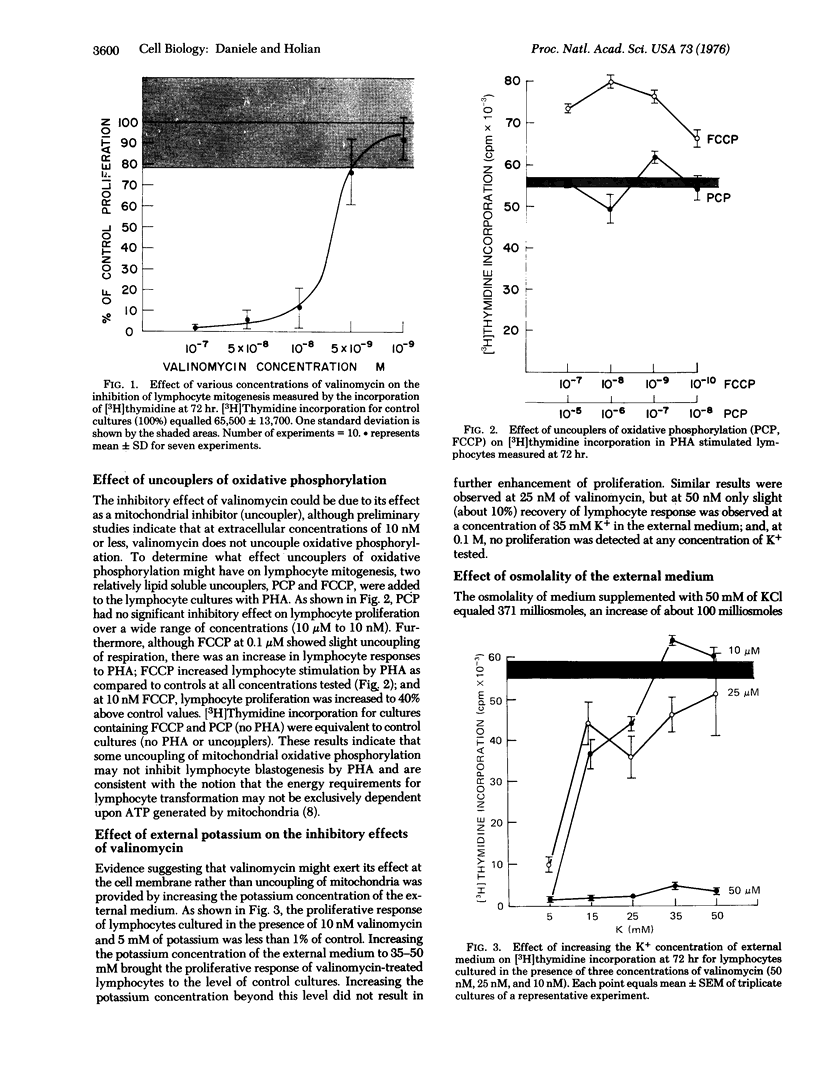
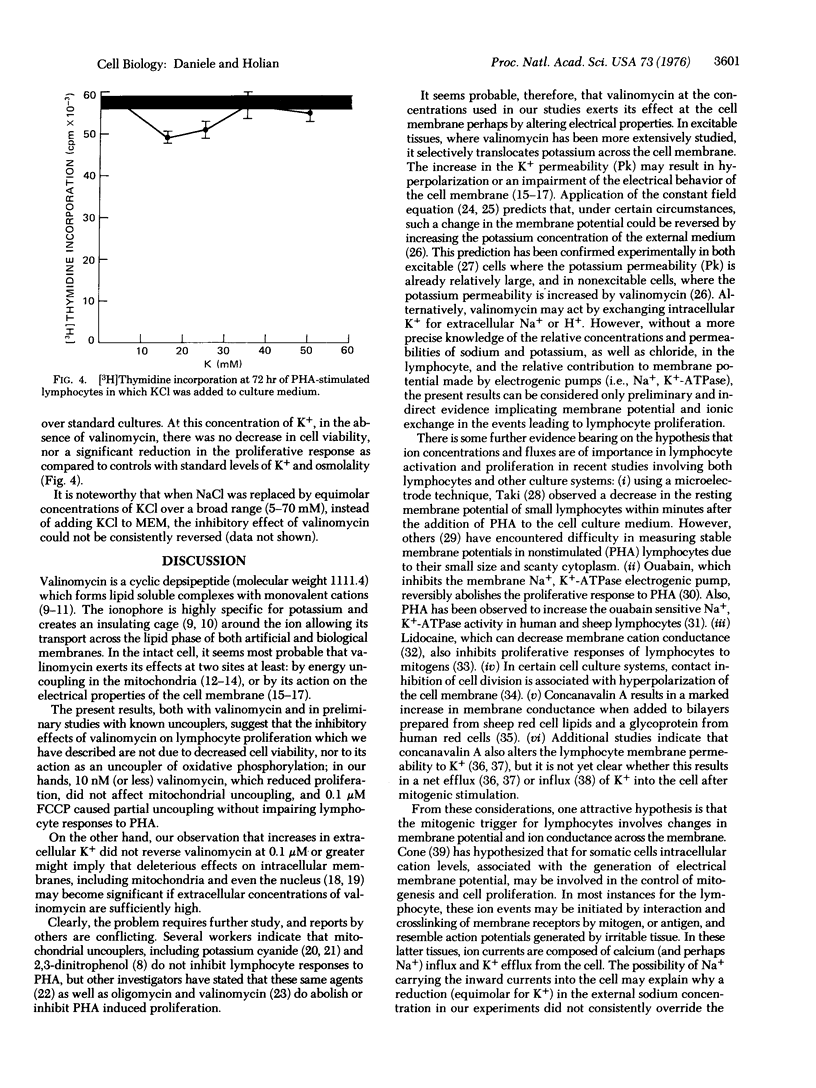
Valinomycin is a depsipeptide antibiotic which selectively translocates potassium across biologic membranes. This potassium ionophore was observed to inhibit phytohemagglutinin-stimulated blastogenesis and proliferation in human lymphocytes. The effect was not due to toxicity to the cells, nor appeared to be due to the effects of valinomycin as an uncoupler of oxidative phosphorylation. Furthermore, the inhibitory effect on phytohemagglutinin stimulated lymphocytes was prevented by increasing the potassium concentration of the external media. These results suggest that the interaction of mitogens with specific receptors at the cell membrane may involve mechanisms affecting cation fluxes and membrane potential. These ionic events may play a role in the transduction of membrane signals for lymphocyte stimulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKINRIMISI E. O., BONNER J., TSO P. O. BINDING OF BASIC PROTEINS TO DNA. J Mol Biol. 1965 Jan;11:128–136. doi: 10.1016/s0022-2836(65)80178-4. [DOI] [PubMed] [Google Scholar]
- Alford R. H. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J Immunol. 1970 Mar;104(3):698–703. [PubMed] [Google Scholar]
- Averdunk R., Lauf P. K. Effects of mitogens on sodium-potassium transport, 3H-ouabain binding, and adenosine triphosphatase activity in lymphocytes. Exp Cell Res. 1975 Jul;93(2):331–342. doi: 10.1016/0014-4827(75)90458-9. [DOI] [PubMed] [Google Scholar]
- Blondin G. A. Isolation of a divalent cation ionophore from beef heart mitochondria. Biochem Biophys Res Commun. 1974 Jan;56(1):97–105. doi: 10.1016/s0006-291x(74)80320-7. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation and removal of lymphocytes from bone marrow of rats and guinea-pigs. Scand J Clin Lab Invest Suppl. 1968;97:91–106. [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971 Jan;30(1):151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- Covino B. G. Local anesthesia. 1. N Engl J Med. 1972 May 4;286(18):975–983. doi: 10.1056/NEJM197205042861805. [DOI] [PubMed] [Google Scholar]
- Cullen B. F., Chretien P. B., Leventhal B. G. The effect of lignocaine on PHA-stimulated human lymphocyte transformation. Br J Anaesth. 1972 Dec;44(12):1247–1252. doi: 10.1093/bja/44.12.1247. [DOI] [PubMed] [Google Scholar]
- Eisenman G. Ion permeation of cell membranes and its models. Fed Proc. 1968 Nov-Dec;27(6):1249–1251. [PubMed] [Google Scholar]
- FARNES P., BARKER B. E., BROWNHILL L. E., FANGER H. MITOGENIC ACTIVITY IN PHYTOLACCA AMERICANA (POKEWEED). Lancet. 1964 Nov 21;2(7369):1100–1101. doi: 10.1016/s0140-6736(64)92616-9. [DOI] [PubMed] [Google Scholar]
- Green E., Reible S. Paired moving charges in mitochondrial energy coupling. II. Universality of the principles for energy coupling in biological systems. Proc Natl Acad Sci U S A. 1975 Jan;72(1):253–257. doi: 10.1073/pnas.72.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle M., Van der Kloot W. The effects of valinomycin on striated muscles of the frog and the crayfish. Comp Biochem Physiol A Comp Physiol. 1973 Oct 1;46(2):269–278. doi: 10.1016/0300-9629(73)90417-9. [DOI] [PubMed] [Google Scholar]
- Hoffman J. F., Laris P. C. Determination of membrane potentials in human and Amphiuma red blood cells by means of fluorescent probe. J Physiol. 1974 Jun;239(3):519–552. doi: 10.1113/jphysiol.1974.sp010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezzi M. Differential gene activation in isolated chromosomes. Int Rev Cytol. 1970;29:127–168. doi: 10.1016/s0074-7696(08)60034-0. [DOI] [PubMed] [Google Scholar]
- Maino V. C., Green N. M., Crumpton M. J. The role of calcium ions in initiating transformation of lymphocytes. Nature. 1974 Sep 27;251(5473):324–327. doi: 10.1038/251324b0. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Nowell P. C., Daniele R. P., Winger L. A. Kinetics of human lymphocyte proliferation: proportion of cells responsive to phytohemagglutinin and correlation with E rosette formation. J Reticuloendothel Soc. 1975 Jan;17(1):47–56. [PubMed] [Google Scholar]
- Oliveira-Castro G. M., Barcinski M. A., Cukierman S. Intercellular communication in stimulated human lymphocytes. J Immunol. 1973 Nov;111(5):1616–1619. [PubMed] [Google Scholar]
- Orr C. W., Yoshikawa-Fukada M., Ebert J. D. Potassium: effect on DNA synthesis and multiplication of baby-hamster kidney cells: (cell cycle-membrane potential-synchronization-transformation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):243–247. doi: 10.1073/pnas.69.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar P. R., Foster J. M., Cooperband S. R. Glycolysis as an energy source for stimulation of lymphocytes by phytohemagglutinin. Exp Cell Res. 1968 Feb;49(2):231–237. doi: 10.1016/0014-4827(68)90174-2. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Early stimulation of potassium uptake in lymphocytes treated with PHA. Exp Cell Res. 1970 Nov;63(1):230–233. doi: 10.1016/0014-4827(70)90360-5. [DOI] [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Inhibition by ouabain of human lymphocyte transformation induced by phytohaemagglutinin in vitro. Nature. 1968 Jul 13;219(5150):198–200. doi: 10.1038/219198a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res. 1973 Mar 15;77(1):127–135. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- Schellekens P. T., Eijsvoogel V. P. Lymphocyte transformation in vitro. 3. Mechanism of stimulation in the mixed lymphocyte culture. Clin Exp Immunol. 1970 Aug;7(2):229–239. [PMC free article] [PubMed] [Google Scholar]
- Segel G. B., Hollander M. M., Gordon B. R., Klemperer M. R., Lichtman M. A. A rapid phytohemagglutinin induced alteration in lymphocyte potassium permeability. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):327–335. doi: 10.1002/jcp.1040860404. [DOI] [PubMed] [Google Scholar]
- Shamoo A. E., Albers R. W. NA + -selective ionophoric material derived from electric organ and kidney membranes. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1191–1194. doi: 10.1073/pnas.70.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Palfrey C., Littauer U. Z. Enhancement of the electrical excitability of neuroblastoma cells by valinomycin. Nature. 1975 Mar 13;254(5496):121–124. doi: 10.1038/254121a0. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Ingram M. A method for counting phytohemagglutinin-stimulated lymphocytes. Blood. 1967 Apr;29(4 Suppl):628–639. [PubMed] [Google Scholar]
- Taki M. Studies on blastogenesis of human lymphocytes by phytohemagglutinin, with special reference to changes of membrane potential during blastoid transformation. Mie Med J. 1970 Jan;19(3):245–262. [PubMed] [Google Scholar]
- Tosteson M. T., Lau F., Tosteson D. C. Incorporation of a functional membrane glycoprotein into lipid bilayer membranes. Nat New Biol. 1973 May 23;243(125):112–114. [PubMed] [Google Scholar]
- Wecksler M., Levy A., Jaffé W. G. Acción mitogénica de extractos de Canavalia ensiformis y Concanavalina A. Acta Cient Venez. 1968;19(4):154–156. [PubMed] [Google Scholar]