Abstract
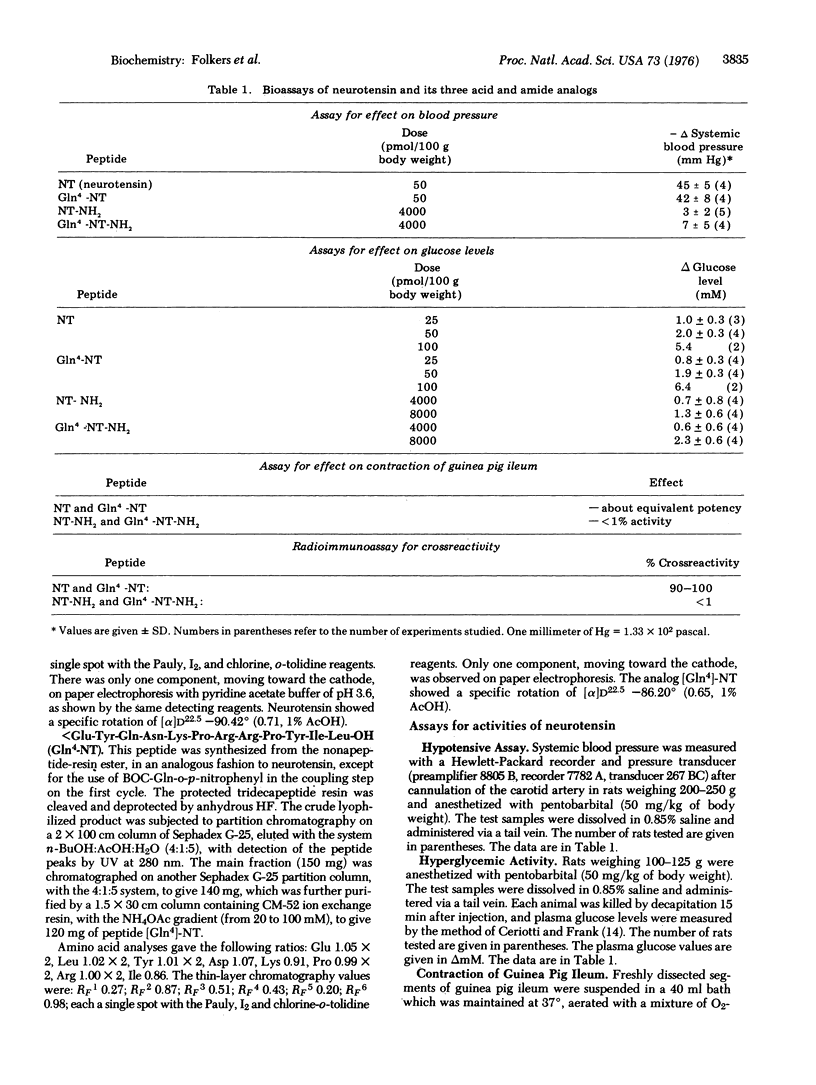
It was considered, a priori, that the isolation of the tridecapeptide, neurotensin, might have inadvertently sllowed the hydrolysis of either the [Gln4]- or the [Leu13-NH2]-moieties. Neurotensin and its three acid and amide analogs, i.e., [Gln4]-neurotensin, neurotensin-NH2, and [Gln4]-neurotensin-NH2 were synthesized. Neurotensin and [Gln4]-neurotensin were indistinguishable by the hypotensive assay, hyperglycemic assay, contraction of the ileum, and radioimmunoassay. Neurotensin-NH2 and [Gln4]-neurotensin-NH2 showed less than 1% of these neurotensin activities. Present information does not elucidate whether the glutamic acid residue in position 4 of neurotensin in situ is present as Glu4 or as Gln4. At high levels, neurotensin released the luteinizing hormone, follicle stimulating hormone, and thyrotropin; [Gln4]-neurotensin-NH2 released thyrotropin, and [Gln4]-neurotensin released luteinizing hormone and follicle stimulating hormone, but these activities do not appear biologically significant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carraway R., Leeman S. E. The amino acid sequence of a hypothalamic peptide, neurotensin. J Biol Chem. 1975 Mar 10;250(5):1907–1911. [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854–6861. [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. The synthesis of neurotensin. J Biol Chem. 1975 Mar 10;250(5):1912–1918. [PubMed] [Google Scholar]
- Ceriotti G., De Nadai Frank A. An improved proceure for blood glucose determination with o-toluidine. Clin Chim Acta. 1969 May;24(2):311–313. doi: 10.1016/0009-8981(69)90330-1. [DOI] [PubMed] [Google Scholar]
- Currie B. L., Johansson K. N., Greibrokk T., Folkers K., Bowers C. Y. Identification and purification of factor A-GHRH from hypothalami which releases growth hormone. Biochem Biophys Res Commun. 1974 Sep 23;60(2):605–609. doi: 10.1016/0006-291x(74)90283-6. [DOI] [PubMed] [Google Scholar]
- Currie B. L., Johansson N. G., Folkers K. On the chemical existence and partial purification of the hypothalamic follicle stimulating hormone releasing hormone. Biochem Biophys Res Commun. 1973 Jan 4;50(1):14–19. doi: 10.1016/0006-291x(73)91056-5. [DOI] [PubMed] [Google Scholar]
- Greibrokk T., Currie B. L., Johansson N. K., Hansen J. J., Folkers K. Purification of a prolactin inhibiting hormone and the revealing of hormone D-GHIH which inhibits the release of growth hormone. Biochem Biophys Res Commun. 1974 Jul 24;59(2):704–709. doi: 10.1016/s0006-291x(74)80037-9. [DOI] [PubMed] [Google Scholar]
- Johansson K. N., Currie B. L., Folkers K., Bowers C. Y. Identification and purification of factor B-GHRH from hypothalami which releases growth hormone. Biochem Biophys Res Commun. 1974 Sep 23;60(2):610–615. doi: 10.1016/0006-291x(74)90284-8. [DOI] [PubMed] [Google Scholar]
- Johansson K. N., Greibrokk T., Currie B. L., Hansen J., Folkers K. Factor C-LHIH which inhibits the luteinizing hormone from basal release and from synthetic LHRH and studies on purification of FSHRH. Biochem Biophys Res Commun. 1975 Mar 3;63(1):62–68. doi: 10.1016/s0006-291x(75)80011-8. [DOI] [PubMed] [Google Scholar]
- Lenard J., Robinson A. B. Use of hydrogen fluoride in Merrifield solid-phase peptide synthesis. J Am Chem Soc. 1967 Jan 4;89(1):181–182. doi: 10.1021/ja00977a057. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H., Robinson A. B. Deamidation of asparaginyl residues as a hazard in experimental protein and peptide procedures. Anal Biochem. 1971 Aug;42(2):565–568. doi: 10.1016/0003-2697(71)90074-1. [DOI] [PubMed] [Google Scholar]


