Introduction
Herpesviruses comprise a family of relatively large double-stranded DNA viruses, which are widespread among humans and other animals. Both the Cytomegalovirus and the Roseolovirus genera in the Betaherpesvirinae subfamily include viruses found in humans. The Roseolovirus genus is composed of three human viruses: the closely related human herpesvirus 6A (HHV-6A) and human herpesvirus 6B (HHV-6B) and the more distantly related human herpesvirus 7 (HHV-7) (Pringle, 1998). Although HHV-6A and HHV-6B were originally considered as two variants of the same virus (D. Ablashi, 1993), evidence of genetic, biological and epidemiologic differences between HHV-6A and HHV-6B has led to their recent classification as distinct virus species (Adams and Carstens, 2012).
Roseoloviruses are among the most prevalent viruses in the human population with primary infection occurring during early childhood (Hall et al., 2006; Zerr et al., 2005). Primary infections with HHV-6B and HHV-7 have been reported to cause exanthema subitum (Tanaka et al., 1994; Yamanishi et al., 1988) while the clinical symptoms associated with HHV-6A are still unclear. These clinical features associated with roseolovirus infections are generally characterized by low morbidity, as the virus infection becomes latent. However, severe pathological conditions can arise due to active viral replication, often in immunocompromised hosts. In transplant patients HHV-6B reactivation is associated with frequent complications including encephalitis, acute graft versus host disease, CMV reactivation and bone marrow suppression (Zerr, 2012). In this setting, the causal involvement of HHV-7 reactivation is less well documented. HHV-6B is also implicated in a majority of pediatric cases of febrile status epilepticus (Epstein et al., 2012). HHV-6A active replication on the other hand is associated with autoimmune diseases such as Hasimoto’s thyroiditis (Caselli et al., 2012) and enhances disease progression in HIV infected individuals (Ablashi et al., 1998; Boutolleau et al., 2004; Lusso et al., 2007).
Both HHV-6B and HHV-7 are believed to be transmitted by exposure to saliva. Infectious virus particles can be isolated from saliva samples and both viruses have been detected in salivary glands (Di Luca et al., 1995). In contrast HHV-6A is rarely detected in saliva (Aberle et al., 1996) and it is likely that transmission occurs following other types of exposure including sexual contact (Leach et al., 1994). To date, no significant differences in prevalence of roseolovirus infections in men and women have been reported. However, large variations in prevalence have been observed in different geographic locations (Krueger et al., 1998; Magalhaes et al., 2010).
All roseoloviruses were initially characterized by their tropism for lymphocytes (Frenkel et al., 1990; Salahuddin et al., 1986). However, since these viruses can infect and replicate in a wide range of tissues, a broader cellular tropism is likely. Although HHV-6 and HHV-7 have been detected in a similar panel of tissues (Chen and Hudnall, 2006; Kempf et al., 1998; Mori, 2009), it is likely that they infect different cell types since each virus relies on a different membrane receptor for viral entry (Lusso et al., 1994b; Santoro et al., 1999; Tang et al., 2013).
To our knowledge, no roseolovirus homolog has been identified in any of the three major macaque species used in research, including long-tailed macaques (Macaca fascicularis (Mfa)), Rhesus macaques (Macaca mulata (Mmu)), and pig-tailed macaques (Macaca nemestrina (Mne)). The only non-human primate roseloviruses discovered to date are a HHV-6 homolog in chimpanzee (PanHV6) (Lacoste et al., 2005) and a roseolovirus in drill monkeys (MndHVbeta) (Lacoste et al., 2000). Both viral homologs were identified in DNA isolated from blood by nested PCR using the consensus-degenerate hybrid oligonucleotide primer (CODEHOP) approach. Nonetheless, HHV-6 reactive antibodies have been detected in a number of primate species, including macaque species, orangutans and African green monkeys, suggesting the existence of additional roseolovirus homologs (Higashi et al., 1989).
In the current study, we investigated whether pig-tailed macaques maintained at the Washington National Primate Research Center (WaNPRC) were naturally infected with Roseolovirus homologs using a CODEHOP PCR approach specifically designed to detect novel roseoloviruses. We identified multiple DNA fragments in pig-tailed macaques with strong sequence homology to HHV-6A, HHV-6B, and HHV-7. Phylogenetic analysis revealed that these DNA fragments corresponded to two novel Roseoloviruses belonging to the beta-herpesvirus subfamily. One viral sequence clustered closely with HHV-6A and -6B and panHV-6 suggesting that it was derived from the pig-tailed macaque homolog of HHV-6, provisionally termed MneHV-6. The other viral sequence clustered closely with HHV-7, suggesting that it was derived from the pig-tailed macaque homolog of HHV-7, provisionally termed MneHV-7. Using specific quantitative TaqMan PCR assays, we determined that the prevalence and tissue tropism of MneHV-6 and MneHV-7 were similar to HHV-6 and HHV-7 in humans. Our studies suggest that pig-tailed macaques naturally infected with MneHV-6 and MneHV-7 can be used as biologically relevant animal models to study HHV-6 and HHV-7 infections and associated pathologies in the human host.
MATERIALS AND METHODS
Saliva and tissue samples
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood collected from 283 pig-tailed macaques during the biannual colony health screening at the Washington National Primate Research Center (WaNPRC). Saliva samples were from stored aliquots from pig-tailed macaques that had been part of a previous simian immunodeficiency (SIV) vaccine study. All animals in this study were housed and cared for according to the Guide for the Care and Use of Laboratory Animals at the Washington National Primate Research Center (WaNPRC), an Association for Assessment and Accreditation of Laboratory Animal Care International accredited institution. The experimental procedures were approved by the Institutional Animal Care and Use Committee (2370-20) at the University of Washington and conducted in compliance with the Public Health Services Policy on Humane Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf). The animals were kept under deep sedation during the saliva collection procedures with ketamine HCl at the dose of 10–15 mg/kg intramuscularly to alleviate any pain and discomfort. The animals were monitored by the Animal Technician or Veterinary Technologist while under sedation. Tissues collected at necropsy were either frozen or fixed in 10% neutral buffered formalin and embedded in paraffin.
Preparation of DNA from saliva and tissues
To separate whole saliva into a cell-free fraction and cell pellet, 600 µl of whole saliva was centrifuged (5 min, 2,500 rpm) to pellet any cells. Supernatants were collected and filtered using a 0.45 micron acrodisk filter (PALL, Port Washington, NY). Oral swab samples were obtained using sterile polyester tipped swabs (Puritan, Guilford, ME) by passing the swab over the inside of both cheeks, along the upper and lower gum-lines, around the hard palate and across the soft palate. Formalin-fixed paraffin-embedded tissue was treated with xylene to remove paraffin, followed by extensive ethanol washes prior to DNA isolation. DNA was extracted from whole saliva, saliva supernatant and cell pellet, oral swab and tissue samples using proteinase K at 56°C and the QIAamp DNA Mini Kit (Qiagen, Valencia, CA)), following the manufacturer’s protocols.
COnsensus DEgenerate Hybrid Oligonucleotide Primer (CODEHOP) design
Several CODEHOP primer pairs targeting the highly conserved DNA polymerase (DNA pol) and glycoprotein B (gB) genes were designed as previously described (Rose, 2005) from an alignment of available human and non-human primate beta-herpesvirus protein sequences (see below) as well as one bat beta-herpesvirus sequence (Wibbelt et al., 2007). The iCODEHOP software (Boyce et al., 2009) was used to determine short conserved amino acid motifs in the DNA pol and gB genes from which the degenerate core for each CODEHOP primer was designed. The consensus portion for each CODEHOP primer was designed from the consensus of all aligned beta herpesvirus nucleotide sequences flanking the degenerate core. Additional modified CODEHOPs were designed by modifying core and/or consensus sequences to bias the primers to resemble more closely the roseolovirus nucleotide sequences in the alignment. Optimal primer combinations were chosen after comprehensive testing of multiple CODEHOP primer combinations. PCR assays were performed across a temperature gradient from 50°C to 70°C using as template the human T-cell line HBS-2 infected with the HHV-6B strain Z29. The CODEHOP primer pairs with the best sensitivity for HHV-6B detection were used to screen macaque DNA samples. CODEHOP primer pairs targeting roseolovirus DNA pol sequences are shown with the predicted amplicon sizes: SLYP-F (5’-GCCTACTGTGGTGTTTGATTTTCAGAGYYTSTAYCC-3’) × YGD-R (5’-CTGACAGACATAAAGATGCTATCCGTATCACCATA-3’), 549 bp; DIEC-F (5’-GGCCTTTATATGGATGCTGGTCCTTCGAYATWGARTG -3’) × CNS-R (5’-GCCGCTCCCGTCACACCGTACACIGARTTRCA -3’), 1,218 bp. The CODEHOP primer pair targeting roseolovirus gB sequencsis shown with the predicted amplicon size: PLEN-F (5’-CTAAAGATCGATCCTCTAGAAAATGCNGATTTTA-3’) × NPFG-R (5’-ATCAACATGAGACCACCCCCRAATGGATTT-3’), 326 bp.
Protocol for CODEHOP PCR
PCR and thermal denaturation were conducted on a Roche LightCycler 480 II, with accompanying software release 1.5.0. The amplification reaction mix included 1× PCR buffer (Life Technologies, Grand Island, NY), 0.4 mM each dNTP (Bioline, Taunton, MA), 2 mM MgCl2, 0.5 U/reaction HS Taq polymerase (Kapa Biosystems, Wilmington, MA), 0.4 µM of each the forward and the reverse CODEHOP primer pools, and 2 µL of template DNA. For the initial CODEHOP primer optimization, quantitative PCR was performed by adding 0.4× SYBRgreen (Life Technologies, Grand Island, NY) to the reaction mix. The amplification protocol for CODEHOP PCR was as follows: incubation of amplification reaction mix (25 µl/assay) at 94°C for 2.5 min, followed by 45 cycles at 94°C for 15s, annealing at 50°C or 60°C for 30s (or gradient from 50°C to 65°C for 30s for gB screening), and 72°C for 15s, then cooled for 1 min at 37°C, all at maximum ramp speed of 4.4°C/s.
Sequence analysis of CODEHOP amplicons by Sanger sequencing
PCR amplicons obtained from the CODEHOP PCR reactions were analyzed by gel electrophoresis and bands of the appropriate size were excised and prepared for sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit following the manufacturer’s instructions (Life technologies, Grand Island, NY).
Comparative and phylogenetic analysis
Sequence data was evaluated using the Geneious software package (v6.1.6; Biomatters Ltd., Auckland, NZ). Amplicon sequences were aligned with known beta-herpesvirus sequences using Muscle (for amino acid sequences) or the Geneious alignment algorithm (for nucleotide sequences) and sequence identity was determined. Phylogenetic analysis was performed using the Maximum Likelihood method (PhyML) with the JC69 substitution model (Guindon and Gascuel, 2003) from Muscle or Geneious alignments of herpesvirus nucleotide or amino acid sequences. The number of substitution rate categories was set at 4, and the best of both NNI and SPR topology searches was combined into the final tree. The percent likelihood of branching within the phylogenetic tree (Fig. 3) was obtained by using the approximate likelihood ratio test returning Chi2-based parametric branch supports (Anisimova and Gascuel, 2006). The final phylogenetic trees were visualized using Geneious or FigTree v1.3.1 [http://tree.bio.ed.ac.uk/software/figtree/].
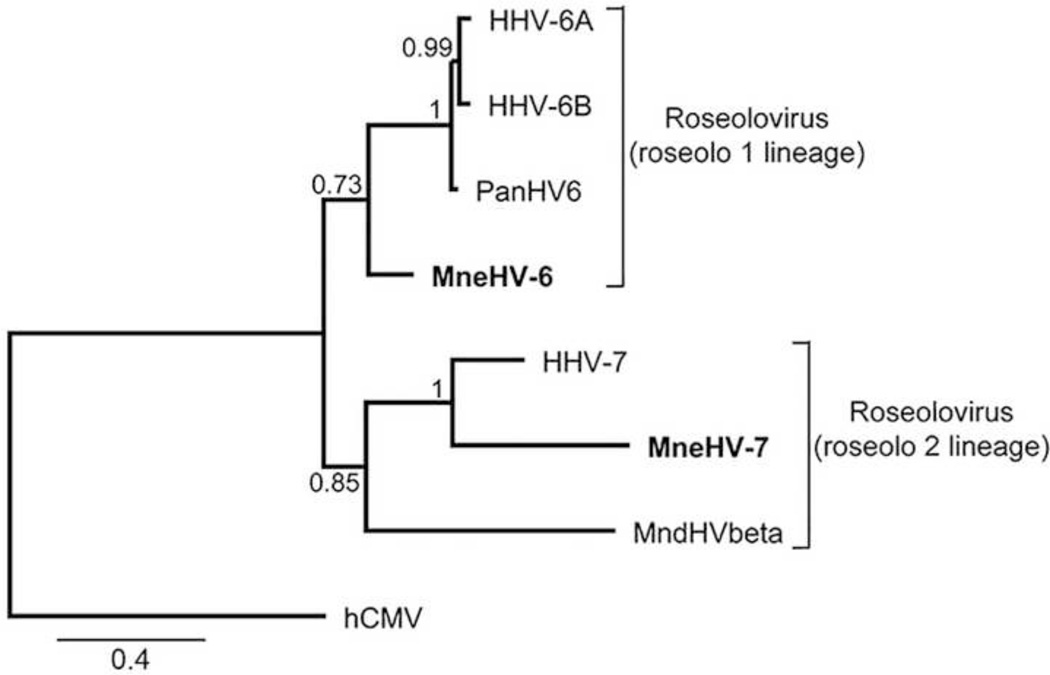
Figure 3. Phylogenetic relationship of the novel MneHV-6 and MneHV-7 DNA pol nucleotide sequences within the Roseolovirus genus.
The 478 bp nucleotide sequences of the MneHV-6 and MneHV-7 DNA pol SLYP-YGD CODEHOP amplicons were compared to analogous nucleotide sequences from other Old World primate roseoloviruses (see Materials and methods section for accession records) using maximum likelihood analysis. The proposed roseolo 1 and roseolo 2 lineages of the Betaherpesvirinae subfamily are indicated. The cytomegalovirus hCMV / HHV5 DNA pol sequence was used as out-group. Branch support values were obtained by using the approximate likelihood ratio test (aLRT) with Chi2-based parametric statistics. The substitutions per site are indicated.
GenBank references
The following herpesvirus DNA pol protein sequences were used to construct the phylogenetic tree for Figure 2: Alphaherpesvirinae: a) Varicellovirus: HHV3 / VZV (NP_040151), CeHV9. Simian VZV (NP_077443), b) Simplexvirus: McHV1 / macaque Herpes B (NP_851890), HSV1 (NP_044632), HSV2 (NP_044500), PanHValpha1 (AFV26919); Betaherpesvirinae: a) Cytomegalovirus: McHV3 / MmuCMV (O71121), MfaCMV (AAW55912), MndCMV (AAM89282), HHV5 / hCMV (YP_081513), PnHV2 / panCMV (NP_612698); b) Roseolovirus: (roseolo1 lineage): PanHV6 (AAW55996), HHV6A (P28857), HHV6B (Q9QJ32); (roseolo2 lineage): MndHVβ (AAG39065), HH7 (AAC40752); Gammaherpesvirinae : a) Lymphocryptovirus : PnHV1 / PanLCV (ADY05316), HHV4 / EBV (P03198), MndLCV1 (ADY05317), MfaLCV1 (ADY05313), McHV4 / MmuLCV1 (YP_068007); b) Rhadinovirus : (RV1 lineage): RFHVMn (AAF81662), MfaRV1 (AAN35122), McHV5 / RFHVMm (AAC57976), MndRHV1 (AAG39066), HHV8 / KSHV (AAC57974),PanRV1 (AAN35124); (RV2 lineage): PanRV2 (ABU52897), MndRHV2 (AAG39063), MfaRV2 (ABU52895), RRV (NP_570750), MneRV2 (AF204167). Nucleotide accession numbers used for sequence comparisons and phylogenetic analysis in Figure 3 are as follows: HHV6A (NC_001664), HHV6B (NC_000898), PanHV6 (AY854171), HHV7 (NC_001716), MndHVbeta (AF282942).
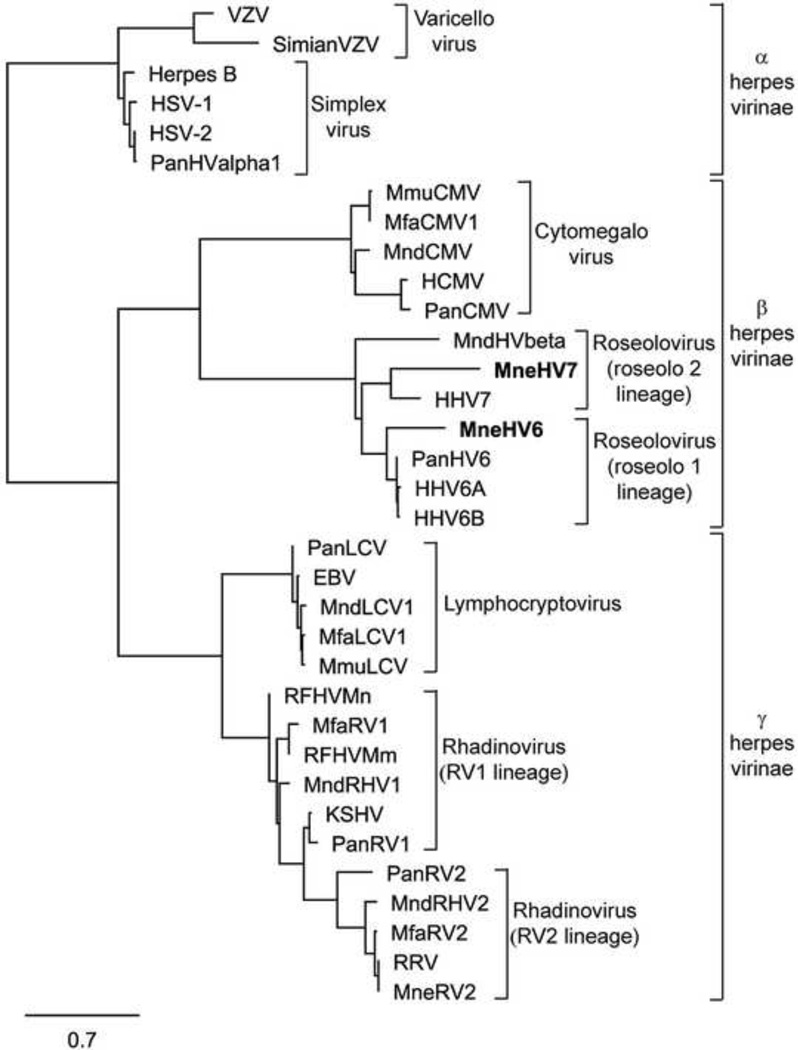
Figure 2. Phylogenetic relationship of the novel MneHV-6 and MneHV-7 DNA pol protein fragments within the Herpesviridae family.
The 159aa sequences encoded by the MneHV-6 and MneHV-7 478bp DNA pol SLYP-YGD CODEHOP amplicon sequences were compared to analogous protein sequences from other Old World primate herpesvirinae (see Materials and methods section for accession records) using maximum likelihood analysis. Members of the Varicellovirus genus and Simplexvirus genus of the Alphaherpesvirinae subfamily, the Cytomegalovirus genus (CMV) and Roseolovirus genus (provisional roseolo 1 and roseolo 2 lineages) of the Betaherpesvirinae subfamily as well as the Lymphocryptovirus genus (LCV) and Rhadinovirus genus (RV1 and RV2 lineages) of the Gammaherpesvirinae subfamily are shown. The substitutions per site are indicated. HSV-1 (Herpes simplex virus 1, HHV-1); HSV-2 (Herpes simplex virus 2, HHV-2); VZV (Varizellavirus, HHV-3); EBV (Epstein-Barr virus, HHV-4); CMV (cytomegalovirus, HHV-5); RFHV (retroperitoneal fibromatosis-associated herpesvirus); RRV (Rhesus rhadinovirus); KSHV (Kaposi’s sarcoma-associated herpesvirus, HHV-8 ).
MneHV-6 and MneHV-7 specific TaqMan qPCR assays
PCR primers and TaqMan probes for quantitative real-time PCR assays (qPCR) specifically targeting either the MneHV6 or the MneHV7 virus were designed based on the sequences of the partial MneHV6 gB and MneHV7 DNA pol gene segments amplified from the CODEHOP assays, using the modified Primer3 software available on the IDT website (Integrated DNA Technologies, Coralville, IA). Probe and primer sequences were as follows: MneHV7 DNA pol probe (5’-[6FAM]ACTGGTGCAACACATAGCTTATTACCGT[BHQ1] -3’); forward primer: MneHV7-pol-F (5’-GTGCAAAGACCCTACGTTAATTATG-3’); reverse primer: MneHV7-pol-R (5’-CTTGTTACCGAAGCAGCAATAG-3’); MneHV6 gB probe (5’-[6FAM]CGCCGTCGTATGTCAATGGCC[BHQ1] -3’); forward primer: MneHV6-gB-F (5’-TCCCCGGATGAATTGAGTAG-3’); reverse primer: MneHV6-gB-R (5’-TAGGTACAGGGTTGGGATCG-3’). PCR and thermal denaturation were conducted on a Roche LightCycler 480 II, as described above, using 1× PCR buffer (Life Technologies, Grand Island, NY, USA), 0.2 mM each dNTP (Bioline, Taunton, MA), 3 mM MgCl2, 0.5 U/reaction HS Taq polymerase (Kapa Biosystems, Wilmington, MA), 1 µM of each the forward and the reverse CODEHOP primer, 0.1 µM probe and 1 µL of template DNA. The amplification protocol for qPCR was as follows: incubation of amplification reaction mix (25 µl/assay) at 94°C for 2.5 min, followed by 45 cycles of 94°C for 30s, 60°C for 30s and 72°C for 30s (single acquisition), then cooled for 1 min at 37°C, all at maximum ramp speed of 4.4°C/s. All samples were run in duplicate reactions. Viral load was determined by comparing the Ct values obtained with the MneHV-6 and MneHV-7 qPCR assays with the Ct value obtained from a single copy cellular gene, oncostatin M, as described previously (Bruce et al., 2005). Standard deviation was calculated based on the delta delta CT (Livak and Schmittgen, 2001).
Standardized plasmids
The CODEHOP SLYP-YGD (MneHV-7) and PLEN-NPFG (MneHV-6) amplicons were excised from 1% agarose gels, ligated into the pJET vector and transformed into competent DH5α cells. Selected colonies were sequenced to confirm the DNA insert and plasmid DNA was prepared as standardized templates for the MneHV-6 and MneHV-7 qPCR assays.
Statistics
Statistics was performed using GraphPad Prism version 6.01 for Windows, (GraphPad Software, La Jolla California USA. The statistical significance of differences in viral loads for groups of samples was calculated using the unpaired two-tailed Mann-Whitney t-test or the Wilcoxon matched-pairs signed rank test with 95% confidence intervals. To compare viral loads between saliva and salivary gland or pyloric stomach samples (Fig. 12), respectively, two-tailed Spearman nonparametric correlation coefficients (r2) and corresponding P-values were calculated using 95% confidence intervals.
Results
Development of CODEHOP PCR assays to identify novel roseoloviruses
In order to identify novel members of the Roseolovirus genus among the Betaherpesvirinae subfamily in Old World monkey species, we used the Consensus-Degenerate Hybrid Oligonucleotide Primer (CODEHOP) technique (Rose et al., 1998) to develop PCR primers targeting the highly conserved DNA polymerase (DNA pol) and glycoprotein B (gB) genes, as done previously (Rose, 2005). Amino acid motifs conserved within the known member species of the Betaherpesvirinae subfamily were used to design CODEHOP primer pairs that would preferentially amplify members of the betaherpesvirus family. Several primer pairs were designed using the iCODEHOP software (Boyce et al., 2009) targeting multiple conserved sites within the DNA pol and the gB genes. CODEHOP primers with low degeneracy and with a sequence bias for the known roseolovirus sequences were selected. Inosine was introduced into the degenerate cores of some primers to further lower degeneracy, and some primer consensus sequences were adjusted to more specifically target roseolovirus sequences. Primers were then paired based on compatible annealing temperatures and suitable amplicon lengths. Forty-six DNA pol-specific and 16 gB-specific CODEHOP primer pairs targeting different regions of the DNA pol and gB genes were tested with DNA from the HBS-2 cell line infected with the HHV-6A strain U1102 (Downing et al., 1987). Since the optimal annealing temperature of each CODEHOP primer pool to a novel template is not known, initial PCR reactions were performed using a temperature gradient from 50°C to 70°C. PCR products were then resolved on agarose gels (data not shown). Five DNA pol-specific and fifteen gB-specific CODEHOP primer pairs displayed robust amplification of HHV-6A over the entire temperature gradient and showed minimal non-specific amplification.
Identification of a pig-tailed macaque homolog of HHV-6
Since HHV-6 is often detected in human saliva, whole saliva from macaques was used as a source of DNA to screen for the presence of a macaque roseolovirus homolog. Archived saliva samples were available from five pig-tailed macaques that had been challenged with SHIV in a previous vaccine study at the WaNPRC (Polacino et al., 2008). Two of the animals (M03126 and M03182) had detectable viral loads of SHIV in their plasma and three animals (M02383, M02156 and L02393) were negative. DNA isolated from the five saliva samples was tested by PCR using the CODEHOP primers SLYP-F (forward) and YGD-R (reverse). These primers, which target the highly conserved “SLYP” and “YGD” amino acid motifs within the DNA pol gene (Fig. 1), had given a strong amplification signal with DNA from the HHV-6A infected HSB-2 cell line. This primer pair is similar to CODEHOP primers we have used previously to identify unknown macaque gamma-herpesviruses (Rose, 2005), but is biased towards roseoloviruses. Using the SLYP-F / YGD-R primer pair with an annealing temperature of 60°C, we detected a PCR amplicon of the expected size (~550 bp) in one of the five macaques tested (Table 1). Following gel purification and Sanger sequencing, we obtained a 478 bp nucleotide sequence of the amplified DNA fragment between the flanking primer sequences. We compared the nucleotide sequence to the non-redundant GenBank nucleotide database (NCBI) using BLASTN. The closest matches were partial DNA pol nucleotide sequences from different herpes 6 viruses (Pan troglodytes herpesvirus 6 (PanHV6), HHV-6B (strain Z29) and HHV-6A (strain U1102)) with at least 75% identity (Table 2). This suggested that we had identified a novel roseolovirus DNA pol sequence corresponding to a pig-tailed macaque homolog of HHV-6, provisionally named MneHV-6.
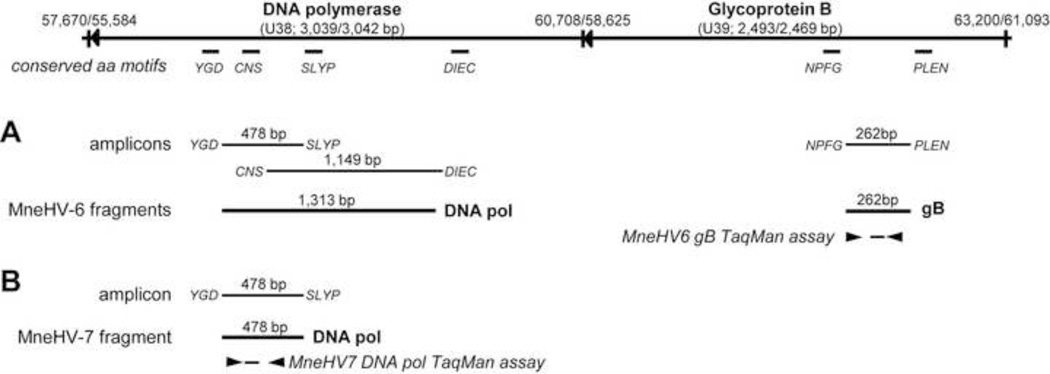
Figure 1. Schematic representation of targeted CODEHOP motifs, novel macaque roseolovirus amplicons and TaqMan assays relative to the HHV-6B and HHV-7 DNA pol and gB genes.
The genomic structure of the DNA pol and gB genes of HHV-6B (NC_000898) and the HHV-7 (NC_001716) are shown with the highly conserved amino acid motifs targeted by the CODEHOP PCR primers. CODEHOP amplicons with flanking primers and sequenced gene fragments are shown for A) MneHV-6 and B) MneHV-7.
Table 1.
Discovery of MneHV-7 and MneHV-6 in saliva of pig-tailed macaques by CODEHOP PCR.
| YGD-SLYP pol1,3 | NPFG-PLEN gB2,3 | |||
|---|---|---|---|---|
| Animal # | 50°C4 | 60°C4 | 50°C4 | 60°C4 |
| M02156 | HHV-7 | HHV-6 | HHV-6 | HHV-6 |
| M02383 | HHV-7 | nd | nd | nd |
| L02393 | HHV-7 | nd | nd | nd |
| M03126 | HHV-7 | nd | nd | nd |
| M03182 | HHV-7 | nd | nd | nd |
nd not detected
roseolovirus-specific CODEHOPs targeting DNA pol gene;
roseolovirus-specific CODEHOPs targeting glyB gene;
detection of novel roseolovirus sequences was based on acrylamide gel analysis of CODEHOP amplicons;
annealing temperatures used.
Table 2.
Sequence comparisons of macaque CODEHOP DNA pol and gB fragments with known Beta-herpesvirus sequences
| MneHV-6 | MneHV-7 | |||
|---|---|---|---|---|
| Beta- herpesvirinae |
DNA pol (YGD-SLYP; 478bp) % nucl / aa identity |
DNA pol (YGD-DIEC; 1,313 bp) % nucl / aa identity |
gB (NPFG-PLEN; 262bp) % nucl / aa identity |
DNA pol (YGD-SLYP; 478 bp) % nucl / aa identity |
| HHV-6A | 75.6 / 80.5 | 77.8 / 84.0 | 76.7 / 86.0 | 60.0 / 61.6 |
| HHV-6B | 76.4 / 80.5 | 77.8 / 84.0 | 77.9 / 86.0 | 59.7 / 61.6 |
| PanHV6 | 77.8 / 80.4 | 78.8 / 83.5 | 76.7 / 87.2 | 60.3 / 61.4 |
| HHV-7 | 65.3 / 71.1 | 67.6 / 72.7 | 63.7 / 65.1 | 69.3 / 69.2 |
| MndHVbeta1 | 61.2 / 63.5 | 61.4 / 63.5 | na | 60.2 / 55.3 |
Betaherpesvirinae used for sequence comparisons including GenBank accession numbers: HHV-6A (Human herpesvirus 6A, strain U1102; NC_001664); HHV-6B (Human herpesvirus 6B, strain Z29; NC_000898); HHV-7 (Human herpesvirus 7, strain RK; NC_001716); PanHV6 (common chimpanzee herpesvirus 6; AY854171); MndHVbeta (Mandrill herpesvirus beta; AF282942).
For MndHVbeta a DNA pol fragment of 478 bp (159aa) but no gB sequence was available for comparison with macaque HV sequences.
To obtain additional sequence for MneHV-6, additional CODEHOP primer pairs were used to amplify DNA from the same previously positive animal (M02156). The DNA pol-specific CODEHOP primer pair DIEC-F/CNS-R produced an amplicon at 60°C, which contained a sequence that overlapped the previous MneHV-6 DNA fragment, generating a 1,313 bp long contiguous sequence of the MneHV-6 DNA pol gene (Fig. 1). Alignment of this sequence with other roseolovirus DNA pol sequences revealed highest nucleotide identity with known human and chimpanzee herpesviruses 6 (Table 2). We also used a CODEHOP primer pair (NPFG/PLEN) targeting motifs conserved in the roseolovirus gB sequences. The NPFG/PLEN assay produced 326 bp PCR amplicons at both 50°C and 60°C annealing temperatures from the saliva of only one animal (M02156). No PCR products were detected in the other macaque samples. Both amplicons were gel-purified and sequenced revealing the same 262 bp sequence flanked by the primers (Figure 1, Table 1). Nucleotide sequence comparisons with known roseolovirus gB sequences showed again the highest identity with HHV-6B, HHV-6A, and with the chimpanzee PanHV6 gB sequences. Sequence analysis from two independently isolated gene fragments confirmed our identification of a novel pigtailed macaque homolog of HHV-6.
Identification of a pig-tailed macaque homolog of HHV-7
To continue the search for other roseolovirus homologs, we tested the same 5 saliva samples using the DNA pol-specific CODEHOP primers (SLYP-F, YGD-R) at a lower annealing temperature (50 °C). Low annealing temperatures allow primers to bind to a broader range of mismatched templates, enhancing the possibility of capturing more distantly related sequences. Using this annealing temperature, we detected PCR amplicons of the expected sizes (~550 bp) in all five of the macaque saliva samples. All PCR products were gel-purified and the amplified DNA fragments were sequenced, yielding the same 478 bp sequence. A comparison of this sequence with the MneHV-6 DNA pol sequence mentioned above showed only 62% nucleotide identity. BLASTN search of the NCBI database revealed a closer match with the DNA pol sequence of HHV-7 (Table 2). Alignment with other roseolovirus DNA pol sequences showed lower nucleotide identities. We were unable to obtain additional overlapping sequences using the remaining DNA pol CODEHOP primers, nor did we detect another PCR amplicon with the gB CODEHOP primers at either annealing temperature. The close similarity of the 478bp DNA pol sequence with HHV-7 suggests that we have identified a novel pig-tailed macaque homolog of HHV-7, provisionally termed MneHV-7. This virus appeared to be more widely distributed than MneHV-6, since all 5 animals were positive in our initial PCR screen (Table 1).
Phylogenetic relationship of MneHV-6 and MneHV-7
To establish the phylogenetic relationship between the putative macaque roseoloviruses and known herpesviruses, we aligned the protein sequences encoded by the 478 bp DNA fragments of the putative MneHV-6 and MneHV-7 DNA pol genes with the corresponding sequences from other herpesviruses, using the MUSCLE software and determined the phylogenetic relationship based on the Maximum Likelihood algorithm (PhyML). The MneHV-6 DNA pol sequence clustered with the DNA pol sequences of HHV-6A, HHV-6B and chimpanzee PanHV6 within the beta-herpesvirus subfamily (Fig. 2). This clustering supported the identity of MneHV-6 as a HHV-6 homolog in the pig-tailed macaque. Conversely, the MneHV-7 DNA pol sequence clustered with the DNA pol sequences of HHV-7 supporting the identity of MneHV-7 as a.HHV-7 homolog in the pig-tailed macaque.
To investigate more closely the relationship between the primate roseolovirus sequences, we performed a maximum likelihood analysis of the nucleotide sequences of the CODEHOP DNA pol fragments of MneHV-6 and MneHV-7 and the corresponding sequences from known roseoloviruses. As seen with the amino acid analysis, the MneHV-6 DNA pol nucleotide sequence clustered with the HHV-6A, HHV-6B and PanHV6 sequences confirming the close association between these HHV-6 homologs (Fig. 3). The clustering relationship of the MneHV-6, PanHV6 and the two human HHV-6 species is consistent with a long term co-evolution of these HHV-6 homologs with their primate host species and further supports the identity of MneHV-6 as a macaque homolog of HHV-6. The MneHV-7 DNA pol nucleotide sequence clustered with the HHV-7 sequence, further confirming its identity as a macaque homolog of HHV-7 (Fig. 3).
Development of MneHV-6 and MneHV-7 specific quantitative PCR assays
To conduct more sensitive and comprehensive screening of pig-tailed macaques for roseolovirus infection and to determine viral loads in various tissues, we developed specific quantitative real-time PCR assays (qPCR) based on primers and TaqMan probes targeting the MneHV-7 DNA pol or the MneHV-6 gB gene segment identified with our CODEHOP assays (Fig. 1). For assay optimization and accurate quantification of assay efficiencies, we created standardized plasmids as control templates with inserts containing the MneHV-6 gB and the MneHV-7 DNA pol regions targeted by the CODEHOP PCR primers. Several primer combinations and candidate TaqMan probes were evaluated for efficiency and sensitivity using decreasing concentrations of the recombinant plasmids. Based on these criteria, a set of primers and probe was selected for each virus. For both quantitative assays, the standard curves were linear (r2=1.0) and their slope coefficients indicated an amplification efficiency superior to 90% (data not shown).
Detection of MneHV-6 and MneHV-7 in saliva from pig-tailed macaques by qPCR
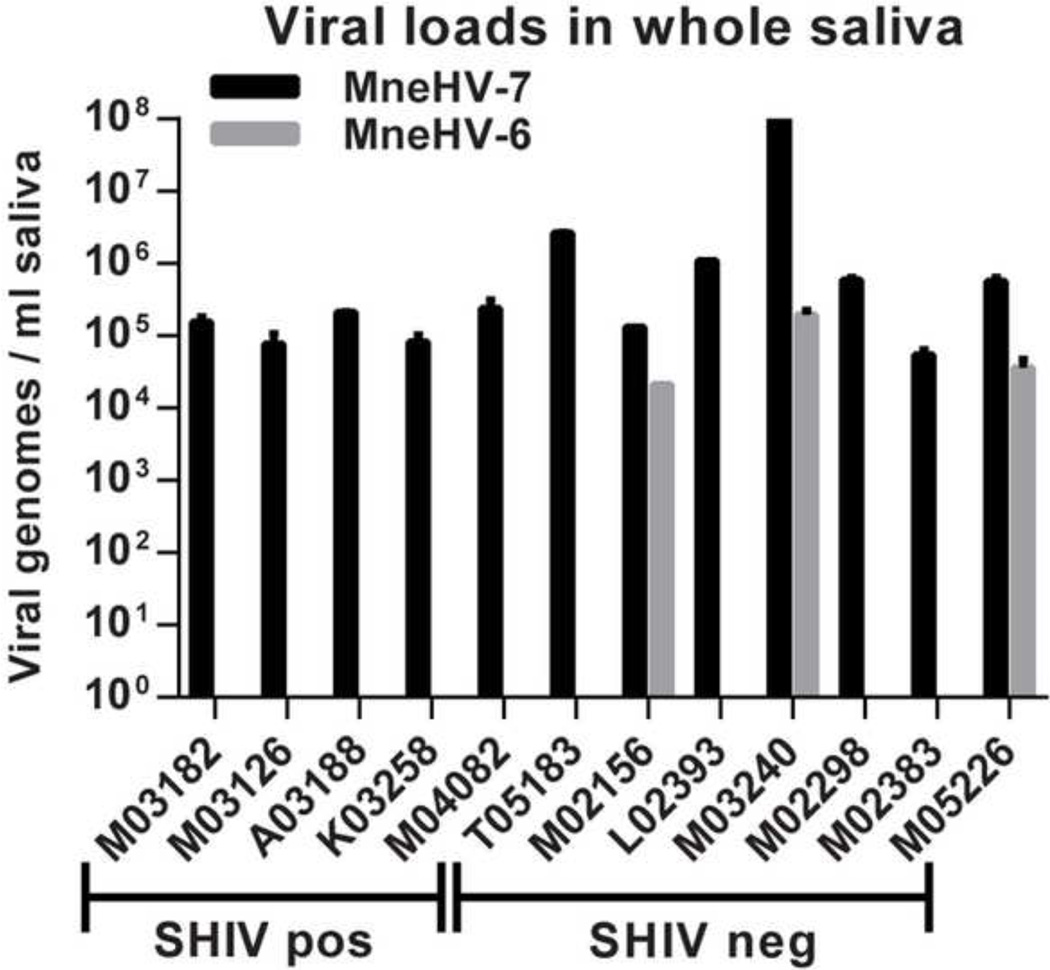
Using the qPCR assays, we determined viral loads for both MneHV-6 and MneHV-7 in archived whole saliva samples available from twelve pig-tailed macaques from the SIV vaccination study, including the five animals tested originally by CODEHOP PCR. Of the 12 animals, five were classified as long term non-progressors, since they were able to control the SHIV infection with only moderate levels of detectable SHIV in plasma (SHIV-positive). The remaining seven animals had developed a sterilizing immunity to the SHIV challenge, with no detectable SHIV in their blood (SHIV-negative). MneHV-7 was detected in all 12 saliva samples, while MneHV-6 was detected in only three SHIV-negative negative animals (Fig. 4). Although the MneHV-7 viral loads detected in the SHIV-positive animals (median: 1.5×105 genome copies / ml) were 4-fold lower than in the SHIV-negative animals (median: 6×105 genome copies /ml), this increase was not significantly different (p=0.14) These qPCR data confirmed our previous CODEHOP PCR results and showed that in contrast to MneHV-6, MneHV-7 was highly prevalent in macaque saliva at high viral loads in this study population.
Figure 4. Detection and quantification of MneHV-6 and MneHV-7 DNA in macaque saliva.
DNA isolated from whole saliva from five SHIV-positive and six SHIV-negative pig-tailed macaques that had been challenged with SHIV strain SF162P4 in a previous SIV vaccine study was tested in duplicate using the MneHV-6 and MneHV-7 qPCR assays and viral loads were expressed as viral genome copies per ml of saliva.
Longitudinal analysis of MneHV-6 and MneHV-7 oral shedding in pig-tailed macaques
Viral DNA detected in saliva can have different origins. One source is infectious virions released from infected oral epithelium and salivary glands, which is an indication of active viral replication. Other sources include viral DNA in infected cells sloughed from the oral epithelium or viral DNA released from lysed/disrupted infected cells. To assess the level of cell-associated viral DNA, we fractionated the saliva by centrifugation to obtain a cell pellet and a cell-free supernatant, which was filtered through a 0.45 micron filter to remove cellular contamination. The supernatant contains either DNA from infectious virions that passed through the filter or soluble viral DNA released from lysed infected cells. More recently, oral swabs have been used to quantitate virus in the oral cavity by gently scraping the accessible epithelial tissue (Malamud, 2011). The swab captures cells from the outer epithelial layer and can also absorb a small volume of saliva. Quantitation of virus in saliva and oral swabs has been used as a measure of viral shedding and replication. However, the respective ability of the different collection methods to accurately quantitate and characterize virus in the oral cavity has not been well established.
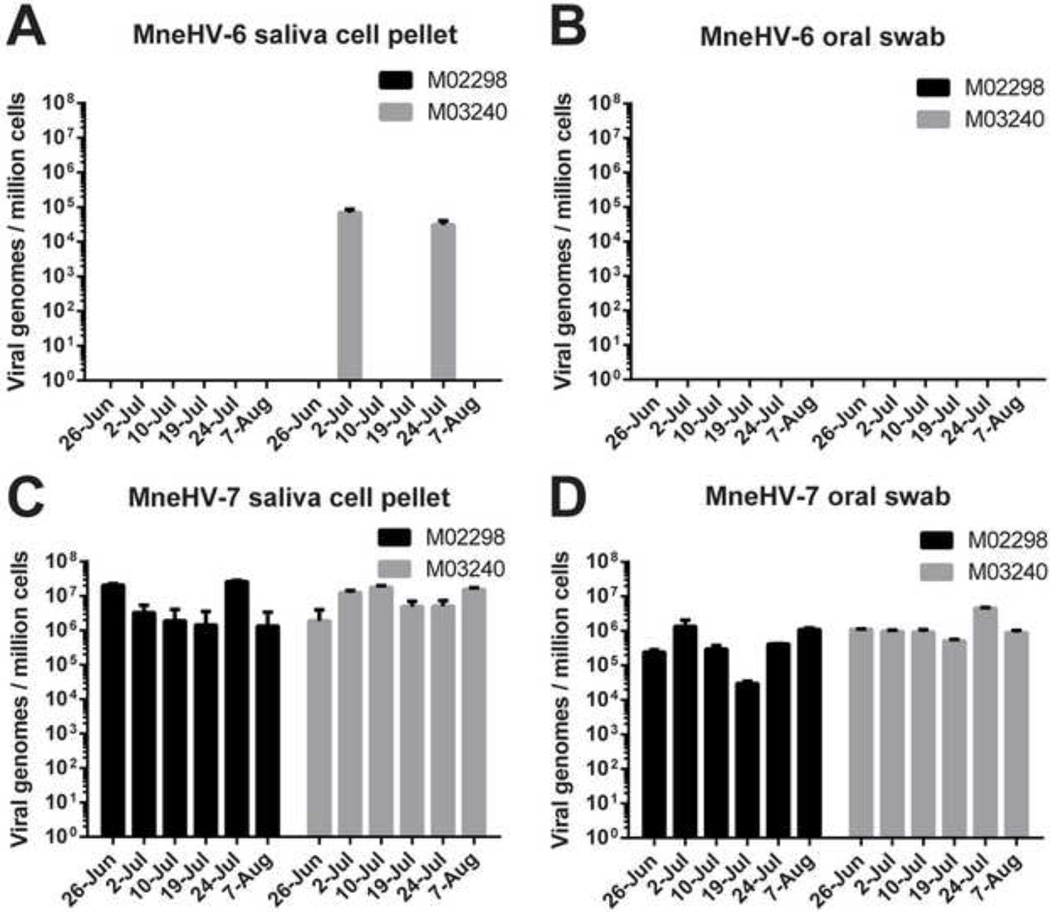
To study the shedding patterns of MneHV-6 and MneHV-7 in the oral cavity and to obtain evidence of roseolovirus activation and replication, archived longitudinal saliva samples from two SHIV-negative macaques (M02298 and M03240) from the same SIV vaccine study were assayed for the presence of both viruses. Both animals had tested positive for MneHV-7 in the initial screen of a single saliva sample, and one (M03240) was also positive for MneHV-6 (Fig. 4). Saliva and oral swabs had been collected weekly from these animals for a total period of 6 weeks. Whole saliva had been fractionated into a filtered cell-free supernatant and a cell pellet, and oral swabs had been collected to sample the exposed epithelium in the oral cavity. DNA was extracted from the different samples, and MneHV-6 and MneHV-7 viral DNA was quantitated by specific TaqMan qPCR assays. MneHV-7 was systematically detected in both cell-free and cell-associated saliva fractions from the two macaques over the entire time period (Fig. 5C and D). The MneHV-7 viral DNA loads in both fractions varied from week to week for both animals. In contrast, MneHV-6 was detected sporadically, at only two time points over the same time period in only one of the two macaques (M03240) (Fig. 5A and B). MneHV-6 was detected in both the cell-free supernatant and cell pellet at only one time point and in the cell pellet only at an additional time point. In this animal, the MneHV-6 loads were ~1,000 fold lower in the cell-free supernatant and ~100 fold lower in the cell pellet than the corresponding MneHV-7 loads.
Figure 5. Longitudinal analysis of MneHV-6 and MneHV-7 DNA in saliva.
Saliva samples were collected weekly for 6 weeks from two SHIV-negative pig-tailed macaques (M03240 and M02298) from a previous SIV vaccine study. DNA was isolated from either filtered cell-free saliva supernatants (A, C) or pelleted saliva cells (B, D) from the same saliva samples. Viral loads for MneHV-6 (A, B) and MneHV-7 (C, D) were determined using the specific qPCR assays in duplicate and expressed as viral genome copies per ml of saliva.
For the majority of time points tested, the viral loads for both MneHV-6 and MneHV-7 were higher in the cell pellet than in the cell-free supernatant (Fig. 5). Over the entire collection period, the median level of MneHV-7 genomes/ml of saliva was 13 and 2 fold higher in the cell pellet compared to the cell-free supernatant in animals M02298 and M03240, respectively. These viral loads values were significantly different in the case of macaque M02298 (p=0.004) but not for M03240 (p=0.093). At the time point where MneHV-6 was detected in both fractions in M03240, the level of MneHV-6 was more than 10 fold higher in the cell pellet than in the cellfree supernatant. At the other time point in which M03240 was positive for MneHV-6, no MneHV-6 was detected in the cell-free supernatant while still detected in the cell pellet. Currently, we are unable to determine whether the viral DNA present in the cell-free supernatant is from infectious virus particles due to the lack of an adequate in vitro model for MneHV-6 and MneHV-7 propagation. However, our results indicate that the vast majority of MneHV-7 genomes present in whole saliva of M02298 and M03240 represents cell associated viral DNA (93% and 73%, respectively) and not cell-free virus particles.
To further analyze the cell-associated viral DNA, the qPCR data for MneHV-6 and MneHV-7 were normalized using the OSM cellular gene qPCR to determine viral genome copies per cell. The calculated ratio represents an average number of viral genome copies per cell based on all cells collected in each sample and as such, underestimates the number of viral genome copies present only among infected cells in the sample. The viral genome copies per cell for MneHV-6 in the cell associated fraction of whole saliva from M0320 from the two time points was at most (on 02-Jul) one virus copy for every 14 cells (Fig. 5B). In contrast, the median viral load for MneHV-7 in the cell fraction of whole saliva was 2.6×106 and 8.7×106 viral genomes/ million cells, in M02298 and M03240, respectively (Fig. 5D). This average number of viral genomes is sufficient to have all of the cells present in whole saliva infected with 2–8 viral genomes per cell, consistent with an overall latent infection in every cell. If only a fraction of the cells present in whole saliva were infected then each infected cell would contain a much higher viral load, suggestive of active viral replication.
To further investigate the origin of the cell-associated viral DNA, we compared viral loads in cells present in whole saliva with viral loads in cells sampled from the gum and cheek epithelial surfaces using the oral swab. MneHV-6 was not detected in the oral swabs from either macaque at any time point including those times when virus was detected in the cell-associated fractions of whole saliva from M03240 (Figs. 6A and B). As seen with whole saliva, MneHV-7 DNA was systematically detected in oral swabs from both animals over the entire collection period (Figs. 6C and D). The median viral loads for MneHV-7 in oral swab samples were 3.5×105 and 9.3×105 viral genomes per million cells in M02298 and M03240, respectively. Thus, the MneHV-7 load in the cells sampled by the oral swab was 7 to 9 fold lower than the viral load in the cell pellet from whole saliva at the same time point (Figs. 6C and D; p=0.03 for both M02298 and M03240). This would indicate that there is either a lower prevalence of MneHV-7 infected cells in the oral epithelium sampled by the swab when compared to cells naturally sloughed into saliva, or a lower level of virus replication in the oral epithelium sampled by the swab. Surprisingly, there was no correlation between MneHV-7 loads detected in cells present in whole saliva and cells sampled from the epithelium by the swab at identical time points (r2 = 0.02, p =1.0 and r2 = −0.25, p =0.65 for M02298 and M03240 respectively).
Figure 6. Comparison of cell-associated MneHV-6 and MneHV-7 viral loads in whole saliva and oral swab samples.
The viral loads of MneHV-6 (A, B) and MneHV-7 (C, D) were determined from DNA isolated from the cell fraction of whole saliva (A, C) and from DNA isolated from oral swabs (B, D) collected at the same time point from two pig-tailed macaques (M03240 and M02298) using the MneHV-6 and MneHV-7 qPCR assays in duplicate. Viral loads are expressed as viral genome copies per cell, as described in Materials and Methods. MneHV-6 was not detectable in oral swab samples collected from either animal over the entire time period (B).
Prevalence of MneHV-6 and MneHV-7 in PBMC of pig-tailed macaques
While we have shown that saliva samples are a good source of macaque roseolovirus DNA, there is no general archive of macaque saliva available at the WaNPRC to examine viral prevalence. Human herpesviruses 6 and 7 were originally isolated from peripheral blood lymphocytes (Frenkel et al., 1990; Salahuddin et al., 1986), and peripheral blood mononuclear cells (PBMC) are routinely collected as part of the WaNPRC colony health monitoring program. Therefore, we obtained 283 archived PBMC DNA samples from the WaNPRC to screen for MneHV-6 and MneHV-7 using our specific qPCR assays. Samples were considered positive if both qPCR replicates tested positive. Based on the efficiencies for both assays,, the limit of detection is less than 50 genomes in ~300 ng DNA typically used as template amounts in the PCR reactions. Overall, MneHV-6 was detected in 29 of the 283 PBMC samples (10% prevalence) (Table 3). MneHV-7 was detected in 72 of the 283 PBMC samples (25% prevalence). In addition, 12 macaques (4%) had detectable levels of both viruses in their saliva. To further characterize roseolovirus prevalences, animals were grouped based on gender, age, and origin. Virus prevalence among females was 11% for MneHV-6 and 24% for MneHV-7, while prevalence among males was 9% for MneHV-6 and 27% for MneHV-7 (Figs. 7A and B, respectively), showing no significant difference between males and females.
Table 3.
Prevalence of MneHV-6 and MneHV-7 DNA in macaque PBMC.
| MneHV-6 pos1 | MneHV-7 pos1 | |
|---|---|---|
| Overall (n=283) | 29 (10.2%) | 72 (25.4%) |
| Females (n=153) | 19 (11.0%) | 42 (24.4%) |
| Males (n=101) | 10 (9.0%) | 30 (27.0%) |
Presence of MneHV-6 or MneHV-7 sequence in PBMC was assessed by virus-specific qPCR to determine the percent positive (%). Samples were considered positive if both qPCR replicates tested positive.
Figure 7. Age-related prevalence of MneHV-6 and MneHV-7 in males and females.
The MneHV-6 and MneHV-7 qPCR results of the 283 pig-tailed macaques were sorted by age (in years) and gender. MneHV-6 (A) and MneHV-7 (B) prevalence in PBMC is reported as percentage of monkeys with a detectable viral load in each age group. The numbers of samples per age group are indicated.
We also assessed virus prevalence for different age groups. Since more females than males are used for breeding purposes, female animals tend to have a larger representation among older animals at the WaNPRC. In order to compare prevalence between males and females, we limited the analysis to an age range of 2 to 10 years where animals from both genders had a more equal representation. MneHV-6 prevalence differed remarkably between populations of females and males. In young male macaques, MneHV-6 DNA was only detected between the ages of 2 and 4 (Fig. 7A) with a prevalence of 13% (9/69). Males between the ages of 5 to 10 were negative for MneHV-6 (0/31). In contrast, MneHV-6 was not detectable in young females, ages 2–4 (0/57). Females between the ages of 5 to 10 were positive for MneHV-6, with an overall prevalence of 16% (14/83). The highest prevalence of MneHV-6 DNA was observed in older females at 9 years of age (30%). For MneHV-7, virus was detected throughout the ages 2–7 for both males and females with overall prevalences of 32% for males (N=90) and 33% for females (N=113). The highest prevalence for MneHV-7 was observed in 4 year old males (37%) and 3 year old females (77%) (Fig. 7B).
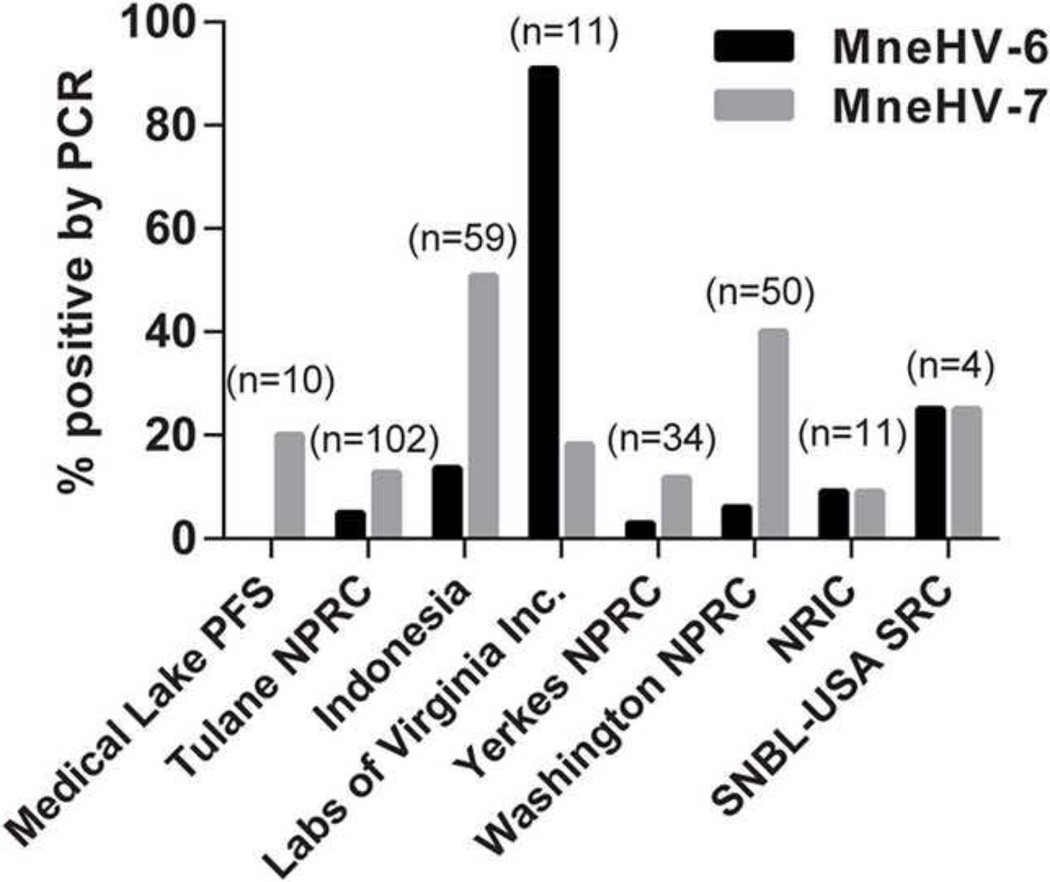
The WaNPRC manages one of the largest colonies of pig-tailed macaques in the country. Animals are obtained from several breeding colonies located in- and outside the United States (Kanthaswamy et al., 2012). In addition to the Seattle breeding colony, animals have been obtained from the Medical Lake Primate Field Station, the Tulane National Primate Research Center, New Iberia Research Center, USA-Scientific Resource Center, and from Indonesia. Additional macaques were also purchased from animal facilities such as Labs of Virginia Inc., Osage Research Primates, and the Yerkes National Primate Research Center. Animals originating from all locations are screened biannually at the WaNPRC as part of the colony health testing. To determine whether there was a relationship between virus prevalence and facility of origin, macaques were stratified by colony/facility of origin based on the available information from the animal records (Fig. 8). The highest MneHV-7 prevalence was observed in pig-tailed macaques originating from the wild breeding colony in Indonesia (50%) and the lowest prevalence was observed in animals originating from the New Iberia Research Center (9%). MneHV-6 was most prevalent in animals obtained from the Labs of Virginia (90%) and was much lower or absent in animals from all other locations. Only one pig-tailed macaque had been originally purchased from Osage Research Primates company and tested negative by qPCR for both MneHV-6 and MneHV-7 (data not shown).
Figure 8. Variation in prevalence of MneHV-6 and MneHV-7 among pig-tailed macaques from different breeding colonies.
Pig-tailed macaques maintained at the WaNPRC are acquired from different breeding colonies. The prevalence of MneHV-6 and MneHV-7 was determined in PBMC samples from macaques grouped by their breeding colony of origin. Prevalence is reported as percentage of samples with detectable viral loads in PBMCs for each location of origin. The numbers of samples per colony are indicated. (Medical Lake Primate Field Station: Medical Lake PFS, Tulane National Primate Research Center: Tulane NPRC, Yerkes National Primate Research Center: Yerkes NPRC, Washington National Research Station: WaNPRC , New Iberia Research Center: NIRC, Shin Nippon Biomedical Laboratories USA-Scientific Resource Center: SNBL USA SRC).
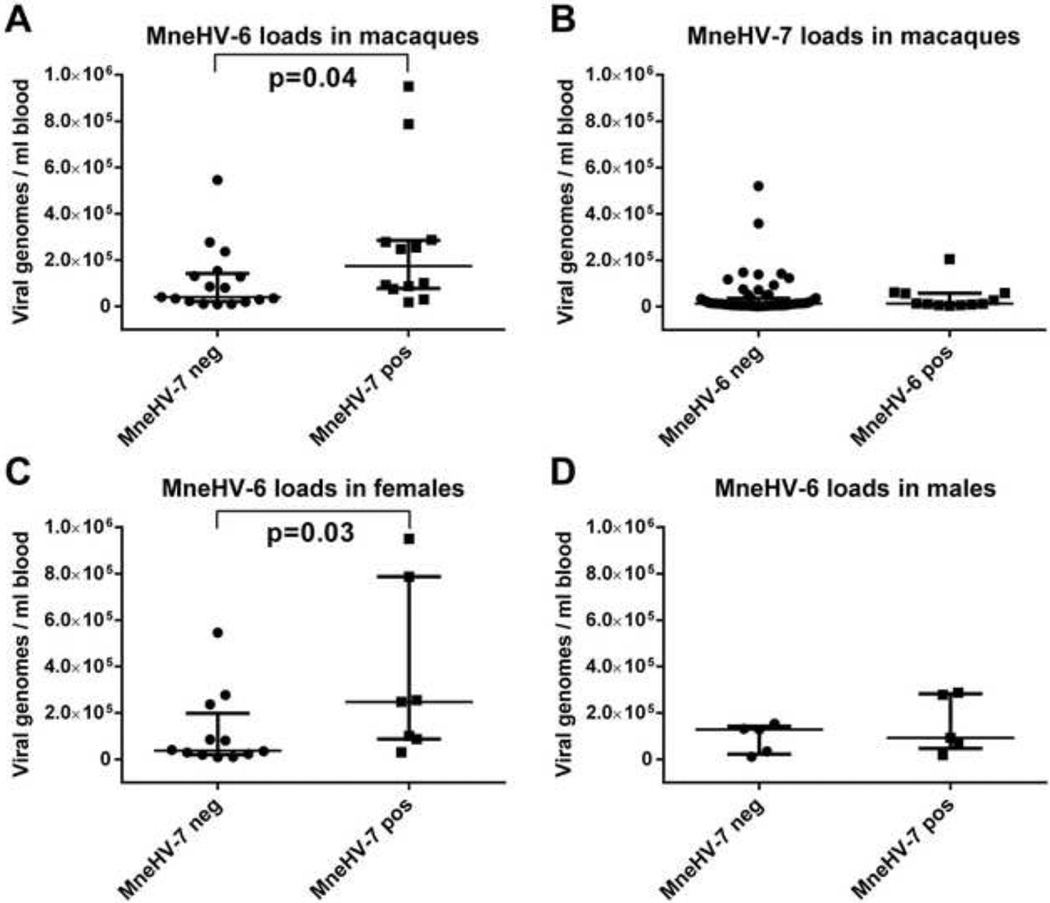
Examination of viral loads in the 283 macaque PBMC samples revealed an overall median viral load of MneHV-6 (~8.0×104 genomes/ml blood) that was 7 fold higher than the median viral load of MneHV-7 (~1.0×104 genomes/ml blood), which was significantly different (p<0.0001). Analysis of PBMC samples containing both viruses revealed a 4 fold higher median viral load of MneHV-6 (~1.5×105 viral genomes/ml blood) in animals with detectable levels of MneHV-7 compared to animals without MneHV-7 DNA (~4.0×104 viral genomes/ml blood), which was statistically significant (p=0.04) (Fig. 9A). In contrast, the MneHV-7 median viral load was equivalent in PBMC with or without detectable MneHV-6 DNA (~1.0×104 vs ~1.0×104 viral genomes/ml blood, respectively) (Fig. 9B). Further analysis revealed that the increase in MneHV-6 viral loads in MneHV-7 infected PBMC was only observed in the female population (p=0.03) (Fig. 9C)
Figure 9. Effect of coinfection on MneHV-6 and MneHV-7 viral loads in PBMC.
The viral copy number of MneHV-6 and MneHV-7 DNA in PBMC was compared between singly and co-infected animals. A) MneHV-6 viral loads in MneHV-7 negative and positive macaques; B) MneHV-7 viral loads in MneHV-6 negative and positive macaques; C) MneHV-6 viral loads in MneHV-7 negative and positive female macaques; D) MneHV-6 viral loads in MneHV-7 negative and positive male macaques. Results are expressed as viral genome copies per ml of blood. Statistical comparison of groups was performed using an unpaired two-tailed Mann-Whitney t-test and the mean and 95% confidence interval is indicated.
MneHV-6 and MneHV-7 tissue tropism in naturally infected macaques
HHV-6A has a broad cellular tropism most likely due to the ubiquitous expression of its essential membrane receptor CD46 on almost all primate cells. On the other hand, HHV-6B who uses CD134 as entry receptor is most frequently detected in PBMCs but surprisingly also in a wide range of tissues (Di Luca et al., 1996). Similarly , while the known HHV-7 membrane receptor CD4 is only expressed on a limited set of cell types, HHV-7 has been detected in many different types of tissues in the human body (Kempf et al., 1998).
To investigate the tissue tropisms of MneHV-6 and MneHV-7 in naturally infected pigtailed macaques, the MneHV-6 and MneHV-7 viral loads were determined for a panel of tissue samples collected from the initial group of 12 pig-tailed macaques. The twelve animals were age-matched female pig-tailed macaques. DNA was isolated from formalin-fixed paraffin-embedded tissue sections from biopsies collected from different organs at necropsy and analyzed by qPCR. Viral loads per cell for each tissue section were determined using the OSM specific qPCR.
While only 3 of the 12 animals had tested positive for MneHV-6 DNA in the available saliva samples (Fig. 4), 8 animals had detectable MneHV-6 DNA in one or more tissues (Table 4), indicating that the prevalence of MneHV-6 DNA in saliva significantly underestimated the prevalence of MneHV-6 infection in these animals. Four animals had MneHV-6 DNA in salivary glands (parotid gland) and two of these (M02156 and M05226) had been positive for MneHV-6 DNA in saliva. None of the animals had detectable MneHV-6 in gingival and tonsil samples and only one, M02156, had MneHV-6 DNA in the cheek sample. In the stomach, tissue biopsies were collected from three histologically different regions: cardiac, fundic and pyloric. The cardiac region is located near the esophageal orifice and contains mainly mucous secreting glands. The fundic region composes the largest part of the stomach. Gastric glands in the fundic stomach produce digestive enzymes and hydrochloric acid. The pyloric region is found proximal to the pyloric sphincter which leads to the duodenum. Glands in the pyloric epithelium contain specialized cells secreting either mucus or the peptide hormone gastrin. Seven of the 8 MneHV-6 positive animals had MneHV-6 DNA in the fundic stomach tissue. Animals M03182 and M05226 also had MneHV-6 DNA in the cardiac and pyloric stomach tissue, while T05183 was also positive in the pyloric stomach. M05226, which was positive in saliva, salivary glands, and all three regions of the stomach, was also positive in spleen, pancreas and liver. All other animals had no detectable MneHV-6 DNA in spleen, pancreas, or liver. None of the animals had detectable MneHV-6 DNA in skin or kidney tissue. The highest viral loads of MneHV-6 were observed in the fundic region of the stomach, with up to 6 viral genomes / cell (M03240). This average ratio of viral genome copies per cell is largely underestimated since it is likely that not all the cell types found in the stomach tissue are infected. Interestingly, none of the SHIV-positive animals had detectable MneHV-6 in saliva or oral tissues. Furthermore, the only animal (M05226) with widespread MneHV-6 infection in tissues other than oral and gastric, was SHIV-negative. No significant difference was detected in the MneHV-6 viral loads in the fundic stomach of the SHIV-positive and SHIV-negative animals.
Table 4.
MneHV-6 tissue distribution in naturally infected pig-tailed macaques.
| Animal | SHIV1 | Saliva2 | Skin | Cheek | Gingiva | Tonsil | Parotid gland |
Fundic stomach |
Cardiac stomach |
Pyloric stomach |
Spleen | Pancreas | Liver | Kidney |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M03182 | + | − | − | − | − | − | − | 2*103 | 7*103 | 4.9*103 | − | − | − | − |
| K03258 | + | − | − | − | − | − | − | 5.4*104 | − | − | − | − | − | − |
| M04082 | + | − | − | − | − | − | − | 3.1*106 | − | − | − | − | − | − |
| T05183 | − | − | − | − | − | − | 5.7*104 | 9.5*102 | − | 9*103 | − | − | − | − |
| M02156 | − | 2.1*104 | − | 1*104 | − | − | 8.1*101 | − | − | − | − | − | − | − |
| L02393 | − | − | − | − | − | − | 1.1*106 | 2.5*104 | − | − | − | − | − | − |
| M03240 | − | 2.0*105 | − | − | − | − | − | 6.2*106 | − | − | − | − | − | − |
| M05226 | − | 3.6*104 | − | − | − | − | 3.7*104 | 1.9*104 | 7.8*104 | 1*105 | 1*104 | 2.7*104 | 1.9*104 | − |
DNA was isolated from formalin-fixed paraffin-embedded tissue sections obtained at necropsy from five SHIV-positive long-term non-progresssors and seven SHIV-negative macaques that had been differentially vaccinated and challenged with SHIV strain SF162 P4 in a previous SIV vaccine study. Viral loads were determined using the MneHV-6 and cellular OSM qPCR assays in duplicate and are reported as average MneHV-6 genome copies per million cells, as described in Materials and Methods. Two of the SHIV-positive and two of the SHIV-negative animals had undetectable levels of MneHV-6 in all samples tested (not shown).
SHIV status of the animals was determined by weekly testing of SHIV viral loads in plasma by SHIV qPCR
MneHV-6 genome copies / ml whole saliva, which had been collected at various time points at or before necropsy.
MneHV-7 DNA was detected in tissues from all 12 macaques of the original vaccine cohort, which had all tested positive for MneHV-7 in saliva (Fig 5., Table 5). All of these animals had MneHV-7 DNA in salivary gland tissues. Although MneHV-6 DNA was only detected in saliva and salivary glands of SHIV-negative animals, MneHV-7 DNA was detected in saliva and salivary glands of both SHIV-positive and SHIV-negative animals. However, the highest MneHV-7 viral loads in saliva and salivary gland were detected in a SHIV-negative animal (M03240) and the median MneHV-7 viral loads in the saliva and salivary gland were 4 and 27 fold higher in the SHIV-negative animals compared to the SHIV-positive animals, respectively. Since two of the seven SHIV-negative animals had low levels of MneHV-7, these differences were not significant. Six animals had MneHV-7 in other oral tissues, including cheek, gingiva and/or tonsils. All animals except for M03126 had MneHV-7 in the stomach with 6/12 positive in the fundic region, 7/12 positive in the cardiac region and 9/12 positive in the pyloric region. Two of the 12 animals were positive for MneHV-7 in spleen, while none were positive in skin, pancreas, liver or kidney.
Table 5.
MneHV-7 tissue distribution in naturally infected pig-tailed macaques.
| Animal | SHIV1 | Saliva2 | Skin | Cheek | Gingiva | Tonsil | Parotid gland |
Fundic stomach |
Cardiac stomach |
Pyloric stomach |
Spleen | Pancreas | Liver | Kidney |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M03182 | + | 1.5*105 | − | 9.6*102 | − | 2*102 | 4.6*104 | − | 5.7*102 | 8.9*103 | 4*104 | − | − | − |
| M03126 | + | 7.7*104 | − | − | − | − | 1.6*105 | − | − | − | − | − | − | − |
| A03188 | + | 2.1*105 | − | − | − | − | 4.2*104 | − | 8.7*102 | − | − | − | − | − |
| K03258 | + | 8.2*104 | − | − | − | 7*101 | 5.1*104 | 5.8*103 | − | − | − | − | − | − |
| M04082 | + | 2.4*105 | − | 6*102 | − | 2*103 | 1.8*105 | 1.9*106 | − | 3.1*103 | − | − | − | − |
| T05183 | − | 2.7*106 | − | − | 1.9*103 | − | 3.2*106 | 2.8*104 | 5.2*104 | 3.3*106 | 9.9*102 | − | − | − |
| M02156 | − | 1.3*105 | − | − | − | − | 1.4*106 | − | 2.1*103 | 1.4*105 | − | − | − | − |
| L02393 | − | 1.1*106 | − | − | − | − | 1.4*106 | 8.2*103 | 1.3*104 | 8.3*106 | − | − | − | − |
| M03240 | − | 2.0*108 | − | 1.6*103 | − | − | 2.4*107 | 2*105 | − | 2.5*105 | − | − | − | − |
| M02298 | − | 5.9*105 | − | − | − | − | 2.1*107 | − | 9.3*103 | 1.9*103 | − | − | − | − |
| M02383 | − | 5.5*104 | − | 4.7*102 | − | − | 8*103 | − | − | 8.3*103 | − | − | − | − |
| M05226 | − | 5.7*105 | − | − | − | − | 7*103 | 4.7*103 | 2.1*104 | 2.3*104 | − | − | − | − |
DNA was isolated from formalin-fixed paraffin-embedded tissue sections obtained at necropsy from five SHIV-positive long-term non-progressors and seven SHIV-negative macaques that had been differentially vaccinated and challenged with SHIV strain SF162 P4 in a previous SIV vaccine study. Viral loads were determined using the MneHV-7 and cellular OSM qPCR assays in duplicate and are reported as the average MneHV-7 genome copies per million cells, as described in Materials and Methods.
SHIV status of the animals was determined by weekly testing of SHIV viral loads in plasma by SIV qPCR
MneHV-7 genome copies / ml whole saliva, which had been collected at various time points at or before necropsy
The highest MneHV-7 viral loads were detected in salivary glands attaining an average ratio of 24 viral genomes per cell (M03240). This viral load strongly suggests that active viral replication was ongoing in salivary gland tissues. High viral loads were also detected in the stomach, with loads reaching an average of 8 viral genomes per cell in the pyloric stomach sample (L02393). This average ratio of viral genome per cell is unlikely to represent a widespread latent infection but rather a lytic replication in a limited number of cells present in these tissue samples.
The spread of MneHV-7 infection in spleen, tonsil, cheek and gingival epithelium was variable in the 12 animals with low viral loads. While there was no statistically significant difference between MneHV-7 levels in SHIV positive and SHIV negative animals for most tissues (salivary glands: p=0.25; fundic stomach: p=0.49; and cardiac stomach: p=0.33), the MneHV7 viral loads in the pyloric stomach were significantly higher in SIV-negative animals compared to SIV-infected animals (P=0.016).
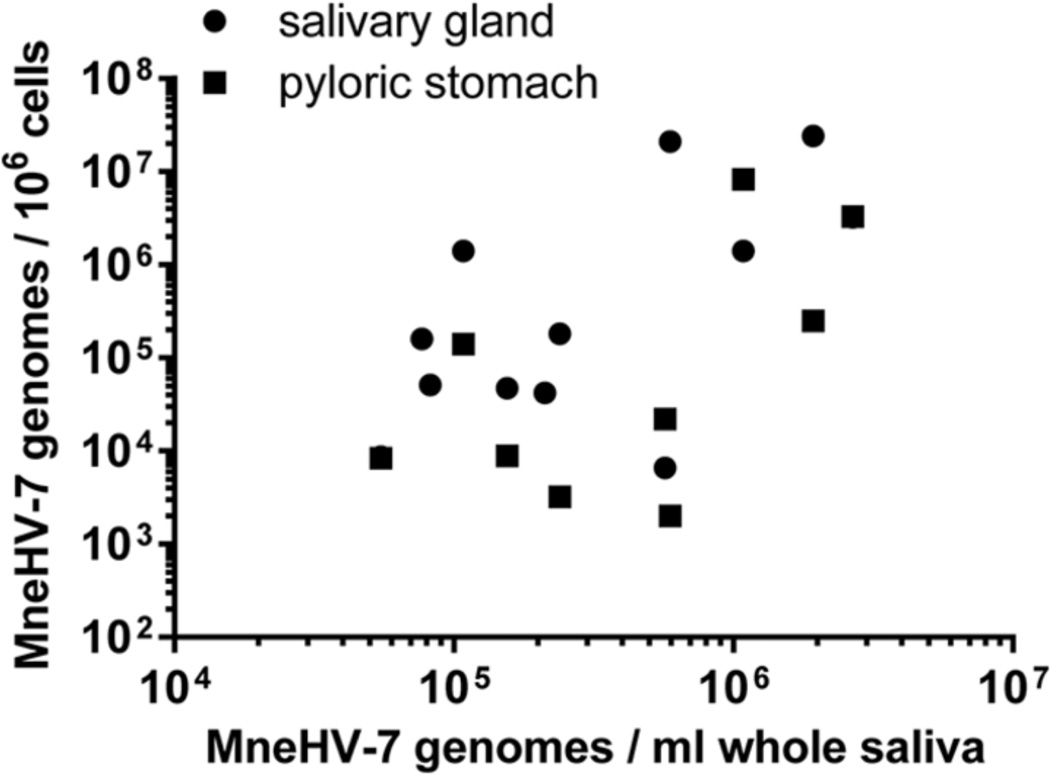
We also examined the relationship between the viral load of MneHV-7 in whole saliva and in orogastric tissues (salivary glands and pyloric stomach) for all twelve macaques. Interestingly, a strong correlation was detected not only between MneHV-7 loads in whole saliva and salivary glands (r2=0.61, p=0.039) but also between whole saliva and the pyloric stomach (r2=0.62, p=0.034) (Figure 10). This data suggests that MneHV-7 infection status in different organs within the orogastric system is reflected in the viral loads detected in whole saliva.
Figure 10. Correlation between MneHV-7 loads in saliva and orogastric tissues.
The MneHV-7 viral loads in whole saliva (genome copies/ml saliva) were compared to the viral loads in the salivary gland and pyloric stomach tissue (genome copies/ million cells). Tissues were obtained at necropsy, while saliva samples were taken at various earlier time points. Statistical significance of the correlations was assessed by two-tailed Spearman nonparametric correlation with 95% confidence intervals (salivary glands: r2=0.61, p=0.039; pyloric stomach r2=0.62, p=0.034).
Discussion
This study provides the first identification and characterization of two new roseolovirus homologs of HHV-6 and HHV-7 naturally infecting pig-tailed macaques (Macaca nemestrina). Pig-tailed macaques are one of the major macaque species maintained at the WaNPRC, and are commonly used for biomedical research since they develop disease pathologies similar to those seen in humans. Using a CODEHOP PCR primer approach, we identified the provisionally named MneHV-6 and MneHV-7 viruses based on the amplification of a 1,575 bp and a 478 bp DNA segment with homology to the HHV-6 and HHV-7 genome sequences, respectively. It was of interest that the same CODEHOP PCR primers targeting the conserved roseolovirus DNApol gene amplified the MneHV-6 sequence at one annealing temperature (60°C) and the MneHV-7 sequence at a lower annealing temperature (50°C), highlighting the importance of a comprehensive molecular strategy for the discovery of novel viruses even in closely related species. Complete sequencing of the MneHV-6 and MneHV-7 genomes will be needed to fully determine the structural and functional similarities between these viruses and their counterparts in humans.
Phylogenetic analysis of the DNA pol gene fragments confirmed that MneHV-6 and MneHV-7 belong to separate Roseolovirus lineages. The MneHV-6 sequence clustered together with sequences of the two HHV6 viruses (6A) and (6B) and PanHV6, a chimpanzee virus closely related to HHV6, within a lineage we have provisionally termed roseolo 1. MneHV-7 clustered together with HHV7 and MndHVbeta, a herpesvirus from mandrills, an Old World monkey related to baboons, within a tentatively named roseolo 2 lineage. While only one chimpanzee roseolovirus PanHV6 and one mandrill roseolovirus, MndHVbeta have been identified so far, our discovery of MneHV6 and MneHV7 in macaques clearly demonstrates that the two Roseolovirus lineages existed before the divergence of monkeys and hominoids, some 30 million years ago. We observed significantly longer branch lengths in the DNA PhyML analysis of the roseolo 2 lineage DNA pol gene fragments (Fig. 3), suggesting a higher rate of evolutionary divergence than seen in the roseolo 1 lineage. Analysis of additional gene fragments will be necessary to confirm this evolutionary difference. Within the roseolo 1 lineage, the HHV-6A and HHV-6B sequences clustered closely together in our analysis (Fig. 3), distinct from the PanHV6 and MneHV-6, suggesting that the divergence of the two HHV-6 species occurred after the divergence of chimpanzees from humans. Thus, we would not expect to see separate macaque homologs of HHV-6A and HHV-6B.
Using the available sequence information, we designed specific PCR assays to detect and quantitate the levels of MneHV-6 and MneHV-7 in tissue to determine virus prevalence, oral shedding patterns, and tissue tropism. Initially, we examined available saliva samples from a cohort of twelve pig-tailed macaques that were part of an experimental SIV vaccination study (Polacino et al., 2008). While these macaques had undergone different vaccination protocols, they had all been challenged with SHIV (Vlasak and Ruprecht, 2006). Four to six years post challenge the macaques were still healthy, with five showing evidence for a low level ongoing SHIV infection, while the remaining seven animals showed no evidence of SHIV in plasma samples. We fractionated the saliva to determine the respective contribution of cell-free and cell-associated virus to the viral DNA shed into saliva. While MneHV-6 and MneHV-7 DNA was detected in both cell free supernatant and the cell pellet of whole saliva, the ratio of cell-free to cell-associated viral DNA was variable. In most instances, the cell-associated DNA constituted the vast majority of the viral DNA in whole saliva. Thus, the quantitation of viral DNA in whole saliva does not appear to be a valid measure of the level of cell-free infectious virions present in saliva.
Oral swabs have been used to monitor different herpesviruses in the oral cavity and increases in the level of viral DNA in the oral material collected by the swab has been attributed to active virus replication. Such data have been used to monitor disease progression and response to therapy in herpesvirus-associated diseases, such as Kaposi’s sarcoma (Casper et al., 2008). The oral swab collection procedure used in our study was modeled after similar procedures used in human studies, and samples primarily surface cells from cheek and gingival epithelia with minimal absorption of liquid saliva. We compared the level of MneHV-6 and MneHV-7 DNA detected by oral swabs with the level of viral DNA in whole saliva, assaying both the cell-free supernatant, which is predicted to contain free infectious virions and the cell pellet containing cell-associated latent or replicating viral genomes. The origin of cells naturally sloughed into saliva is not clear, but previous studies have indicated that these cells may be a mixture of oral epithelial cells and lymphocytes (Thiede et al., 2000). We saw no correlation between viral loads in the cell-free supernatant and cells in whole saliva or cells collected by oral swab. The lack of correlation between the infection status of the swab-sampled epithelial cell populations and the saliva-associated cell populations suggests that these two populations are not the same and have different epithelial origins in the oral cavity with different susceptibility to roseolovirus infection and/or activation. A possible tissue site of more highly infected cells is the tongue epithelium which has been reported to host different herpesviruses including HHV-6 (Chen and Hudnall, 2006). Further studies are needed to determine the nature and origin of the MneHV-6 and MneHV-7 infected cells in whole saliva. While our studies specifically targeted the oral presence of the macaque homologs of HHV-6 and HHV-7, our results suggest caution in interpreting the level of herpesvirus DNA obtained by oral swab collection as indicative of virus replication in saliva. Similar to what has been reported for HHV-7 (Fujiwara et al., 2000), our data suggests that MneHV-7 is more easily detected in saliva samples than in oral swabs. While we cannot rule out special circumstances for infection and/or activation of virus in our cohort of 12 vaccinated macaques, it is clear that viral infection and/or activation was not equivalent for the two viruses, as only three out of twelve animals had detectable MneHV-6 in whole saliva while MneHV-7 levels were consistently high in all animals. We also observed that MneHV-7 was more prevalent than MneHV-6 in a survey of PBMC from 283 macaques. Overall, 25% of the animals had detectable MneHV-7 DNA in PBMC, 10% had detectable MneHV-6 DNA, and 4% of the animals were co-infected with both viruses. The higher prevalence of viral DNA in tissue biopsies suggests that the actual prevalence of MneHV-6 and MneHV-7 infections at the WaNPRC may be considerably higher. Previous studies have shown that roseolovirus prevalence varies by geographic location (Braun et al., 1997; Cleghorn et al., 1995; Krueger et al., 1998). Our data showed that overall prevalences of MneHV-6 and MneHV-7 varied between macaque populations from different breeding colonies. Examination of the animal records indicated that the PBMC samples from the colony health screen had been obtained from animals originating from the WaNPRC and a variety of other primate centers or facilities. While the majority of macaques from geographically dispersed facilities showed a much higher prevalence of MneHV-7 DNA in PBMC than MneHV-6, the percentage of infected animals varied remarkably. Similar to their human homologs, the macaque roseoloviruses are likely to be transmitted by exposure to saliva, suggesting that virus prevalence is affected by differences in housing conditions and frequency of social contact with other macaques in each colony.
Disease manifestations associated with viral infections often vary depending on the patient’s sex and age (Eshima et al., 2012). A previous study has reported that HHV-6 acquisition is more frequent in girls than boys (Zerr et al., 2005). In our studies, the overall prevalence of MneHV-6 and MneHV-7 DNA in PBMC was similar for male and female macaques and mirrored the prevalence for the combined population. MneHV-7 was detected in both males and females, with a higher prevalence in the younger animals that tended to decrease with age. In contrast, we observed a strong gender bias for MneHV-6 when samples were compared by age groups. MneHV6 was only detected in young males and older females. Multiple studies have reported a possible association between HHV-6 and disease exacerbation among patients with multiple sclerosis (MS) (Alvarez-Lafuente et al., 2004; Behzad-Behbahani et al., 2011). MS occurs later in life and is known to be more common among females than males (Milo and Kahana, 2010). In this context, our observation of a higher prevalence of MneHV-6 infections in PBMC of older female pig-tailed macaques is of interest, as it suggests activation of the HHV-6 homolog in this population.
During the broad screen of macaque PBMC samples, we detected significantly higher viral loads of MneHV-6 DNA compared to MneHV-7. This was mostly due to the fact that MneHV-6 viral loads were much higher in animals co-infected with both MneHV-6 and MneHV-7 than in animals in which only MneHV-6 was detected. In contrast, the viral load of MneHV-7 was not increased in animals co-infected with MneHV-6. This suggests that in vivo gene expression and/or replication of MneHV-7 can significantly increase MneHV-6 viral loads in the peripheral blood and confirms previous in vitro observations for HHV-6B and HHV-7 (Katsafanas et al., 1996; Tanaka-Taya et al., 2000). We also found that this increase was most prominent among females. A possible factor is that the co-infected animals were older among the females than the males. Currently, we are investigation whether there are lymphocyte subpopulations that are co-infected with both MneHV-6 and MneHV-7 to identify mechanisms by which MneHV-7 might trigger MneHV-6 reactivation.
As is the case for other herpesviruses, primary infection with either HHV-6 A/B or HHV-7 is followed by a lifelong chronic infection (Jarrett et al., 1990; Kidd et al., 1996). Virus persists latently in reservoirs of infection with occasional episodes of viral reactivation. HHV-6 and HHV-7 are frequently detected in oro-pharyngeal, gastrointestinal and lymphoid tissues (Chen and Hudnall, 2006). Our qPCR data indicated that MneHV-7 was present in tissue samples from all 12 pig-tailed macaques studied, while MneHV-6 was only detected in 8 of the 12 monkeys. Our mapping of anatomical sites of infections is in agreement with previous studies performed in humans (Chen and Hudnall, 2006; Kempf et al., 1998), although HHV-6 and HHV-7 were detected in a broader range of tissues. This discrepancy might be due to the fact that screening for HHV-6 and HHV-7 mostly relied on nested PCR assays that have a higher sensitivity than our assays allowing for detection of low, often latent, tissue infection.
The viral loads detected in tissues by our qPCR assays were often lower for MneHV-6 compared to MneHV-7. Both viruses were frequently detected in different parts of the stomach and in salivary glands. The highest measured viral loads were detected in tissue samples from these two anatomical sites indicating that viral reactivation and active replication could take place in these organs. Occasionally, MneHV-6 and MneHV-7 were also detected in lymphoid organs like tonsil or spleen at viral loads compatible with a latent stage of infection as described for their human homologs (Kempf et al., 2000; Roush et al., 2001).
Absence or low levels of MneHV-7 detected by the qPCR assay in oral epithelia from the cheek and gingiva mirrored the results from the oral swab samples and suggested the presence of low levels of latent, non-replicating MneHV-7 genomes. Conversely, the MneHV-7 loads present in cells in whole saliva were much higher, indicative of virus activation and genome replication. A strong correlation was detected between the MneHV-7 viral loads in whole saliva and viral loads in both the stomach and salivary glands, suggesting concomitant virus activation in different regions of the orogastric epithelium. Apparently, these desquamated oral epithelial cells are different from the cells sampled using the oral swab technique. It is tempting to speculate that infected cells present in saliva, when swallowed, play an important role in the observed MneHV-6 and MneHV-7 infections in the different regions of the stomach.
HHV-6A/B and HHV-7 have been increasingly recognized as important pathogens associated with severe complications in transplant patients (Razonable et al., 2009). HHV-6 in particular is often associated with acute liver failure and pancreatitis (Cacheux et al., 2005; Randhawa et al., 1997). Although we detected MneHV-6 in a smaller number of tissue samples than MneHV-7, MneHV-6 was still uniquely detected in pancreas and liver tissue samples from a naturally infected pig-tailed macaque. An inherent problem facing roseolovirus studies in humans is the difficulty of obtaining biopsy specimens from asymptomatic healthy persons for obvious ethical reasons. Hence, the availability of an animal model for natural infection with roseoloviruses is of particular interest since it would allow for a better understanding of the life cycles of these viruses in different organs including in the context of immunosuppression. Efforts to further characterize tissue location and cellular tropism using histological staining are currently underway.
Although our study was not designed to evaluate the impact of SHIV exposure on MneHV-6 and MneHV-7 infections, we observed that ongoing SHIV infection (SHIV-positive) in long term non progressing animals was associated with decreased levels of MneHV-6 and MneHV-7 in saliva and gastrointestinal tissues when compared to SHIV-negative macaques. Furthermore, only SHIV-negative macaques had detectable MneHV-6 in saliva, even though several SHIV-positive animals were infected with MneHV-6. Since HIV infection has been associated with salivary gland hypertrophy and marked secretory hypofunction (Fox, 1991) it is possible that the adverse effect of SHIV infection on salivary glands functions resulted in a decrease of roseolovirus DNA loads released into saliva. Further studies will be needed to evaluate more comprehensively the interactions between SIV, MneHV-6 and MneHV-7 in co-infected macaques.
The existence of roseolovirus homologs in non-human primates had been suggested based on serological data (Higashi et al., 1989). Attempts to find HHV-6-like sequences in pig-tailed macaques by HHV-6-specific PCR failed, which led to the assumption that these animals are not commonly infected by a virus related to HHV-6 (Lusso et al., 1994a). Our studies clearly show that pig-tailed macaques are naturally infected with two distinct roseoloviruses closely related to HHV-6 and HHV-7, suggesting that MneHV-6 and MneHV-7 infections in macaques would be an important non-human primate model of roseolovirus infections and pathology. Taken together, our discovery and initial analysis of two novel roseolovirus homologs in macaques offers a unique opportunity to develop relevant animal models using viruses native to the host to answer various unresolved questions surrounding their biology and respective roles as etiological agents in diseases.
Highlights.
We identified DNA fragments in macaques with sequence homology to HHV 6 and HHV7.
Phylogenetic analysis confirmed our discovery of novel macaque Roseoloviruses.
The newly identified viruses were provisionally named MneHV 6 and MneHV 7.
The prevalence and tissue tropism were similar to HHV 6 and HHV 7 in humans.
Acknowledgements
We thank Meei-Li Huang (FHCRC) for providing the HHV-6A infected HSB-2 cell lines. We thank Leon Flanary (WaNPRC), Yonghou Jiang (WaNPRC), Naoto Iwayama (WaNPRC) and Mish McEntire (WaNPRC) for help with sample collection and shipment. Blood DNA samples were kindly provided by the Primate Diagnostic and Services Laboratory (PDSL) of the WaNPRC. We thank Randy McClain (PDSL) and Michael B. Agy, (WaNPRC) for access and help with the blood DNA samples. The PDSL is supported by the WaNPRC NIH Grant RR00166. We thank Timothy Rose (UW, SCRI) for macaque saliva and tissue samples and helpful advice on the development of CODEHOP PCR primers and critical reading of the manuscript.
This project was supported by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number RR023343 to Timothy Rose.
Contributor Information
Jeannette P. Staheli, Email: jstaheli@seattlechildrens.org.
Michael R. Dyen, Email: michael.dyen@seattlechildrens.org.
Patrick Lewis, Email: patrick.lewis@seattlechildrens.org.
Serge Barcy, Email: serge.barcy@seattlechildrens.org.
References
- Aberle SW, Mandl CW, Kunz C, Popow-Kraupp T. Presence of human herpesvirus 6 variants A and B in saliva and peripheral blood mononuclear cells of healthy adults. Journal of clinical microbiology. 1996;34:3223–3225. doi: 10.1128/jcm.34.12.3223-3225.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablashi DV, Marsh S, Kaplan M, Whitman JE, Jr, Pearson GR. HHV-6 infection in HIV-infected asymptomatic and AIDS patients. Intervirology. 1998;41:1–9. doi: 10.1159/000024909. [DOI] [PubMed] [Google Scholar]
- Adams MJ, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012) Archives of virology. 2012;157:1411–1422. doi: 10.1007/s00705-012-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, De las Heras V, Bartolome M, Picazo JJ, Arroyo R. Relapsing-remitting multiple sclerosis and human herpesvirus 6 active infection. Archives of neurology. 2004;61:1523–1527. doi: 10.1001/archneur.61.10.1523. [DOI] [PubMed] [Google Scholar]
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Systematic biology. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- Behzad-Behbahani A, Mikaeili MH, Entezam M, Mojiri A, Pour GY, Arasteh MM, Rahsaz M, Banihashemi M, Khadang B, Moaddeb A, Nematollahi Z, Azarpira N. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. Journal of microbiology, immunology, and infection. 2011;44:247–251. doi: 10.1016/j.jmii.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Boutolleau D, Bonduelle O, Agut H, Gautheret-Dejean A, French ALTSG. Is human herpesvirus-7 a marker or a competitor for HIV progression? Aids. 2004;18:358–359. doi: 10.1097/00002030-200401230-00040. [DOI] [PubMed] [Google Scholar]
- Boyce R, Chilana P, Rose TM. iCODEHOP: a new interactive program for designing COnsensus-DEgenerate Hybrid Oligonucleotide Primers from multiply aligned protein sequences. Nucleic Acids Res. 2009;37:W222–W228. doi: 10.1093/nar/gkp379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clinical microbiology reviews. 1997;10:521–567. doi: 10.1128/cmr.10.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AG, Bakke AM, Thouless ME, Rose TM. Development of a real-time QPCR assay for the detection of RV2 lineage-specific rhadinoviruses in macaques and baboons. Virol J. 2005;2:2. doi: 10.1186/1743-422X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacheux W, Carbonell N, Rosmorduc O, Wendum D, Paye F, Poupon R, Chazouilleres O, Gozlan J. HHV-6-related acute liver failure in two immunocompetent adults: favourable outcome after liver transplantation and/or ganciclovir therapy. Journal of internal medicine. 2005;258:573–578. doi: 10.1111/j.1365-2796.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Caselli E, Zatelli MC, Rizzo R, Benedetti S, Martorelli D, Trasforini G, Cassai E, degli Uberti EC, Di Luca D, Dolcetti R. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto's thyroiditis. PLoS Pathog. 2012;8:e1002951. doi: 10.1371/journal.ppat.1002951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, Hamilton S, Huang ML, Wald A. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. The Journal of infectious diseases. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Hudnall SD. Anatomical mapping of human herpesvirus reservoirs of infection. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19:726–737. doi: 10.1038/modpathol.3800584. [DOI] [PubMed] [Google Scholar]
- Cleghorn FR, Maybank KA, Jack N, Pate E, Mingle J, Levine PH, Manns A. Comparison of HHV-6 antibody titers in West Africa and the Caribbean. Annals of epidemiology. 1995;5:497–500. doi: 10.1016/1047-2797(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Ablashi HA D, Berneman Z, Campadelli-Fiume G, Carrigan D, Ceccerini-Nelli L, Chandran B, Chou S, Collandre H, Cone R, Dambaugh T, Dewhurst S, DiLuca D, Foa-Tomasi L, Fleckenstein B, Frenkel N, Gallo R, Gompels U, Hall C, Jones M, Lawrence G, Martin M, Montagnier L, Neipel F, Nicholas J, Pellett P, Razzaque A, Torrelli G, Thomson B, Salahuddin S, Wyatt L, Yamanishi K. Human herpesvirus-6 strain groups: a nomenclature. Archives of virology. 1993;129:363–366. doi: 10.1007/BF01316913. [DOI] [PubMed] [Google Scholar]
- Di Luca D, Mirandola P, Ravaioli T, Bigoni B, Cassai E. Distribution of HHV-6 variants in human tissues. Infectious agents and disease. 1996;5:203–214. [PubMed] [Google Scholar]
- Di Luca D, Mirandola P, Ravaioli T, Dolcetti R, Frigatti A, Bovenzi P, Sighinolfi L, Monini P, Cassai E. Human herpesviruses 6 and 7 in salivary glands and shedding in saliva of healthy and human immunodeficiency virus positive individuals. Journal of medical virology. 1995;45:462–468. doi: 10.1002/jmv.1890450418. [DOI] [PubMed] [Google Scholar]
- Downing RG, Sewankambo N, Serwadda D, Honess R, Crawford D, Jarrett R, Griffin BE. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987;2:390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Shinnar S, Hesdorffer DC, Nordli DR, Hamidullah A, Benn EK, Pellock JM, Frank LM, Lewis DV, Moshe SL, Shinnar RC, Sun S, team Fs. Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia. 2012;53:1481–1488. doi: 10.1111/j.1528-1167.2012.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshima N, Tokumaru O, Hara S, Bacal K, Korematsu S, Karukaya S, Uruma K, Okabe N, Matsuishi T. Age-specific sex-related differences in infections: a statistical analysis of national surveillance data in Japan. PloS one. 2012;7:e42261. doi: 10.1371/journal.pone.0042261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PC. Saliva and salivary gland alterations in HIV infection. Journal of the American Dental Association. 1991;122:46–48. doi: 10.14219/jada.archive.1991.0331. [DOI] [PubMed] [Google Scholar]
- Frenkel N, Schirmer EC, Wyatt LS, Katsafanas G, Roffman E, Danovich RM, June CH. Isolation of a new herpesvirus from human CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Namba H, Ohuchi R, Isomura H, Uno F, Yoshida M, Nii S, Yamada M. Monitoring of human herpesvirus-6 and -7 genomes in saliva samples of healthy adults by competitive quantitative PCR. Journal of medical virology. 2000;61:208–213. doi: 10.1002/(sici)1096-9071(200006)61:2<208::aid-jmv6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hall CB, Caserta MT, Schnabel KC, McDermott MP, Lofthus GK, Carnahan JA, Gilbert LM, Dewhurst S. Characteristics and acquisition of human herpesvirus (HHV) 7 infections in relation to infection with HHV-6. The Journal of infectious diseases. 2006;193:1063–1069. doi: 10.1086/503434. [DOI] [PubMed] [Google Scholar]
- Higashi K, Asada H, Kurata T, Ishikawa K, Hayami M, Spriatna Y, Sutarman Yamanishi K. Presence of antibody to human herpesvirus 6 in monkeys. J Gen Virol. 1989;70(Pt 12):3171–3176. doi: 10.1099/0022-1317-70-12-3171. [DOI] [PubMed] [Google Scholar]
- Jarrett RF, Clark DA, Josephs SF, Onions DE. Detection of human herpesvirus-6 DNA in peripheral blood and saliva. Journal of medical virology. 1990;32:73–76. doi: 10.1002/jmv.1890320113. [DOI] [PubMed] [Google Scholar]
- Kanthaswamy S, Ng J, Penedo MC, Ward T, Smith DG, Ha JC. Population genetics of the Washington National Primate Research Center's (WaNPRC) captive pigtailed macaque (Macaca nemestrina) population. Am J Primatol. 2012;74:1017–1027. doi: 10.1002/ajp.22055. [DOI] [PubMed] [Google Scholar]
- Katsafanas GC, Schirmer EC, Wyatt LS, Frenkel N. In vitro activation of human herpesviruses 6 and 7 from latency. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9788–9792. doi: 10.1073/pnas.93.18.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf W, Adams V, Mirandola P, Menotti L, Di Luca D, Wey N, Muller B, Campadelli-Fiume G. Persistence of human herpesvirus 7 in normal tissues detected by expression of a structural antigen. The Journal of infectious diseases. 1998;178:841–845. doi: 10.1086/515339. [DOI] [PubMed] [Google Scholar]
- Kempf W, Muller B, Maurer R, Adams V, Campadelli Fiume G. Increased expression of human herpesvirus 7 in lymphoid organs of AIDS patients. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2000;16:193–201. doi: 10.1016/s1386-6532(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Kidd IM, Clark DA, Ait-Khaled M, Griffiths PD, Emery VC. Measurement of human herpesvirus 7 load in peripheral blood and saliva of healthy subjects by quantitative polymerase chain reaction. The Journal of infectious diseases. 1996;174:396–401. doi: 10.1093/infdis/174.2.396. [DOI] [PubMed] [Google Scholar]
- Krueger GR, Koch B, Leyssens N, Berneman Z, Rojo J, Horwitz C, Sloots T, Margalith M, Conradie JD, Imai S, Urasinski I, de Bruyere M, Ferrer Argote V, Krueger J. Comparison of seroprevalences of human herpesvirus-6 and -7 in healthy blood donors from nine countries. Vox sanguinis. 1998;75:193–197. [PubMed] [Google Scholar]
- Lacoste V, Mauclere P, Dubreuil G, Lewis J, Georges-Courbot MC, Rigoulet J, Petit T, Gessain A. Simian homologues of human gamma-2 and betaherpesviruses in mandrill and drill monkeys. Journal of virology. 2000;74:11993–11999. doi: 10.1128/jvi.74.24.11993-11999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste V, Verschoor EJ, Nerrienet E, Gessain A. A novel homologue of Human herpesvirus 6 in chimpanzees. J Gen Virol. 2005;86:2135–2140. doi: 10.1099/vir.0.81034-0. [DOI] [PubMed] [Google Scholar]
- Leach CT, Newton ER, McParlin S, Jenson HB. Human herpesvirus 6 infection of the female genital tract. The Journal of infectious diseases. 1994;169:1281–1283. doi: 10.1093/infdis/169.6.1281. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lusso P, Crowley RW, Malnati MS, Di Serio C, Ponzoni M, Biancotto A, Markham PD, Gallo RC. Human herpesvirus 6A accelerates AIDS progression in macaques. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5067–5072. doi: 10.1073/pnas.0700929104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P, Secchiero P, Crowley RW. In vitro susceptibility of Macaca nemestrina to human herpesvirus 6: a potential animal model of coinfection with primate immunodeficiency viruses. AIDS research and human retroviruses. 1994a;10:181–187. doi: 10.1089/aid.1994.10.181. [DOI] [PubMed] [Google Scholar]
- Lusso P, Secchiero P, Crowley RW, Garzino-Demo A, Berneman ZN, Gallo RC. CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America. 1994b;91:3872–3876. doi: 10.1073/pnas.91.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes IM, Martins RV, Cossatis JJ, Cavaliere RM, Afonso LA, Moyses N, Oliveira SA, Cavalcanti SM. Detection of human herpesvirus 6 and 7 DNA in saliva from healthy adults from Rio de Janeiro, Brazil. Memorias do Instituto Oswaldo Cruz. 2010;105:925–927. doi: 10.1590/s0074-02762010000700015. [DOI] [PubMed] [Google Scholar]
- Malamud D. Saliva as a diagnostic fluid. Dental clinics of North America. 2011;55:159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmunity reviews. 2010;9:A387–A394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Mori Y. Recent topics related to human herpesvirus 6 cell tropism. Cellular microbiology. 2009;11:1001–1006. doi: 10.1111/j.1462-5822.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Polacino P, Larsen K, Galmin L, Suschak J, Kraft Z, Stamatatos L, Anderson D, Barnett SW, Pal R, Bost K, Bandivdekar AH, Miller CJ, Hu SL. Differential pathogenicity of SHIV infection in pig-tailed and rhesus macaques. J Med Primatol. 2008;37(Suppl 2):13–23. doi: 10.1111/j.1600-0684.2008.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle CR. The universal system of virus taxonomy of the International Committee on Virus Taxonomy (ICTV), including new proposals ratified since publication of the Sixth ICTV Report in 1995. Archives of virology. 1998;143:203–210. doi: 10.1007/s007050050280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa PS, Jenkins FJ, Nalesnik MA, Martens J, Williams PA, Ries A, Pham S, Demetris AJ. Herpesvirus 6 variant A infection after heart transplantation with giant cell transformation in bile ductular and gastroduodenal epithelium. The American journal of surgical pathology. 1997;21:847–853. doi: 10.1097/00000478-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Razonable RR, Zerr DM, Practice ASTIDCo. HHV-6, HHV-7 and HHV-8 in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(Suppl 4):S97–S100. doi: 10.1111/j.1600-6143.2009.02899_1.x. [DOI] [PubMed] [Google Scholar]
- Rose TM. CODEHOP-mediated PCR - a powerful technique for the identification and characterization of viral genomes. Virol J. 2005;2:20. doi: 10.1186/1743-422X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose TM, Schultz ER, Henikoff JG, Pietrokovski S, McCallum CM, Henikoff S. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Res. 1998;26:1628–1635. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush KS, Domiati-Saad RK, Margraf LR, Krisher K, Scheuermann RH, Rogers BB, Dawson DB. Prevalence and cellular reservoir of latent human herpesvirus 6 in tonsillar lymphoid tissue. American journal of clinical pathology. 2001;116:648–654. doi: 10.1309/Y2HH-B1CK-0F5L-U7B8. [DOI] [PubMed] [Google Scholar]
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- Tanaka-Taya K, Kondo T, Nakagawa N, Inagi R, Miyoshi H, Sunagawa T, Okada S, Yamanishi K. Reactivation of human herpesvirus 6 by infection of human herpesvirus 7. Journal of medical virology. 2000;60:284–289. doi: 10.1002/(sici)1096-9071(200003)60:3<284::aid-jmv6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kondo T, Torigoe S, Okada S, Mukai T, Yamanishi K. Human herpesvirus 7: another causal agent for roseola (exanthem subitum) The Journal of pediatrics. 1994;125:1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- Tang H, Serada S, Kawabata A, Ota M, Hayashi E, Naka T, Yamanishi K, Mori Y. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9096–9099. doi: 10.1073/pnas.1305187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede C, Prange-Krex G, Freiberg-Richter J, Bornhauser M, Ehninger G. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone marrow transplantation. 2000;25:575–577. doi: 10.1038/sj.bmt.1702170. [DOI] [PubMed] [Google Scholar]
- Vlasak J, Ruprecht RM. AIDS vaccine development and challenge viruses: getting real. Aids. 2006;20:2135–2140. doi: 10.1097/QAD.0b013e328010beb5. [DOI] [PubMed] [Google Scholar]
- Wibbelt G, Kurth A, Yasmum N, Bannert M, Nagel S, Nitsche A, Ehlers B. Discovery of herpesviruses in bats. J Gen Virol. 2007;88:2651–2655. doi: 10.1099/vir.0.83045-0. [DOI] [PubMed] [Google Scholar]
- Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Zerr DM. Human herpesvirus 6 (HHV-6) disease in the setting of transplantation. Current opinion in infectious diseases. 2012;25:438–444. doi: 10.1097/QCO.0b013e3283553362. [DOI] [PubMed] [Google Scholar]
- Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, Corey L. A population-based study of primary human herpesvirus 6 infection. The New England journal of medicine. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]