Abstract
Late wilt, a severe vascular disease of maize caused by the fungus Harpophora maydis, is characterized by rapid wilting of maize plants before tasseling and until shortly before maturity. The pathogen is currently controlled by resistant maize cultivars, but the disease is constantly spreading to new areas. The plant’s late phenological stage at which the disease appears suggests that plant hormones may be involved in the pathogenesis. This work revealed that plant growth hormones, auxin (Indole-3-acetic acid) and cytokinin (kinetin), suppress H. maydis in culture media and in a detached root assay. Kinetin, and even more auxin, caused significant suppression of fungus spore germination. Gibberellic acid did not alter colony growth rate but had a signal suppressive effect on the pathogens’ spore germination. In comparison, ethylene and jasmonic acid, plant senescing and defense response regulators, had minor effects on colony growth and spore germination rate. Their associate hormone, salicylic acid, had a moderate suppressive effect on spore germination and colony growth rate, and a strong influence when combined with auxin. Despite the anti-fungal auxin success in vitro, field experiments with dimethylamine salt of 2,4-dichlorophenoxyacetic acid (that mimics the influence of auxin) failed to suppress the late wilt. The lines of evidence presented here reveal the suppressive influence of the three growth hormones studied on fungal development and are important to encourage further and more in-depth examinations of this intriguing hormonal complex regulatory and its role in the maize-H. maydis interactions.
Keywords: Auxin, Harpophora maydis, Kinetin, Late wilt, Maize, Plant hormones
Introduction
Late wilt, or black bundle disease, is a vascular wilt disease of Zea mays (corn, maize) caused by the soil-borne and seed-borne fungus, Harpophora maydis (Michail et al. 1999; Samra et al. 1966) with synonyms Cephalosporium maydis and Acremonium maydis (Gams 1971; Samra et al. 1966). The fungus reproduces asexually, and no perfect stage has been identified (Zeller et al. 2000). Late wilt was reported in Egypt (Sabet et al. 1961), India (Payak et al. 1970), Hungary (Pecsi and Nemeth 1998), Israel (Drori et al. 2012), Spain and Portugal (Molinero-Ruiz et al. 2011). Serious economic losses from late wilt have been reported in Egypt and Israel, where 100 % infection occurs in some fields, and in India, with an incidence as high as 70 % and economic losses up to 51 % (Johal et al. 2004). Maize and Lupinus (lupine) (Sahab et al. 1985) are the only known hosts of H. maydis, although localized temporary lesions occur on young cotton hypocotyls (Bahteem 185 cultivar) (Sabet et al. 1966). The Egyptian, Indian and Hungarian isolates of H. maydis differ in morphology, pathogenicity and route of infection (Warren 1983). In Egypt, there are four clonal lineages of H. maydis that differ in colonization ability and virulence on maize (El-Assiuty et al. 1999; Saleh et al. 2003; Zeller et al. 2000, 2002).
Late wilt disease is characterized by relatively rapid wilting of maize plants, typically at the age of 70 to 80 days, before tasseling and until shortly before maturity. First symptoms appear approximately 60 days after sowing (Sabet et al. 1970) and include drying out of the lower leaves. Later, drying out ascends upwards in the plant and causes leaf yellowing and dehydration, color alteration of the vascular bundles to a yellow-brown hue and then the appearance of red-brown stripes on the lower internode (Sabet et al. 1966). With disease progression, the lower stem dries out (particularly at the internodes) and has a shrunken and hollow appearance, with dark yellow to brownish macerated pith and brownish-black vascular bundles. Late wilt symptoms are often enhanced by secondary invaders infection such as Helminthosporium acremonium, Sclerotium bataticola, Fusarium verticillioides and various bacterial rots to present a “stalk rot complex” (El-Shafey and Claflin 1999; Samra et al. 1962). Fewer ears are produced, and kernels that form are poorly developed (Drori et al. 2012) and may be infected with the pathogen. Seed quantity is correlated negatively to disease severity (Shehata 1976).
Plant hormones regulate plant development and are involved in the plant’s responses to various environmental signals, including biotic stresses. Three phytohormones – salicylic acid (SA), jasmonates (JA) and ethylene (ET) – are known to play major roles in regulating plant defense responses against various pathogens, pests and abiotic stresses, such as wounding and exposure to ozone (Bari and Jones 2009). SA plays a crucial role in plant defense and is generally involved in the activation of defense responses against pathogens that require a living host (biotrophs) and hemi-biotrophic pathogens (those that are also capable of destroying host cells, often through the production of phytotoxins and cell-wall degrading enzymes, and then feed on the contents), as well as the establishment of systemic acquired resistance (SAR) (Grant and Lamb 2006).
By contrast, JA and ET are usually associated with defense against pathogens that kill the host and feed on the contents (necrotrophs) and herbivorous insects (Bari and Jones 2009). Ethylene acts at trace levels throughout the life of the plant by stimulating or regulating the ripening of fruit, the opening of flowers, and the abscission (or shedding) of leaves (Lin et al. 2009). Ethylene plays a complex role in plant defense. Treatment with ET can cause increased resistance to pathogens; however, ET can also cause increased susceptibility (van Loon et al. 2006). Ethylene causes the formation of barriers within the plant, which prevents the advancement of the pathogen. Although SA and JA/ET defense pathways are mutually antagonistic, evidence of synergistic interactions has also been reported (Beckers and Spoel 2006). This suggests that the defense signaling network activated and utilized by the plant is dependent on the nature of the pathogen and its mode of pathogenicity. In addition, the lifestyles of different pathogens are not often readily classifiable as purely biotrophic or necrotrophic. Therefore, the positive or negative cross talk between SA and JA/ET pathways may be regulated depending on the specific pathogen (Adie et al. 2007).
Plant growth hormones cytokinin (CK) and auxin (Indole-3-acetic acid, IAA) are also involved in these pathways (Jameson 2000). SA and auxin signaling pathways interact, for the most part, antagonistically (Wang et al. 2007); elevated auxin signaling correlates with increased susceptibility to biotrophic pathogens (Navarro et al. 2008). Auxin can also interact with the JA signaling pathway, although reports are conflicting (Robert-Seilaniantz et al. 2011). Taken together, emerging evidence suggests that auxin acts as an important component of hormone signaling network involved in the regulation of responses against various biotrophic and necrotrophic pathogens.
Compared to auxin signaling, our understanding of the roles of CKs in disease and its interactions with other hormones is relatively limited (Robert-Seilaniantz et al. 2011). CKs are phytohormones derived from adenine and are involved in the regulation of root and shoot growth and leaf longevity. Evidence suggests that CK is involved in the regulation of plant defense responses against some pathogens (Walters and McRoberts 2006).
Gibberellin (GA), another growth regulator, promotes plant growth in higher plants, but is also produced by fungi and bacteria (MacMillan 2001). It is presumed that GAs in fungi and bacteria are secondary metabolites that act as signaling factors to establish the interaction with host plants. Indeed, emerging evidence suggests that GA signaling components play major roles in plant disease resistance and susceptibility (Robert-Seilaniantz et al. 2011). Since GA stimulates the degradation of growth regulators called DELLA proteins, it is likely that GA promotes resistance to biotrophs and susceptibility to nectrotrophs (Bari and Jones 2009).
Because late wilt disease emerges at a late phenological stage of plant development (soon before ripening), as auxin secretion decreases and ET secretion increases (Beyer 1973; Sexton et al. 1989), these hormones may be involved in controlling fungal development in the disease in susceptible strains. Here, we conducted a first in vitro examination of the influence of the plant hormones on H. maydis under controlled conditions. We used a DNA-sequence-based approach (Degani and Cernica 2014; Drori et al. 2012) along with traditional morphological methods to conduct a controlled inspection of the influence of major plant hormones on H. maydis colonies growth rate, spore germination, and on its ability to infect maize detached roots and field plants.
Materials and methods
Fungal isolates and culture conditions
Four isolates of H. maydis (named Hm-1, Hm-2, Hm-3 and Hm-4, three of which are now deposited in the CBS-KNAW Fungal Biodiversity Center, Utrecht, The Netherlands, under the numbers CBS 133164, CBS 133165 and CBS 133166) were used in this study. These H. maydis strains were recovered from wilting maize plants (Zea mays L., Jubilee cv., Syngenta, Fulbourn, Cambridge, UK) sampled from a maize field in Sdeh Nehemia in the Hula Valley (upper Galilee, northern Israel) in 2001. Pathogenicity of the Israeli H. maydis isolates was confirmed by complying with Koch’s postulates, and the pathogen was characterized by its colony morphology and microscopic traits (Drori et al. 2012). The morphological and microscopic characteristics of the pathogen were identical to those of previously described strains found in Egypt and India (Payak et al. 1970; Samra et al. 1963). Final confirmation was achieved by PCR-based DNA analysis (Degani and Cernica 2014; Drori et al. 2012). All isolates were grown on potato dextrose agar (PDA) (Difco, Detroit, MI, USA) at 28 ± 1 °C in complete darkness.
Effect of plant hormones on H. maydis growth in culture medium
We carried out an in vitro evaluation of seven plant hormones on the radial mycelial growth of H. maydis. These phytohormones included: the CK hormone kinetin (Sigma, Israel); the auxin hormone Indole-3-acetic acid (IAA, Duchefa, The Netherlands); Gibberellic Acid 3 (Duchefa, The Netherlands); the ET precursor amino-cyclopropane-1-carboxylic acid (ACC, Sigma, Israel) or 1-aminocyclopropane-1-carboxylic acid (ACPC, Sigma, Israel); Abscisic acid (ABA, Sigma, Israel); SA (Duchefa, The Netherlands); and JA (Sigma, Israel). All hormones were at high purified grade. Fungal isolates were inspected for phytohormone sensitivity in growth assays on agar plates. The hormones were first scanned at the high 100 mg/L rate separately or in mixtures of 2 hormones together. In the synergism testing groups (combination of 2 different hormones), the final concentration of 100 mg/L was the sum of 50 mg/L of each of the 2 hormones tested.
Those hormones that had a significant decreasing effect on fungal colonies radial growth were selected for further evaluation at 4 rates – 0.1, 1, 10 and 100 mg/L final concentration, unless otherwise indicated. The preparation of each hormone’s stock solution was done by dissolving it in acetic acid, ethanol, NaOH or sterile distilled and deionized water (DDW), according to the manufacturer’s instructions. The final concentration of the stocks was 1 g/l. The stock solutions were filtered using a 0.22 μm syringe filter (Danyel Biotech, Rehovot, Israel). Media for the inhibition-response experiments were prepared by adding the hormones in the required concentration to an autoclaved PDA after it had cooled down to 55 °C. Twenty milliliters of these PDA amended with different hormone rates (0–100 mg/L as described in each figure) were poured into a 9 cm diameter petri dish. Each plate, including the control (PDA without hormone), was inoculated on solidification in the middle with a 6 mm (in diameter) culture agar disk cut from the margins of 4-6-day-old H. maydis colonies. Labeled petri dishes were placed in an incubator at 28 ± 1 °C in the dark. All treatments (hormones at different rates and the control) were replicated six times. Each experiment was repeated at least twice. Radial mycelial growth was taken at two-day intervals (2, 4, 6 and 8 days) after inoculation by measuring the diameter along two perpendicular lines from the underside of the petri dishes. Data collected were subjected to statistical analysis using the student’s t-test.
Growth conditions of maize plants in a growth chamber
The susceptible cultivar of sweet corn Jubilee from Pop Vriend Seeds B.V., Andijk, The Netherlands (supplied by Eden Seeds, Reut, Israel) was selected for the root and field pathogenicity tests. This variety has been tested previously for susceptibility to late wilt (Drori et al. 2012). Maize seeds were sown individually in a plastic seedling nine pot tray about 5 cm beneath the surface. The soil mix (Shacham Givat Ada, Givat Ada, Israel) was commercial and nonsterilized, composed of 65 % coco, 20 % peat, 10 % tuff (4–10 mm volcanic stones) and 5 % Multicote® (slow-release fertilizers, Pt. Multigreen Indonesia, Jakarta, Indonesia), w/w. Watering was done by adding 500 ml DDW at two-day intervals to the plastic seedling pot tray. All the plants used for the detached roots experiment were grown in a growth chamber under constant temperature of 25 ± 2 °C and photoperiod conditions of 12 h of light (from cool-white fluorescent tubes, Philips, Eindhoven, The Netherlands) and 12 h of darkness. Maize seedlings were 20 days old. At this age, the plants’ fourth leaf had emerged, remained partly rolled, and was beginning to expand.
Detached root pathogenicity assay for the influence of plant hormones
Detached roots were used to determine the influence of hormones on the infection ability of H. maydis. This assay is especially important since the exogenous phytohormons influenced the host root physiology as well. A high dose of 100 mg/L of selected plant hormones was chose for the detached root infection assay. This concentration, which is 10 times higher than the concentrations at which plant tissues are normally grown in tissue culture, was chosen in order to induce a strong and short exogenic effect. This acute assay is different from the chronic endogenic influence of the low concentration hormone in normal tissues that is subjected to changes during plant growth and that is in combination with the other plant hormones. Plants were grown under the conditions described above. For the selection of roots, care was taken to collect roots of similar diameter and pigmentation. Lateral, young and white roots, about 2 cm long, were removed from potted 20-day-old maize seedlings (Zea mays L., Jubilee cv.) and washed in tap water to remove the soil. The roots were disinfected using 70 % ethanol solution for 2 min and dried in a pre-sterilized fume hood. Each root was then placed individually in a sterile plastic petri dish lined with Whatman 3 mm filter paper. Ten ml of the selected hormones (100 mg/L) or DDW (as positive control) were used for wetting the filter paper beneath the infected detached roots in a petri dish. A 6-mm-diameter culture agar disk taken from the growing edge of a 4-6-day-old fungal colony (grown at 28 ± 1 °C in the dark) was placed on the cut end of each root. Negative control roots were left untouched. The petri dishes were sealed with Parafilm (a plastic paraffin film) and incubated at 28 ± 1 °C in the dark. Each treatment was done in two independent repeats and the results were similar; data for one representative root of each treatment are shown. The lengths of root infection threads (seen as a dark filament within the root) were identified, measured and photographed 3 and 6 days after inoculation. For DNA extraction, one segment was cut at 1 cm from the infected or uninfected root cut ends, 6 days after inoculation. DNA isolation and PCR were conducted as described below.
Molecular diagnosis
DNA was obtained using the Extract-N-amp plant PCR kit (Sigma, Rehovot, Israel) according to the manufacturer’s instructions from 4-6-day-old fungal colonies (grown at 28 ± 1 °C in the dark) or from detached roots samples. PCR was performed to amplify a specific H. maydis segment (Drori et al. 2012; Saleh and Leslie 2004) with a Rapidcycler (Idaho Technology, Salt Lake City, UT, USA). The A200a primer set [A200a (forward primer): 5’-CCGACGCCTAAAATACAGGA-3’, A200b (reverse primer): 5’-GGGCTTTTTAGGGCCTTTTT-3’] amplifies a specific (Saleh and Leslie 2004) H. maydis segment. The Am42/43 primer set [Am42 (anti-sense primer): 5’-CAACTACGAGCTTTTTAACTGC-3’, Am43 (sense primer): 5’-CAAATTACCCAATCCCGACAC-3’] amplifies eukaryotic ribosomal DNA [18S rRNA gene product, rDNA (Fromont-Racine et al. 2003)] and was used for positive control. Reaction mixtures were contained in a total volume of 20 μl: 1 μl of each primer (20 μM of each primer), 4 μl Red Load Taq Master (Larova, Teltow, Germany), 3 μl DNA sample and 11 μl sterile DDW. Cycling conditions for all primer pairs were 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final step of 72 °C for 5 min. After PCR, a 200-bp amplified DNA band was identified by electrophoresis on a 1.5 % Agarose gel (Lonza, Rockland, USA).
Effect of plant hormones on H. maydis spore germination
To induce sporulation, cultures were grown at 28 ± 1 °C in a humid atmosphere on the surface of PDA. After 4 days, as previously described (Samra et al. 1966), spores were harvested, washed and scraped off the agar surface with 1 ml sterile deionized water. The spore suspension was kept at 4 °C for up to one day. The spores were suspended in watery solutions containing different phytohormone solutions to a final concentration of approximately 50 spore’s μl. All hormones were tested at 100 mg/L – the highest concentration inspected in the plate’s sensitivity assay described above. Two control treatments were introduced: spores in DDW without hormone; and spores in DDW containing pre-boiled kinetin solution at the same concentration (100 mg/L). The second control aimed at negating the osmotic effect of the hormone solution on the spore’s germination, as was previously reported (Degani and Goldblat 2014). The spores’ suspensions in Eppendorf tubes were then incubated in a rotary shaker at 150 rpm at 28 ± 1 °C in the dark. The percentage of germinating conidia was determined after incubation for 0, 10, 13 or 16 h by direct counting in 2 μl drops on a glass slide using a light microscope equipped with a Moticam 5 (Motic Instruments, Richmond, Canada) microscope camera. A representative photo of each treatment is presented. The criterion for germination was the observation of any germ tube emerging from the spores examined. Each assay was performed in six independent replications, and the entire experiment (for each hormone) was repeated twice.
Field experiment for assessing 2,4-dichlorophenoxyacetic acid efficiency in controlling late wilt
The field experiment was conducted to assess the efficiency of Dimethylamine salt of 2,4-dichlorophenoxyacetic acid (2,4-D DMA, 96.9 % active ingredient, comercial name Aminobar, Luxembourg Industries Ltd., Israel) in controlling H. maydis pathogenesis. This chemical is a selective herbicide that kills dicots (but not grasses) by mimicking the growth hormone IAA, which causes uncontrolled growth and eventually death in susceptible plants. The experiment was carried out using the susceptible cultivar of sweet corn Jubilee during the spring and summer of 2009 in the southern area (No. 10) of a maize field in Kibbutz Neot Mordechai in the Hula Valley (upper Galilee, northern Israel), which has been known to be infected with late wilt for many years (Degani et al. 2014; Drori et al. 2012). Plots were arranged in the field using a randomized complete block design. The area included 15 plots, each containing 2 rows (five plots per treatment). Each row was 60 m long and contained 6.5 maize plants m−1. Row spacing was 96.5 cm. This area was part of a large maize field used for grain production. Seeds were pretreated with Thiram, Captan, Carboxin, Metalaxyl-M (manufactured by Rogers/Syngenta Seeds, Boise, ID, USA, supplied by CTS, Tel Aviv, Israel, quota NC7323XLF). The field was watered with 0.6 L/h using a 20 mm drip irrigation line for each row (Dripnet PC1613 F, Netafim USA, Fresno, CA). The drip points were spaced 25 cm apart. Irrigation for each treatment was controlled manually from a manifold. Four- to five-meter-long 20 mm blind affiliates equipped with a manual control valve were installed at the edge of each treatment unit near the water source and used as point of injection and mixing of the 2,4-D DMA with the irrigation water. Water faucets were installed to prevent the chemical from passing to the control treatment. The total amount of water for the season was determined by the Penman-Monteith method to 500 mm. The field was watered twice a week.
Seeding was performed on April 21, 2009 and germination (with a frontal irrigation system) 1 day later. Plants appeared above the ground surface 6 days after planting. Plants were pollinated when they reached 70 % silk on June 20, 2009 (60 days after sowing), and the field was harvested on July 14, 2009 (84 days after sowing). The Jubilee cv. field plants were treated separately with 2,4-D DMA. This treatment was done at a dosage of 150 cm3/0.1 ha and applied 3 times on May 7 (phenological stage V2, four visible leaves), May 21 (phenological stage V4-5, eight visible leaves, 25–30 cm plant height), and June 4 (phenological stage V8, 13–14 visible leaves, 60 cm plant height), 16, 30 and 44 days after sowing. The control group was untreated plants. 2,4-D DMA was injected directly into the drip line using a 5 ml syringe.
Wilt determination was carried out 17 (7/7/09) and 24 (14/7/09) days after fertilization (DAF, 77 and 84 days after sowing) for 100 plants in a sequence. The plants were classified as wilted when dehydration symptoms appeared on the leaf whose cob was located in its axil. Yield assessment was done 24 DAF (14/7/09, 84 days after sowing) and included all the upper part plant cobs in a 20-m-long section of each of the experiment rows.
Molecular diagnosis of late wilt pathogenesis in the field experiment
Three plants were collected arbitrarily from the Jubilee cv. plants at approximately three-week intervals from day 20 after sowing onwards. The last two samplings were made at two-week intervals in order to inspect the disease eruption more closely. Sampling was made on days 20 (11/5/09), 40 (31/5/09), 61 (21/6/09), 75 (5/7/09) and 89 (19/7/09) after seeding. Different plant tissues (root, stem, leaf and seed) were sterilized separately with 70 % ethanol for 2 min and then washed with autoclaved DDW. The plants were ground separately in a mortar with pestle, and DNA was extracted and analyzed in three independent replications, as described above.
Results
Effect of plant hormones on H. maydis growth in culture medium
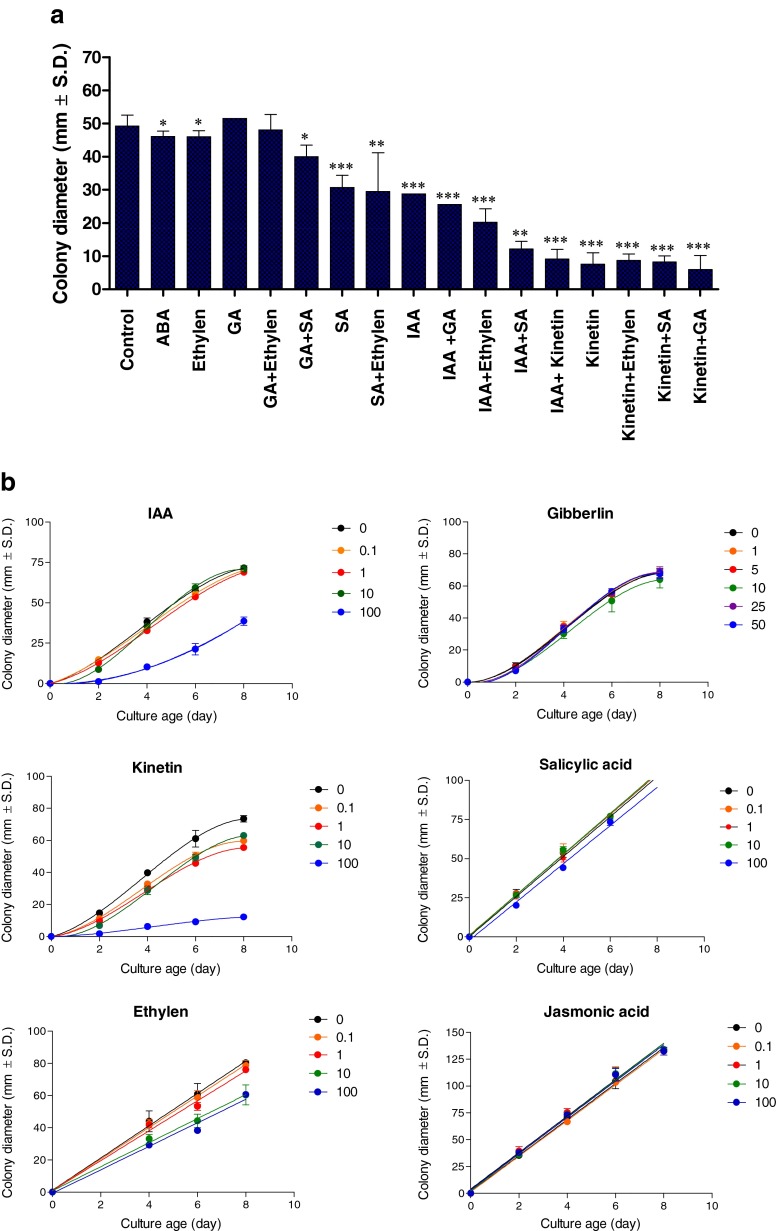
We investigated whether H. maydis can grow efficiently on agar plates containing different plant hormones at a concentration of 100 mg/L active ingredient (Fig. 1a). Our hypothesis was that growth hormones such as IAA and kinetin suppress the fungal pathogenic burst stage as long as they are maintained at relatively high levels in the host plant. Indeed, plant hormones IAA and kinetin significantly (p < 5*10−4) suppress H. maydis in culture plates (Fig. 1a). In comparison to these two hormones, a high concentration of SA causes a lesser, but still significant (p < 0.05), reduction in pathogen colony radial growth (Fig. 1a). Nevertheless, this hormone had a strong (p < 0.005) suppressive influence when combined with auxin (Fig. 1a). Other hormones such as GA, ET (ACPC), JA or ABA had a relatively minor effect on colony growth at the inspected concentration. Combining two hormones together led, in all cases, to a more pronounced effect than the effect of a sole hormone (Fig. 1a).
Fig. 1.
Effect of plant hormones on H. maydis growth in culture medium. Assay plates were inoculated in the middle with a 6-mm (in diameter) culture agar disk cut from the margins of 4-6-day-old H. maydis colonies. H. maydis colonies were grown on potato dextrose agar (PDA) at 28 ± 1 °C in complete darkness. Radial mycelial growth was measured six days after inoculation. Phytohormones were incorporated separately or in a mixture into PDA medium at a high 100 mg/L dosage (a) or at the different concentrations indicated (B, for selected hormones) to evaluate their influence on the colonies’ radial growth. In the synergism testing groups (combination of two different hormones), the final concentration of 100 mg/L was the sum of 50 mg/L of each of the two hormones tested. The following hormones were used: kinetin; auxin (hormone Indole-3-acetic acid, IAA); Gibberellic Acid 3 (GA); the ethylene precursor amino-cyclopropane-1-carboxylic acid (ACC) or 1-aminocyclopropane-1-carboxylic acid (ACPC); abscisic acid (ABA); salicylic acid (SA); and jasmonic acid (JA). Cultures were incubated for 6 (a) or 8 days (b) at 28 ± 1 °C in the dark. Values represent the average of six replicates. Error bars represent standard deviation. Asterisks represent significant (*P = 0.05, ** P = 0.005, *** P < 5*10−4) differences from the control
A dipper evaluation of these plant hormones using the in vitro sensitivity assay was conducted at two-day intervals and at different hormones rates (Fig. 1b). Using this method, we further support our first observation of the IAA and kinetin inhibitory effect, and found that the inhibited mycelial growth (compared to the control) was significant (p < 0.05) from day 2 onwards. Still, in the IAA treatment, this difference was only measured at the high 100 mg/L hormone concentration (Fig. 1b). The inhibitory effect in agar plates of kinetin increased with an increase in concentration (Fig. 1b). Ethylene (ACPC) and SA also caused some growth repression (less pronounced than the IAA or CK effect) when applied at high dosages (Fig. 1b). The other two hormones tested – GA and JA – did not show any significant difference from the control treatment under these conditions (Fig. 1b).
Detached root pathogenicity assay for the influence of plant hormones
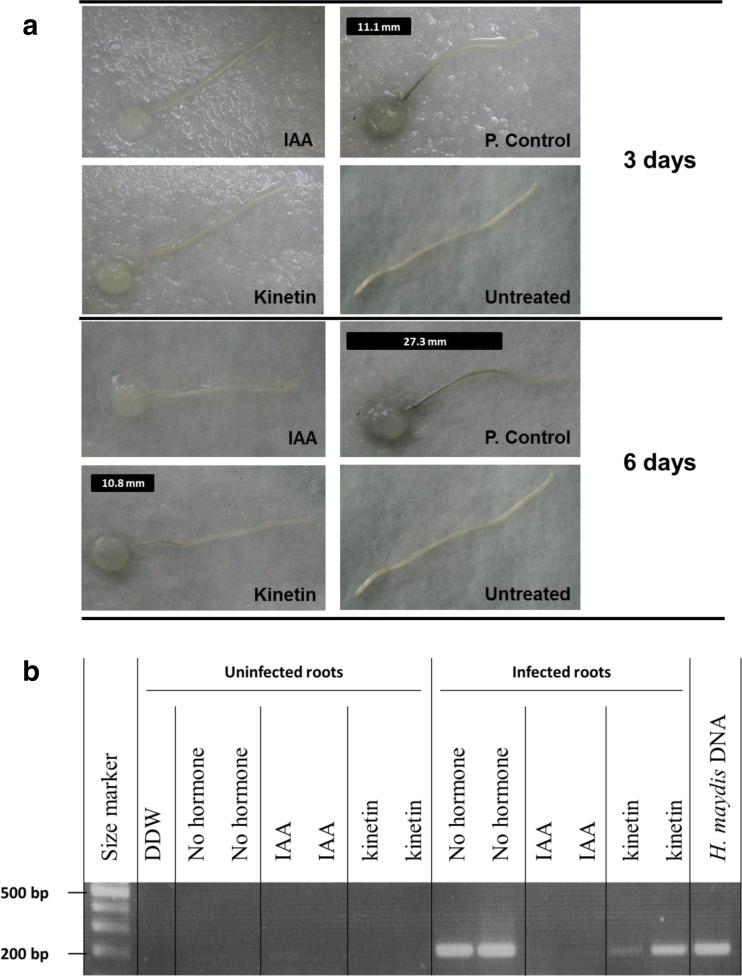
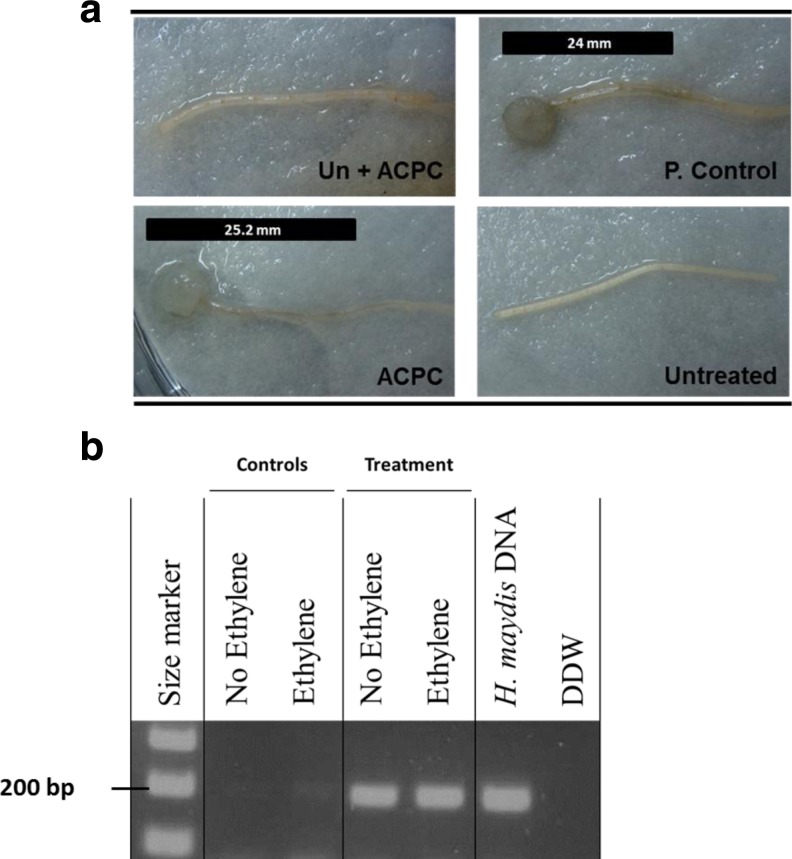
The effect of IAA, kinetin and ET on the ability of H. maydis to infect detached roots was assessed to evaluate their inhibitory effect when the fungus grows on its natural food source. Moreover, detaching the roots may accelerate their senescence and susceptibility to the pathogen (as shown in leaves for the foliar pathogen Cochliobolus heterostrophus (Degani 2014)). The roots themselves may be subject to the hormone influence (CK and IAA inhibit senescence while the ET precursor ACC or ACPC accelerates senescence) that can alter their susceptibility to pathogen invasion (Degani et al. 2004). The positive control inoculated roots (Fig. 2a) had dark brown root infection threads (seen as dark filaments within the root) that were clearly distinguishable from the remaining healthy root, whereas the non-inoculated negative control root remained clear as expected (Fig. 2a). The inoculate experiment groups treated with IAA or kinetin exhibited no sign of infection during the first three days. After six days, the inoculated root lined in kinetin suspension started to develop a short infection thread (10.8 mm, Fig. 2a). This observation differed considerably from the IAA treatment, which on day 6 showed no signs of infection, and from the positive control root, which at this stage was almost entirely infected (27.3 mm, Fig. 2a). The molecular examination made of a sample segment cut 1 cm from the end of the infected or uninfected roots on day 6 supports these observations (Fig. 2b). The DNA bands observed in the kinetin treatment were weaker than those of the positive control. No DNA bands appeared in the IAA treatment. Unlike the plate’s sensitivity assay (Fig. 1b), adding ACPC to the detached roots incubating suspension cause no distinguishable difference from the control although the ACPC treated roots appeared to undergo more rapid senescing, observed as widespread chlorosis (Fig. 3a). This conclusion was supported by the molecular examination (Fig. 3b).
Fig. 2.
Detached root pathogenicity assay for the influence of auxin and kinetin on H. maydis. Side, young and white roots, about 2 cm long, were removed from potted 20-day-old maize seedlings (Zea mays L., Jubilee cv.) and inoculated by placing a 6-mm-diameter culture agar disk taken from the margins of a 4-6-day-old fungal colony (grown at 28 ± 1 °C in the dark) on the cut end of each root. The inoculated roots were placed separately in petri dishes containing auxin (IAA) or kinetin (100 mg/L) or distilled and deionized water, DDW (p. control), and incubated in moist petri dishes at 28 ± 1 °C in the dark. Negative controls are roots without pathogen inoculation inoculates on DDW without hormones (untreated). a. Progression of the pathogen infection thread inside the xylem tissue of each root (seen as a dark filament within the root) was evaluated qualitatively after three and six days of inoculation and marked in the photograph by a black line positioned above each root. b. In order to identify the fungus DNA in the above treated roots tissues, one segment was cut at 1 cm from the cut end of each root, six days after inoculation. DNA isolation and PCR were conducted for the presence of the pathogen using a PCR-based method, amplified at 200 bp H. maydis-specific oligonucleotide. Controls: DDW – distilled and deionized water, a negative control, used as a template in the PCR mixture to ensure the absence of DNA contamination; H. maydis DNA – positive control, obtained from an agar plate colony
Fig. 3.
Detached root pathogenicity assay for ethylene influence on H. maydis. All conditions as described in Fig. 2. Un + ACPC - roots without pathogen inoculates on ACPC. All other abbreviations as in Fig. 2
Effect of plant hormones on H. maydis spore germination
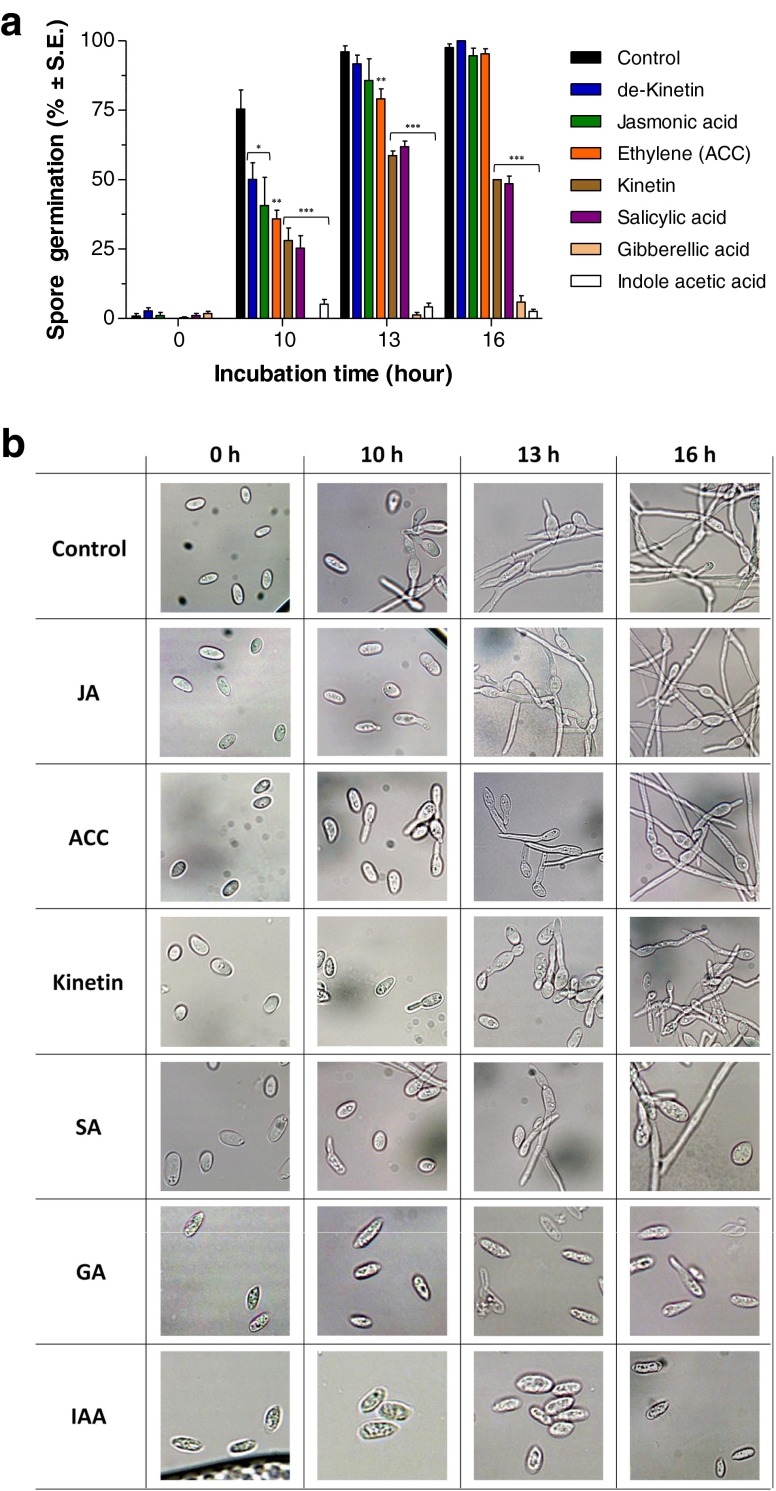
During pathogenesis, H. maydis spores are present in the host plant xylem vessels (Samra et al. 1962) and thus may subjected to the influence of the host hormones. To assay the various phytohormones on H. maydis initial development, we used a spore germination assay. Consistent with the plate’s sensitivity assay (Fig. 1b) and the detached root pathogenicity assay (Fig. 2), IAA and kinetin containing growth media significantly (p < 5*10−4) reduce the spore germination of the pathogen (Fig. 4). As in the detached root pathogenicity assay (Fig. 2), here also IAA had a significantly stronger influence than kinetin (Fig. 4). Surprisingly, GA, which exhibited no effect in the plate’s sensitivity assay (Fig. 1), had a pronounced (p < 5*10−4) decreasing effect on spore germination, similar to that of IAA (Fig. 4). In fact, high levels of IAA or GA totally prevented the fungal spore from germinating at the time period inspected. SA also showed a significant (p < 5*10−4) reducing effect in this assay, similar to the kinetin treatment result (Fig. 4), although it had less remarkable influence on colonies growth rate (Fig. 1). The other three treatments included in this experiment, the pre-boiled kinetin solution (de-Kinetin), JA and ET (ACC), showed significantly different spore germination rates from the DDW control (p < 0.05) after 10 h, but this difference disappeared after 13 h for the de-Kinetin and JA treatments and after 16 h for the ET treatment (Fig. 4).
Fig. 4.
Effect of plant hormones on H. maydis spore germination. To study the effect of phytohormones on colonies’ growth rate, the PDA medium was embedded with the different hormones to a final concentration of 100 mg/L. Plates were inoculated as described in Fig. 1. For the spore germination assay, spores were washed from the agar surface of 4-day-old colonies grown under the above conditions and suspended in watery solutions containing the various hormones described in Fig. 1. The spores’ suspensions in Eppendorf tubes were then incubated in a rotary shaker at 150 rpm at 28 ± 1 °C in the dark. a. The percentage of germinating conidia was determined after 0, 10, 13 and 16 h by direct counting of the spores with any visible germ tube emerging. The controls were spores in DDW without hormones and spores in DDW containing pre-boiled kinetin solution (named de-kinetin) at the same concentration (100 mg/L). Values represent the average of six replicates. Error bars indicate standard error. Asterisks represent significant (*P = 0.05, **P = 0.005, ***P < 5*10−4) differences from the control. b. Representative photograph of the hormones spore solutions at the time interval examined. Abbreviations as in Fig. 1
Field experiment for assessing 2,4-D DMA efficiency in controlling late wilt
The IAA hormone that had the most pronounced effect in restricting late wilt pathogen development in vitro was inspected for potential use in restricting late wilt in a field experiment. Instead of using the high purified grade IAA hormone used in the lab experiments, here we used a 2,4-D DMA, a widely used commercial compound that mimics the IAA effect. This compound was chosen since its application may be more economically realistic and because it does not interfere with normal plant growth. The field experiment was conducted on the Kibbutz Neot Mordechai maize field in the spring-summer of 2009 and 2010 using the susceptible maize Jubilee cv. Approximately two months after sowing, early signs of the disease began to appear.
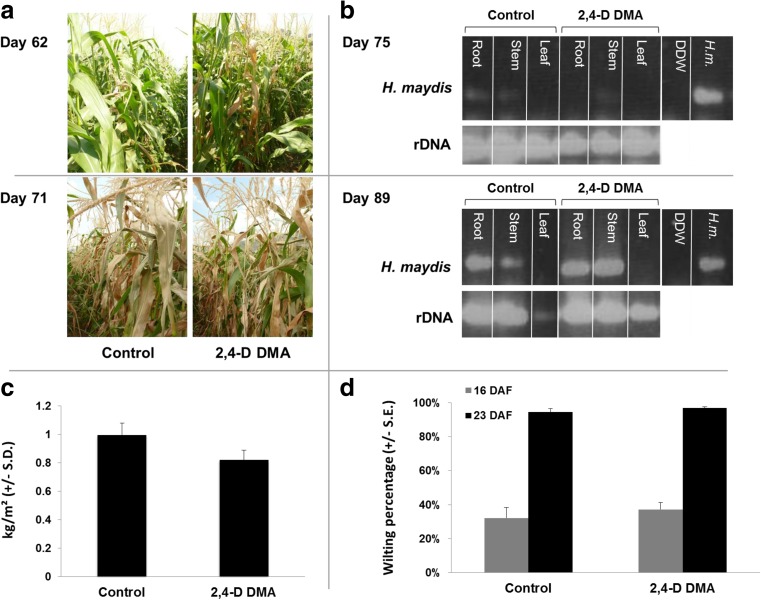
Usually the more severe symptoms of dehydration occur close to the flowering and first stages of seed development. The evident drying out signs of the lower part of the stem (brown strips) and leaves appeared in both the untreated and 2,4-D DMA treated plots about 7 days later than expected (according to our past experience in commercial fields in northern Israel), 2 days after flowering (62 days after sowing) (Fig. 5a). The percentage of wilting was assessed 17 and 24 DAF (77 and 84 days after sowing, respectively), and yield assessment carried out 24 DAF gave a more accurate estimation of these observations. In the control treatment, the percentage of wilting plants was 32 % and 94 %, respectively (Fig. 5d), and cob yield was 995 kg/0.1 ha (Fig. 5c). No remarkable change compared to the control was measured in the 2,4-D DMA treatment, which led to flowing values of 37 % and 97 % wilting plants (17 and 24 DAF, respectively), and cob yield was 820 kg/0.1 ha (Figs. 5c, d).
Fig. 5.
Field experiment for assessing 2,4-D DMA efficiency in controlling late wilt. The experiment was conducted in an infested sweet corn field in the Hula Valley (upper Galilee, northern Israel). The Jubilee cv. field plants were treated separately with Dimethylamine salt of 2,4-D (2,4-D DMA, 96.9 % active ingredient, Aminobar, Luxembourg Industries Ltd., Israel). The treatment was done at a dosage of 150 cm3/0.1 ha and applied three times. a. The control (untreated) and 2,4-D DMA treated groups of Jubilee maize plants in the field were photographed 62 and 71 days after sowing. b. Root, stem and leaf samples were inspected for the presence of pathogen DNA using PCR amplification of the unique H. maydis oligonucleotide (marked as H. maydis) 75 (upper panel) and 89 (lower panel) days after sowing. rDNA – amplified 18S eukaryotic ribosomal DNA; DDW – distilled and deionized water used as a template in the PCR mixture to ensure the absence of DNA contamination; and H. m.– DNA from 7-day-old Hnn maydis in vitro growth cultures used here as positive control for the unique DNA amplification. c. Yield assessment (in kg/m2) done 24 days after fertilization (DAF) (14/7/09, 84 days after sowing) that includes all upper part plant cobs in a 20-m-long section of each of the experiment rows. d. Wilt assessment done 17 (7/7/09) and 24 (14/7/09) DAF (77 and 84 days after sowing) for 100 plants in a sequence. The plants were classified as wilted when wilt symptoms appeared on the leaf whose cob is located in its axil. Bars indicate standard error
The molecular diagnosis carried out to detect pathogen DNA in the host tissues clearly identified the fungus in the root and stem of the plants from day 75 onwards in both the control and 2,4-D DMA treatments (Fig. 5b). The amount of DNA measured was slightly higher in the root compared to the stem, but was identical in both the control and 2,4-D DMA treatments (Fig. 5b). No fungal DNA was detected in the leaves at the time interval examined.
Discussion
Over the past decade, it has become increasingly clear that a plant’s resistance to attack is not brought about by the isolated activation of parallel, linear hormonal circuits, but rather is the consequence of a complex regulatory network that connects the individual pathways, enabling each to assist or antagonize the other (Grant and Lamb 2006; Pieterse et al. 2009). In addition to differential signal signatures, this pathway cross talk provides the plant with a powerful regulatory potential to fine-tune its immune response to different types of attackers.
Harpophora maydis is considered in most parts of the world to be an exotic and unfamiliar pathogen, and because of this, current knowledge of this pathogen’s behavior and its interactions with host maize plants is very limited. This work is the first to investigate the role of the plant hormonal complex regulatory network on maize pathogen H. maydis. It is possible that the host phytohormones regulate the pathogen mode of infection and spread, and that these hormones may be associated with late burst of the disease symptoms. While this hypothesis remains to be established, the lines of evidence found in this work provide the first evidence of this plant hormone influence and encourage further and more in-depth examination. These results include the suppressive influence of the three growth hormones studied on fungal colony development (IAA and CK) and spore germination (IAA, CK and GA) (Figs. 1–4).
Kinetin caused considerable suppression of colony growth (Fig. 1); however, in a detached root assay developed to create conditions that better resemble those in the field, it only delayed the pathogenesis of the fungus (Fig. 2). Conversely, IAA, which suppressed colony growth less than kinetin (Fig. 1), blocked fungal growth and the consequential root infection (Fig. 2). A molecular diagnostic of the infected roots supported these results.
Various effects of IAA on fungi have been reported. IAA and gibberellic acid were reported to affect yeast sporulation and cell elongation, but the effects of IAA were not uniform and varied according to growth conditions, such as vitamin content in the culture medium (Kamisaka et al. 1967). IAA also induced invasive growth in Saccharomyces cerevisiae, suggesting that it activates the pheromone MAP kinase pathway (Prusty et al. 2004). In Neurospora crassa, IAA reduced the ‘spore density effect’ and germination occurred at high densities in the presence of auxin (Nakamura et al. 1982). In Aspergillus nidulans, IAA partially restored cleistothecium formation and fertility of a tryptophan-auxotrophic strain (Eckert et al. 1999). External application of IAA has been shown to have various effects in additional fungal species, but it has been difficult to determine whether the observed phenotypes represent the physiological effects of endogenous fungal IAA (Tsavkelova et al. 2006).
On the other hand, ripening and senescence or stress hormones such as ABA, SA, ET and JA may also be involved in the disease late burst (De Vleesschauwer et al. 2010). These hormones may be up-regulated at a later stage of maize growth or in response to increased pathogenic invasion and spread (Adie et al. 2007). Indeed, recognition of pathogen-derived molecules by plant receptors leads to the activation of a concerted battery of defenses designed to impair further pathogen spread. These inducible defenses are regulated by the coordinated activity of an elaborate matrix of signal transduction pathways in which plant hormones SA, JA and ET act as key signaling molecules (Adie et al. 2007; Grant and Lamb 2006). In response to pathogen attack, plants produce a highly specific blend of SA, JA and ET, resulting in the activation of distinct sets of defense-related genes (Bari and Jones 2009). It is thought that this so-called signal signature, which varies greatly in quantity, timing and composition according to the type of attacker encountered, plays a primary role in the orchestration of the plant’s defense response and eventually determines the specific nature of the defense response triggered (Mur et al. 2006).
Our results showed that in the presence of ET and JA hormones, the pathogen maintained its optimal growth rate and spore germination (Figs. 1, 3,4). An exception is the SA, which at the highest dosage had a suppressive effect on the plate’s sensitivity assay that was enhanced in the presence of auxin (Fig. 1), and a kinetin-like effect on spore germination (Fig. 4). This is surprising since SA and auxin signaling pathways interact, for the most part, antagonistically (Wang et al. 2007). However, this relationship description is likely an oversimplified model, because, similar to the unexpected synergistic actions of SA and JA/ET (Adie et al. 2007; Mur et al. 2006), here also a more complex interaction may exist. Indeed, although considerable research efforts have been made in the past decade, the role of IAA in plant diseases is still ambiguous. Auxin may promote biotroph invasion by suppression of SA-mediated defenses (Bari and Jones 2009; Spoel and Dong 2008). But auxin may also suppress the development of necrotrophic pathogens that thrive on senescing or dead tissues (Degani et al. 2004; Llorente et al. 2008) independent of SA-mediated defense pathways (Llorente et al. 2008). As mentioned in the introduction, lifestyles of different pathogens are not often readily classifiable as purely biotrophic or necrotrophic. Indeed, H. maydis behave both as a hemi-biotroph and necrotroph. The pathogen is spread throughout the host; by flowering (anthesis at 9–10 weeks), it is distributed throughout the stalk and many vessels are blocked with hyphae and a dark gum-like substance (Sabet et al. 1970). So the pathogen is gradually suffocating the host by filling the xylem, causing vascular occlusion. It most probably uses some enzymes for the penetration and host establishment stages, but this is yet to be determined. After killing the host, the pathogen thrives on the remaining dead tissues. So this pathogen is most probably a necrotroph. However, since it survived for a long period (up to 80 days from seeding or more) on living plants, it may also be considered a hemi-biotrophic pathogen.
Although the root pathogenicity assay provides us with a more realistic evaluation method since it inspects fungal development on its natural host, which is also subject to the exogenous hormones effect, it is still based on detached roots. Intact roots are better for modelling host-pathogen interactions in the field. Furthermore, in the intact roots pathogenicity assay, the fungus has to deal with the host’s defense mechanisms and it develops in tissues that alter their structure and properties during plant growth and maturation. So, further research is needed in order to examine the inhibition effect of these hormones on the pathogenesis of H. maydis in greenhouse and field plants. The failure of 2,4-D to suppress late wilt in the field or prevent its symptoms (Fig. 5) may be the result of its application method through the drip lines irrigation system. In the host plant, auxin translocation is driven throughout the plant body, primarily from peaks of shoots to peaks of roots (polar auxin transport) (Friml and Palme 2002). For long distances, relocation occurs via the stream of fluid in phloem vessels, but, for short-distance transport, a unique system of coordinated polar transport directly from cell to cell is exploited. Therefore, in a continuation research, it will be important to examine the application of auxin in the field by spraying.
Field trials are important not only to investigate the plant hormone influence on the relationships between the pathogen and its host, but also to evaluate plant hormone potential as a means of disease control. This is an important issue that should be addressed in future studies. Here, we conducted a first trial (Fig. 5) to asses this possibility, with no positive results so far.
Further research should use the method presented here together with other methods (including greenhouse plants) to address the questions of how the regulation of these plant hormones is associated with H. maydis invasion and how their combined influence affects its spread within the host tissues. Tissue culture may also be used for this purpose (Daub 1986). It is possible, as found in other phytopathogens, that H. maydis itself produces plant hormones or their functional mimics, or induces hormone production by their host as part of its virulence behavior (Bari and Jones 2009; Robert-Seilaniantz et al. 2011). This possibility adds more inquiring questions to be explored in these pathogen-host complex interactions.
Moreover, the mechanistic action of the hormonal antagonistic and cooperative cross talk is yet to be revealed. Transcription factors and effector proteins most surely are critical in the circuitry controlling signal sensitivity and transduction in induced defense, as was found in other host-pathogen pairs (for example, Li et al. 2006; Lorenzo et al. 2004; Brodersen et al. 2006; Degani et al. 2004).
Conclusion
In summary, we have shown that plant growth hormones IAA, CK and GA inhibit the development of maize pathogen H. maydis, in vitro. While SA had the same tendency under some conditions, other maturation, senesces and defense-related hormones (ABA, ET and JA) had no apparent influence on fungal spore germination and/or colony growth. Despite the auxin’s most pronounced decreasing effect on the pathogen in vitro, applying 2,4-D in the field using the drip lines irrigation system failed to suppress late wilt or prevent its symptoms. These lines of evidence are important first steps towards our understanding of the complex interactions between H. maydis and its host plants hormones. It is still remain to be determined if the late outbreak of the disease is influenced by the plant hormones’ alternation, that occurred before tasseling and until shortly before maturity (Beyer 1973; Sexton et al. 1989). Our previous report on the delayed development of H. maydis in resistant maize cultivars (Drori et al. 2012), raise another intriguing question – are the plant hormones play a key role in the resistance mechanism in some maize cultivars?
Acknowledgments
We would like to thank Dr. Tsafrir Weinberg (Galilee Seeds, Research and Development (1989) Ltd), Dr. Onn Rabinovitz (Israel Ministry of Agriculture and Rural Development, Consultation Service) and Mr. Shaul Graph (Migal – Galilee Research Institute) for their helpful advice, Tal Magen, Shahar Menashe and Asaf Blatman (Tel-Hai College, Israel) for their technical assistance. This work was supported by a research grant from the Israel Plant Council, Ministry of Agriculture and a research grant from the Jewish National Fund (Keren Kayemeth LeIsrael).
References
- Adie BA, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano J-J, Schmelz EA, et al. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell Online. 2007;19:1665–1681. doi: 10.1105/tpc.106.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Beckers GJM, Spoel SH. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol. 2006;8:1–10. doi: 10.1055/s-2005-872705. [DOI] [PubMed] [Google Scholar]
- Beyer EM. Abscission support for a role of ethylene modification of auxin transport. Plant Physiol. 1973;52:1–5. doi: 10.1104/pp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Petersen M, Bjørn Nielsen H, Zhu S, Newman MA, Shokat KM, et al. Arabidopsis MAP kinase 4 regulates salicylic acid‐and jasmonic acid/ethylene‐dependent responses via EDS1 and PAD4. Plant J. 2006;47:532–546. doi: 10.1111/j.1365-313X.2006.02806.x. [DOI] [PubMed] [Google Scholar]
- Daub ME. Tissue Culture and the Selection of Resistance to Pathogens. Annu Rev Phytopathol. 1986;24:159–186. doi: 10.1146/annurev.py.24.090186.001111. [DOI] [Google Scholar]
- De Vleesschauwer D, Yang Y, Cruz CV, Höfte M. Abscisic acid-induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase-mediated repression of ethylene signaling. Plant Physiol. 2010;152:2036–2052. doi: 10.1104/pp.109.152702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani O. Pathogenicity assay for Cochliobolus heterostrophus G-protein and MAPK signaling deficiency strains. Am J Plant Sci. 2014;5:1318–1328. doi: 10.4236/ajps.2014.59145. [DOI] [Google Scholar]
- Degani O, Cernica G. Diagnosis and Control of Harpophora maydis, the Cause of Late Wilt in Maize. Adv Microbiol. 2014;4:94–105. doi: 10.4236/aim.2014.42014. [DOI] [Google Scholar]
- Degani O, Goldblat Y. Ambient Stresses Regulate the Development of the Maize Late Wilt Causing Agent, Harpophora maydis. Agric Sci. 2014;5:571–582. [Google Scholar]
- Degani O, Maor R, Hadar R, Sharon A, Horwitz BA. Host physiology and pathogenic variation of Cochliobolus heterostrophus strains with mutations in the G protein alpha subunit, CGA1. Appl Environ Microbiol. 2004;70:5005–5009. doi: 10.1128/AEM.70.8.5005-5009.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani O, Weinberg T, Graph S. Chemical control of maize late wilt in the field. Phytoparasitica. 2014;42:559–570. doi: 10.1007/s12600-014-0394-5. [DOI] [Google Scholar]
- Drori R, Sharon A, Goldberg D, Rabinovitz O, Levy M, Degani O. Molecular diagnosis for Harpophora maydis, the cause of maize late wilt in Israel. Phytopathol Mediterr. 2012;52:16–29. [Google Scholar]
- Eckert SE, Hoffmann B, Wanke C, Braus GH. Sexual development of Aspergillus nidulans in tryptophan auxotrophic strains. Arch Microbiol. 1999;172:157–166. doi: 10.1007/s002030050755. [DOI] [PubMed] [Google Scholar]
- El-Assiuty EM, Ismael AM, Zeller KA, Leslie JF. Relative colonization ability of greenhouse-grown maize by four lineages of Cephalosporium maydis from Egypt. Phytopathology. 1999;89:S23. [Google Scholar]
- El-Shafey HA, Claflin LE, editors. Late Wilt. St. Paul, Mn: APS Press; 1999. [Google Scholar]
- Friml J, Palme K. Polar auxin transport — old questions and new concepts? In: Hagen G, editor. Perrot-Rechenmann C. Springer Netherlands: Auxin Molecular Biology; 2002. pp. 273–284. [PubMed] [Google Scholar]
- Fromont-Racine M, Senger B, Saveanu C, Fasiolo F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. doi: 10.1016/S0378-1119(03)00629-2. [DOI] [PubMed] [Google Scholar]
- Gams W. Cephalosporium-artige Schimmelpilze (Hyphomycetes) Stuttgart: G. Fischer; 1971. [Google Scholar]
- Grant M, Lamb C. Systemic immunity. Curr Opin Plant Biol. 2006;9:414–420. doi: 10.1016/j.pbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Jameson PE. Cytokinins and auxins in plant-pathogen interactions – An overview. Plant Growth Regul. 2000;32:369–380. doi: 10.1023/A:1010733617543. [DOI] [Google Scholar]
- Johal L, Huber DM, Martyn R. Late wilt of corn (maize) pathway analysis: intentional introduction of Cephalosporium maydis. In: Pathways Analysis for the Introduction to the U.S. of Plant Pathogens of Economic Importance; 2004. [Google Scholar]
- Kamisaka S, Yanagishima N, Masuda Y. Effect of Auxin and Gibberellin on Sporulation in Yeast. Physiol Plant. 1967;20:90–97. doi: 10.1111/j.1399-3054.1967.tb07145.x. [DOI] [Google Scholar]
- Li J, Brader G, Kariola T, Tapio Palva E. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006;46:477–491. doi: 10.1111/j.1365-313X.2006.02712.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D. Recent advances in ethylene research. J Exp Bot. 2009;60:3311–3336. doi: 10.1093/jxb/erp204. [DOI] [PubMed] [Google Scholar]
- Llorente F, Muskett P, Sanchez-Vallet A, Lopez G, Ramos B, Sanchez-Rodriguez C, et al. Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol Plant. 2008;1:496–509. doi: 10.1093/mp/ssn025. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell Online. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan J. Occurrence of gibberellins in vascular plants, fungi, and bacteria. J Plant Growth Regul. 2001;20:387–442. doi: 10.1007/s003440010038. [DOI] [PubMed] [Google Scholar]
- Michail SH, Abou-Elseoud MS, Nour Eldin MS. Seed health testing of corn for Cephalosporium maydis. Acta Phytopathol Entomol Hung. 1999;34:35–42. [Google Scholar]
- Molinero-Ruiz ML, Melero-Vara JM, Mateos A. Cephalosporium maydis, the cause of late wilt in maize, a pathogen new to Portugal and Spain. Plant Dis. 2011;94:379–379. doi: 10.1094/PDIS-94-3-0379A. [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Atzorn R, Miersch O, Wasternack C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006;140:249–262. doi: 10.1104/pp.105.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tomita K, Kawanabe Y, Murayama T. Effect of auxin and gibberellin on conidial germination in Neurospora crassa II. “Conidial density effect” and auxin. Plant Cell Physiol. 1982;23:1363–1369. [Google Scholar]
- Navarro L, Bari R, Achard P, Lison P, Nemri A, Harberd NP, et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol : CB. 2008;18:650–655. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Payak MM, Lal S, Lilaramani J, Renfro BL. Cephalosporium maydis - A new threat to maize in India. Indian Phytopathol. 1970;23:562–569. [Google Scholar]
- Pecsi S, Nemeth L. Appearance of Cephalosporium maydis Samra Sabet and Hingorani in Hungary Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen. Univ Gent. 1998;63:873–877. [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Prusty R, Grisafi P, Fink GR. The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. Proc Natl Acad Sci. 2004;101:4153–4157. doi: 10.1073/pnas.0400659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–343. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- Sabet KA, Samra AS, Hingorani MK, Mansour IM. Stalk and root rots of maize in the United Arab Republic. FAO Plant Prot Bull. 1961;9:121–125. [Google Scholar]
- Sabet KA, Samra AS, Dawood NA. Combined infection with stalk-rot fungi. In: Samra AS, Sabet KA, editors. nvestigations on stalk-rot disease of maize in UAR. Cairo: Ministry of Agriculture, Government Printing Offices; 1966. pp. 195–204. [Google Scholar]
- Sabet KA, Zaher AM, Samra AS, Mansour IM. Pathogenic behaviour of Cephalosporium maydis and C. acremonium. Ann Appl Biol. 1970;66:257–263. doi: 10.1111/j.1744-7348.1970.tb06432.x. [DOI] [Google Scholar]
- Sahab AF, Osman AR, Soleman NK, Mikhail MS. Studies on root-rot of lupin in Egypt and its control. Egypt J Phytopathol. 1985;17:23–35. [Google Scholar]
- Saleh AA, Leslie JF. Cephalosporium maydis is a distinct species in the Gaeumannomyces-Harpophora species complex. Mycologia. 2004;96:1294–1305. doi: 10.2307/3762146. [DOI] [PubMed] [Google Scholar]
- Saleh AA, Zeller KA, Ismael AS, Fahmy ZM, El-Assiuty EM, Leslie JF. Amplified Fragment Length Polymorphism Diversity in Cephalosporium maydis from Egypt. Phytopathology. 2003;93:853–859. doi: 10.1094/PHYTO.2003.93.7.853. [DOI] [PubMed] [Google Scholar]
- Samra AS, Sabet KA, Hingorani MK. A new wilt disease of maize in Egypt. Plant Dis Rep. 1962;46:481–483. [Google Scholar]
- Samra AS, Sabet KA, Hingorani MK. Late wilt disease of maize caused by Cephalosporium maydis. Phytopathology. 1963;53:402–406. [Google Scholar]
- Samra AS, Sabet KA, Abdel-Rahim MF. Effect of soil conditions and cultural practices on infection with stalk rots. Government Printing Offices, Cairo, Egypt: U.A.R. Ministry of Agric; 1966. [Google Scholar]
- Sexton R, Tucker ML, del Campillo E, Lewis LN. The Cell Biology of Bean Leaf Abscission. In: Jackson M, editor. Osborne D. Springer Berlin Heidelberg: Cell Separation in Plants; 1989. pp. 69–78. [Google Scholar]
- Shehata FA. The inheritance of resistence to late wilt caused by Cephalosporium maydis in some corn lines Fac Agric. Cairo: Al-Azhar; 1976. [Google Scholar]
- Spoel SH, Dong X. Making Sense of Hormone Crosstalk during Plant Immune Responses. Cell Host Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Tsavkelova E, Klimova SY, Cherdyntseva T, Netrusov A. Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol. 2006;42:117–126. doi: 10.1134/S0003683806020013. [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ. Significance of Inducible Defense-related Proteins in Infected Plants. Annu Rev Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Walters DR, McRoberts N. Plants and biotrophs: a pivotal role for cytokinins? Trends Plant Sci. 2006;11:581–586. doi: 10.1016/j.tplants.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol : CB. 2007;17:1784–1790. doi: 10.1016/j.cub.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Warren HL. Potential disease problems: late wilt of maize. Phytopathology. 1983;73:782. [Google Scholar]
- Zeller KA, Jurgenson JE, El-Assiuty EM, Leslie JF. Isozyme and amplified fragment length polymorphisms from Cephalosporium maydis in Egypt. Phytoparasitica. 2000;28:121–130. doi: 10.1007/BF02981741. [DOI] [Google Scholar]
- Zeller KA, Abou-Serie MI, El-Assuity EM, Fahmy ZM, Bekheet FM, Leslie JF. Relative competitiveness and virulence of four clonal lineages of Cephalosporium maydis from Egypt toward greenhouse-grown maize. Plant Dis. 2002;86:373–378. doi: 10.1094/PDIS.2002.86.4.373. [DOI] [PubMed] [Google Scholar]