Abstract
The induced toxicity of three pesticides (alpha-Hexachlorocyclohexane: α-HCH; Parathion methyl:PM; Carbofuran: CN) in single and four possible combination on human keratinocyte cell line have been investigated. There was no significant change in toxicity (cyto and genotoxicity) on cell line exposed by individual pesticides except α-HCH. But, a synergistic effect was observed when we tested mixture of pesticides. The intracellular ROS and cytotoxicity assay revealed maximum reduction in cell viability (60%) was found in tri mixture of pesticides. All the possible combination of these pesticides demonstrated genotoxic activity in terms of olive tail moment and % tail DNA on cell line at low concentration. The order of toxicity was ranked as α-HCH+PM+CN>α- HCH+CN>PM+CN>α-HCH+PM. Our results call for more research to be undertaken in order to understand the mechanisms behind the synergy observed and quantify the extent of its environmental impacts.
Keywords: In-vitro, Toxicity, Genotoxicity, Pesticides, Cell line
Background
Pesticide is potential chemical pollutants, designed to selectively eliminate variety of pests. The mode of action is strongly connected to their chemical structures which are widely diversified. Indeed, pesticides in our surroundings do not appear singly and usually occurs as complex mixtures and their combined effect may exhibit toxicity to the targeted and non targeted organisms including humans [1]. In India, the pesticides were introduced in sixties and extensively used for agriculture and vector control purposes due to low cost and high effectiveness. Pesticides, especially α-HCH, parathion methyl and Carbofuran are widely used in most of the states of India [2]. In 1990, India banned α-HCH for agricultural use but allow its use for vector born disease eradication programme. These pesticides have carcinogenic risk and cause various degrees of toxicological effects on targeted as well as non targeted organisms [3]. Moreover, cases of acute pesticide poisonings account for significant morbidity and mortality worldwide, especially in developing countries like India, where the pattern of pesticide use is different [4]. Some authors have described several endocrine disruptions, oxidative stress, mutations and chromosomal alterations or DNA damage due to pesticide exposure at very low environmental concentration [5].
Non-linear dose-response toxicity can be observed and combinations with other pesticides or differences in individual susceptibility may increase the effect. As a consequence, effect of pesticides and their combination have to be studied carefully at low concentration. Moreover, we do not know how much environmental exposure of α-HCH, parathion methyl and carbofuran pesticides and their combination at low concentration influence the oxidative stress level and genotoxicity of non targeted organisms. Naturally pesticides are rarely found as single compound. Till date only a few studies have been published relating to the cytotoxic and genotoxic effects of low doses of pesticide mixtures. Hence, the main aim of this study was to evaluate the intracellular ROS generation, cytotoxicity and genotoxicity of the α-HCH, parathion methyl and Carbofuran pesticides in individual and mixed condition using human keratinocyte (HaCaT) cell line.
Methodology
Chemicals:
All pesticides including α-HCH; parathion methyl (PM) and carbofuran (CN) (purity > 98%), were purchased from Sigma− Aldrich, and diluted in DMSO (Dimethyl sulfoxide). Antibiotics and antimycotics were purchased from Sigma− Aldrich. All the chemicals and reagents were of analytical grade.
Cell line and their maintenance:
Toxicity test was performed for evaluation of cytotoxicity by using human keratinocyte (HaCaT) cell line. The cell line was grown in DMEM F-12 HAM culture medium supplemented with 10% FBS, antibiotics and antimycotic solution (1.5%) at 5% CO2 and 95% relative humidity at 37°C.
Sample treatment and quantification of ROS:
Cells were grown in 96-multiwell black plates (2 × 104 cells/well) and treated with seven types (three from each pesticide and four from their possible mixture (C-1: α- HCH+PM; C-2: α-HCH+CN; C-3: PM+CN; C-4: α- HCH+PM+CN) of samples. Cells were then incubated for 30 min at 37 0C with 10 µM carboxy H2-DCFDA in HBSS and exposed. Cells were incubated through the culture medium 24 h postplating with 0.26, 0.6 and 0.2 µM of each pesticide or their mixture having same concentration prepared in DMSO, giving a final concentration of 0.05%. The chosen doses were adapted to cell culture just above of acceptable daily intakes (ADI). Controls received similar amounts of DMSO alone. ADI for α-HCH, parathion methyl and Carbofuran were 0.0013, 0.03 and 0.001 mg/kg of BW/day, respectively. After treatment cells, fluorescence of DCF was measured by using 485 nm excitation and 520 nm emission wavelengths. The generation of intracellular ROS was further substantiated by the administration of NAC (N-acetyl cysteine) (10 and 100 µM) as specific quencher [6]. These experiments were performed in triplicates.
Cell viability assay by MTT:
Cells (2 × 104) were seeded per well in 96-well plates and kept in CO2 incubator for 48 hrs. The treatments were performed with three type pesticides (50µL) by complete medium (200µL) and incubated for 24 hrs. Media was replaced by 3-(4,5- dimethylthiozolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) (5 mg/mL). The absorbance was recorded at 530 nm by using multiwell micro plate reader.
Genotoxicity assessment (Comet):
The exposed cells were harvested with 0.06% trypsin. Olive tail moment (OTM) and percent tail DNA were selected to measure DNA damage. Images from 50 random cells (25 from each replicate slides) were analyzed for each experiment.
Data analysis:
Statistical analyses were performed with Student׳s t-test (one tailed test). Statistical analysis was performed with the SPSS Software. Error bars represent standard error of the mean.
Results
Intracellular ROS generation:
In this study, intracellular ROS levels generated by different pesticides were measured using DCF assay on human cell line (Figure 1). The generation of ROS is as following order C-4>C- 2>C-3>C-1> α-HCH>CN>PM (Figure 1A). Further, quenching of intracellular ROS generation of C-4 by different concentrations of NAC (10 and 100 µM) showed that DCF fluorescence decreased as the concentration of NAC increased (Figure 1a). Figure 1B exhibited DCF-fluorescence photographs of different pesticide samples which also support the above results.
Figure 1.
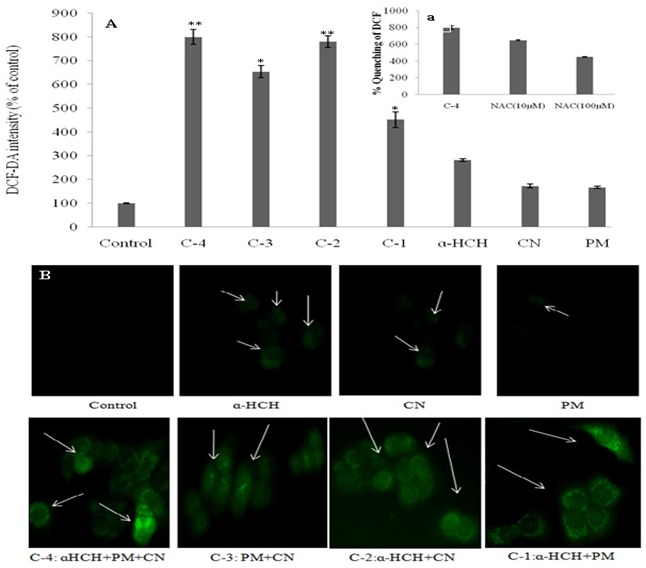
Intracellular ROS generation on HaCaT cell line after 24 hrs incubation: A) Quenching of intracellular ROS by different concentrations of N-acetyl-L-cysteine (10-100 µM) (a). Qualitative analysis of DCF fluorescence under different condition; B) C-1: α- HCH+PM; C-2: α-HCH+CN; C-3: PM+CN; C-4: α-HCH+PM+CN; PM: parathion methyl; CN: carbofuran.
Cytotoxicity:
The cytotoxicity of pesticides was performed through MTT assay. The minimum reduction in cell viability was recorded in control. The result of MTT showed highest reduction in cell viability (60%) in mixture of pesticides (C-4) compare to control. However the C-3, C-2 and C-1 showed reduction in cell viability in 40, 57 and 27%, respectively than control. MTT assay of selected pesticides and their combination visualized in Figure 2A.
Figure 2.
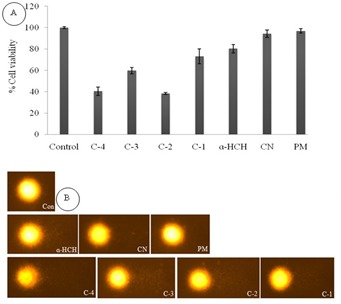
Cytotoxicity A) and genotoxicity; B) assay after incubation of 24 hrs. C-1: α-HCH+PM; C-2: α-HCH+CN; C-3: PM+CN; C-4: α-HCH+PM+CN; PM: parathion methyl; CN: carbofuran.
DNA damage:
The data obtained from alkaline comet assay confirmed that these pesticides were genotoxic in nature (Figure 2B). The OTM and percent DNA in comets formed by medium control (MC) was 0.49±0.03 AU and 6.89±0.48%. The OTM and tail DNA% was 5.01±0.21 AU and 28.09 ±1.40% for C-2; 3.53±0.08 AU and 19.00±0.39% for C-3; 2.01±0.07 AU and 11.4±0.42 for Carbofuran, respectively Table 1 (see supplementary material). The C-4 has highest OTM (6.06±0.04AU) and percent tail DNA (31.13±0.45%), respectively which is the greatest among all pesticide mixture. There was no significant changes were observed in reduction in cell viability as well as DNA damage in cell line exposed by individual pesticides except α- HCH.
Discussion
Nasal, oral, dermal and ocular, are four possible routes for pesticide exposure. Skin or dermal exposure account for about 90 % of all pesticides exposure users received from non fumigated pesticides [7]. It may occur any time a pesticide is mixed, applied or handled, and is often undetected. Dry material-dusts and granules as well as liquid pesticides can be absorbed through skin. Therefore, it is important to assess the toxic response of different pesticides by using human skin cell line as a model. Several studies have described pesticides as potentially harmful for target and non organisms [8]. In this study, three pesticides (α-HCH, Parathion methyl and Carbofuran) and their four possible combinations were selected for in vitro toxicological investigations at low doses. The doses were based on their persistence in the environment and AID which was recommended by different national and international agencies USEPA, 2006. The individual doses of pesticides showed slight reduction in cell viability compared to control illustrating that single pesticides are less toxic then bi (C-1 to C- 3) and tri-mixture (C-4) of these pesticides. The bi and tri-mixture of these pesticides showed higher toxicity with calculated reduction in cell viability at 57 and 60%, respectively. This result indicates that mixture of these three pesticides cause maximum toxicity and is more hazardous for the human. Mechanism of pesticides inside the cell, intracellular ROS and Comet assay were performed to observe the ROS generation and DNA damage [9]. Generally toxicants increase the reactive oxygen species (ROS) level inside the cell which damage different cell organelles which further promote apoptosis [10]. The DCFDA fluorescence is very sensitive method to detect the ROS level inside the cell. Our results of DCFDA on HaCaT cell line showed that as the concentration of pollutants increases, the intracellular ROS also increases thereby increasing the fluorescence. The individual pesticides α-HCH and Parathion methyl showed lesser fluorescence whereas Carbofuran showed minimum fluorescence by DCFDA. This result revealed that Carbofuran are less toxic than all selected pesticide. On other hand mixture of all four possible combinations of pesticides showed maximum fluorescence due additive response of pesticides. Further pretreatment with NAC significantly attenuate the fluorescence intensity of DCF, which confirmed the involvement of ROS in cytotoxicity. Our results were corroborating with that of Jose et al. [11] and Graillot et al. [5]. Data obtained from alkaline comet assay illustrates that the exposure of pesticides in individual and mixed condition causes DNA strands break. In our study, two controls were used, positive control (PC) (data not shown) and medium control (MC). Positive control was used to compare the effectiveness of toxicity and medium control was used to observe the effect of the process of comet assay. Hear the all possible combination of pesticides showed higher OTM and percent tail DNA than individual pesticides and medium control. This might be due synergistic effect of different pesticides which enhanced the toxicity. The magnitude of synergistic effect of pesticides was varied from 2 to 3 fold. Regarding the mixture toxicities caused by pesticides in non targeted species, a number of studies have different interactions were recorded. Liang and Zhou [12] found that interaction between two pesticides exhibited a synergic toxic effect on non targeted organisms. Stepic et al. [13] found that binary combinations of two pesticides atrazine and cyanazine with the organophosphate chlorpyrifos had synergism effect on non target organism. These findings call for more research to be promptly undertaken in order to understand the mechanisms behind the synergy observed and quantify the extent of its environmental impacts.
Conclusion
Humans have a great risk of a cocktail of pesticides exposure at low concentration through several competing pathways. In this context, we tried to appraise a possible link between low dose of three pesticides and their induced toxicity on HaCaT cell line. The intracellular ROS, MTT and comet assay revealed that maximum toxicity was found in mixed pesticide condition compare to individual pesticides and more hazardous to humans. This study helps in better understanding of links between metabolic perturbations of pesticides at low concentration, thus improving the evaluation of the risks to human health posed by exposure to mixtures of pesticides.
Conflict of interest
None
Supplementary material
Acknowledgments
Funding from Council of Science and Technology, Uttar Pradesh is gratefully acknowledged.
Footnotes
Citation:Abhishek et al, Bioinformation 10(12): 716-720 (2014)
References
- 1.Sarkar SK, et al. Environ Int. 2008;34:1062. doi: 10.1016/j.envint.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Mishra K, Sharma RC. Sci Total Environ. 2011;409:4939. doi: 10.1016/j.scitotenv.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Bedi JS, et al. Sci Total Environ. 2013;464:720. doi: 10.1016/j.scitotenv.2013.06.066. [DOI] [PubMed] [Google Scholar]
- 4.Mishra K, et al. Ecotoxicol Environ Saf. 2012;76:215. doi: 10.1016/j.ecoenv.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Graillot V, et al. Mutat Res. 2012;748:8. doi: 10.1016/j.mrgentox.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Bajpayee M, et al. Mutat Res. 2002;520:83. doi: 10.1016/s1383-5718(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 7.George J, Shukla Y. J Proteomics. 2011;74:2713. doi: 10.1016/j.jprot.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Zhou S, et al. J Environ Sci (China) 2011;23:676. doi: 10.1016/s1001-0742(10)60462-7. [DOI] [PubMed] [Google Scholar]
- 9.Matés JM, et al. Free Radic Biol Med. 2010;49:1328. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Valencia A, Kochevar IE. Free Radica Biol Med. 2006;40:641. doi: 10.1016/j.freeradbiomed.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Jose S, et al. Mar Environ Res. 2011;71:169. doi: 10.1016/j.marenvres.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Zhou Q. Bull Environ Contam Toxicol. 2003;71:2258. doi: 10.1007/s00128-003-0228-5. [DOI] [PubMed] [Google Scholar]
- 13.Stepic S, et al. Bull Environ Contam Toxicol. 2013;91:55. doi: 10.1007/s00128-013-1000-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


