Abstract
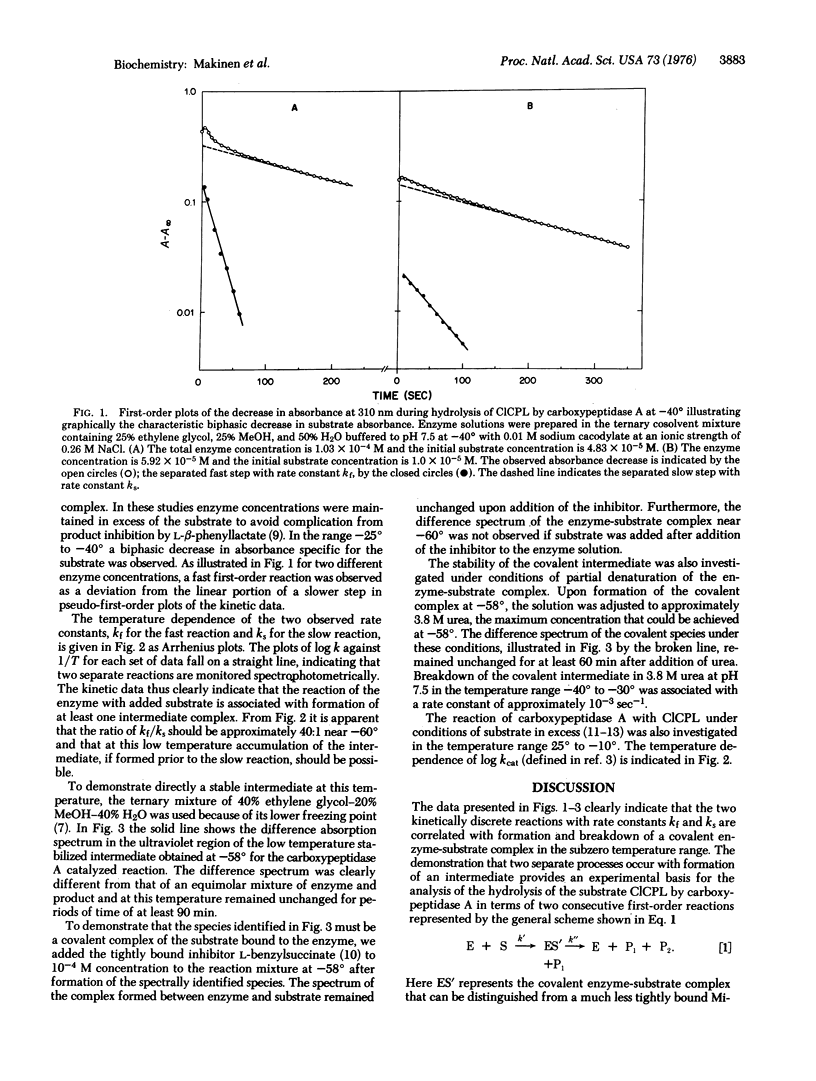
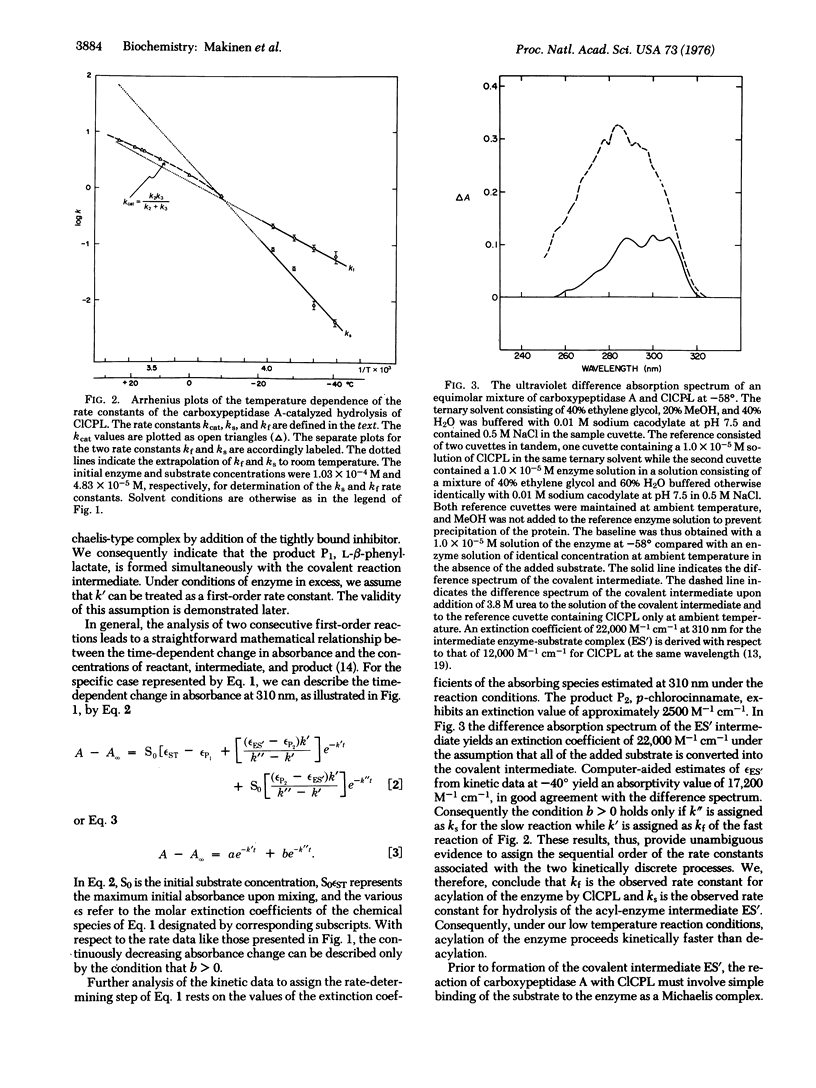
The reaction of carboxypeptidase A (peptidyl-L-amino-acid hydrolase; EC 3.4.12.2) with the specific ester substrate O-(trans-p-chlorocinnamoyl)-L-beta-phenyllactate has been investigated in the temperature range 25 degrees to -40 degrees with use of organic-aqueous cosolvent mixtures. In the subzero temperature range the hydrolysis reaction is characterized by a biphasic decrease in absorbance specific for the substrate. The kinetic data can be unambigously analyzed as two consecutive first-order reactions with formation of a covalent acyl-enzyme intermediate. Deacylation of the covalent intermediate is shown to be rate-limiting in the subzero temperature range, and near -60 degrees it is sufficiently stable for spectral characterization. Consideration of the structure of the active site and of the catalytically functional residues of the enzyme leads to the conclusion that the intermediate is a mixed anhydride in which the gamma-carboxylate of glutamate-270 is acylated by the substrate. The temperature dependence of the rate constants of the acylation and deacylation steps explains why the intermediate of this enzyme-catalyzed reaction is observed only at low temperatures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard S. A., Lau S. J., Noller H. Spectrophotometric identification of acyl enzyme intermediates. Biochemistry. 1965 Jun;4(6):1108–1118. doi: 10.1021/bi00882a020. [DOI] [PubMed] [Google Scholar]
- Byers L. D., Wolfenden R. A potent reversible inhibitor of carboxypeptidase A. J Biol Chem. 1972 Jan 25;247(2):606–608. [PubMed] [Google Scholar]
- Carson F. W., Kaiser E. T. pH dependence of the hydrolysis of O-acetyl-L-mandelate catalyzed by carboxypeptidase A. A critical examination. J Am Chem Soc. 1966 Mar 20;88(6):1212–1223. doi: 10.1021/ja00958a024. [DOI] [PubMed] [Google Scholar]
- Douzou P. Enzymology at sub-zero temperatures. Mol Cell Biochem. 1973 May 11;1(1):15–27. doi: 10.1007/BF01659935. [DOI] [PubMed] [Google Scholar]
- Hall P. L., Kaiser B. L., Kaiser E. T. pPH dependence and competitive product inhibition of the carboxypeptidase A catalyzed hydrolysis of O-(trans-cinnamoyl)-L-beta-phenyllactate. J Am Chem Soc. 1969 Jan 15;91(2):485–491. doi: 10.1021/ja01030a047. [DOI] [PubMed] [Google Scholar]
- Hui-Bon-Hoa G., Douzou P. Ionic strength and protonic activity of supercooled solutions used in experiments with enzyme systems. J Biol Chem. 1973 Jul 10;248(13):4649–4654. [PubMed] [Google Scholar]
- Johansen J. T., Klyosov A. A., Vallee B. L. Circular dichroism-inhibitor titrations of arsanilazotyrosine-248 carboxypeptidase A. Biochemistry. 1976 Jan 27;15(2):296–303. doi: 10.1021/bi00647a009. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Environment and conformation dependent sensitivity of the arsanilazotyrosine-248 carboxypeptidase A chromophore. Biochemistry. 1975 Feb 25;14(4):649–660. doi: 10.1021/bi00675a001. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Suh J., Kaiser E. T. pH dependence of the nitrotyrosine-248 and arsanilazotyrosine-248 carboxypeptidase A catalyzed hydrolysis of O-(trans-p-chlorocinnamoyl)-L-beta-phenyllactate. J Am Chem Soc. 1976 Mar 31;98(7):1940–1947. doi: 10.1021/ja00423a048. [DOI] [PubMed] [Google Scholar]
- Tomalin G., Kaiser B. L., Kaiser E. T. A search for an intermediate in carboxypeptidase A catalyzed ester hydrolyses. J Am Chem Soc. 1970 Oct 7;92(20):6046–6049. doi: 10.1021/ja00723a039. [DOI] [PubMed] [Google Scholar]


