Abstract
Human monocytes activated by toll-like receptor 2/1 ligand (TLR2/1L) show enhanced expression of the vitamin D receptor (VDR) and the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1). The resulting intracrine conversion of precursor 25-hydroxyvitamin D3 (25OHD) to active 1,25-dihydroxyvitamin D (1,25(OH)2D) can stimulate expression of antibacterial cathelicidin (CAMP). To determine whether this response is functional in HIV-infected subjects (HIV+), serum from HIV+ subjects pre- and post-vitamin D supplementation was utilized in monocyte cultures with or without TLR2/1L. Expression of CYP27B1 and VDR was enhanced following treatment with TLR2/1L, although this effect was lower in HIV+ vs HIV- serum (p<0.05). CAMP was also lower in TLR2/1L-treated monocytes cultured in HIV+ serum (p<0.01). In a dose study, supplementation of HIV+ subjects with 4,000IU or 7,000IU vitamin D/day increased serum 25OHD from 17.3±8.0 and 20.6±6.2 ng/ml (43 nM and 51 nM) at baseline to 41.1±12.0 and 51.9±23.1 ng/ml (103 nM and 130 nM) after 12 wks (both p<0.001). Greater percent change from baseline 25OHD was significantly associated with enhanced TLR2/1L-induced monocyte CAMP adjusted for baseline expression (p = 0.009). In a randomized placebo-controlled trial, 7,000IU vitamin D/day increased serum 25OHD from 18.0±8.6 to 32.7±13.8 ng/ml (45 nM and 82 nM) after 12 wks. Expression of CAMP increased significantly from baseline after 52 wks of vitamin D-supplementation. At this time point, TLR2/1L-induced CAMP was positively associated with percent change from baseline in 25OHD (p = 0.029 overall and 0.002 within vitamin D-supplemented only). These data indicate that vitamin D supplementation in HIV-infected subjects can promote improved antibacterial immunity, but also suggest that longer periods of supplementation are required to achieve this.
Keywords: HIV, monocytes, antibacterial, cathelicidin, vitamin D, supplementation
1. Introduction
Vitamin D promotes a range of extra-skeletal responses that may impact on diverse aspects of human physiology [1]. Prominent amongst these are the immunomodulatory effects of active 1,25-dihydroxyvitamin D (1,25(OH)2D), that can influence both innate and adaptive immunity [2, 3]. Responses to 1,25(OH)2D are mediated via the nuclear vitamin D receptor (VDR) which is expressed by many immune cells [4]. However, monocytes are particularly important targets for vitamin D because they express the enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) that catalyzes conversion of pro-hormone 25-hydroxyvitamin D (25OHD) to active 1,25(OH)2D [5, 6]. The resulting intracrine mechanism plays a pivotal role in innate antibacterial responses, where binding of pathogen-associated molecular patterns (PAMPs) to monocyte pattern-recognition receptors (PRR) stimulates expression of both VDR and CYP27B1 [7]. Intracrine conversion of 25OHD to 1,25(OH)2D by monocytes promotes several antibacterial responses, including enhanced expression of antibacterial proteins [7, 8], induction of autophagy [9, 10], and regulation of intracellular iron homeostasis [11]. These effects appear to be dependent on the availability of substrate 25OHD for intracrine conversion to 1,25(OH)2D. As 25OHD is the main circulating form of vitamin D, variations in serum 25OHD status have the potential to influence monocyte antibacterial responses, with vitamin D-deficiency compromising, and vitamin D-supplementation enhancing, antibacterial responses [7, 12].
Vitamin D-induced antimicrobial responses have been reported for several different cell types and may occur in response to a variety of pathogens [3]. In particular, vitamin D-induced antibacterial responses in monocytes have been closely linked to mycobacterial infections such as tuberculosis [13]. The tuberculosis pathogen Mycobacterium tuberculosis (M. tb) is phagocytosed by monocytes and macrophages, but M. tb PAMPs also promote innate immune responses when recognized by PRR such as the toll-like receptor (TLR)2/1 heterodimer [14, 15]. TLR2/1 ligands (TLR2/1L) associated with M. tb have been shown to stimulate monocyte expression of CYP27B1 and VDR, with the resulting intracrine induction of the antibacterial protein cathelicidin (CAMP) acting to promote intracellular killing of M. tb [7].
These observations provide a mechanistic rationale for the historical link between vitamin D and the treatment of tuberculosis, in which ultraviolet light (the primary mode of vitamin D generation in normal physiology) and cod liver oil (a rich source of dietary vitamin D) were at one time used as a treatment for tuberculosis [16, 17]. More recently, epidemiology has shown that vitamin D-insufficiency is associated with increased incidence of tuberculosis [18-21], and several clinical trials of vitamin supplementation and tuberculosis have also been reported with varying degrees of success [21-24]. Vitamin D-deficiency is also prevalent in HIV+ subjects where there is increased risk of infection by pathogens such as M. tb [25, 26]. Supplemental vitamin D may help to promote antibacterial responses in HIV+ subjects by utilizing intracrine vitamin D pathways [27]. However, vitamin D may also stimulate anti-retroviral responses in the setting of HIV-infection, with a recent study showing that 1,25(OH)2D-induced autophagy in macrophages not only stimulates killing of M. tb, but also inhibits replication of HIV [28]. To investigate the possible importance of vitamin D for antibacterial responses in HIV+ subjects, we carried out two vitamin D supplementation trials and used serum from the trial participants to assess monocyte antibacterial activity. Furthermore, we investigated potential differences in anti-bacterial responses in HIV+ subjects compared to healthy control subjects (HIV-) of similar age and serum vitamin D status.
2. Materials and Methods
2.1 Human subjects
Subjects with HIV were recruited from Philadelphia regional urban centers for two vitamin D supplementation trials. Both protocols were approved by Institutional Review Board at CHOP. Written informed consent was obtained from subjects ages 18.0 to 24.9 yrs, emancipated minors presenting for care alone and parents/legal guardians of subjects <18.0 yrs. Healthy control subjects were drawn from two studies, one investigating vitamin D status in subjects with sickle cell disease and one in subjects with renal insufficiency. HIV- control subjects were similar in age and sex to HIV+ subjects. The baseline characteristics of HIV+ subjects from the Dose Study and the RCT compared to the HIV- subjects used in the current analyses are summarized in Supplemental Table 1.
2.2 Vitamin D dose study
Forty-four subjects were recruited from three regional centers and randomized to receive supplemental vitamin D3 at 4000 IU/d (Nutraceutical Science Institute, Lexington, NC) or 7,000 IU/day (NOW Foods, NOW Health Group, Bloomingdale, IL) as described previously [29]. Those unable to swallow capsules took 0.108 mL or 0.189 mL of 1,000 IU/drop vitamin D3 drops (J.R. Carlson Laboratories, Inc. Arlington Heights, IL) in the 4,000 IU or 7,000 IU/day group, respectively. Serum samples were obtained at baseline, and 6 and 12 wks. This trial was registered with ClinicalTrials.gov, number NCT01092338. Eighteen subjects from the Dose Study with baseline 25OHD ≥20 ng/mL were selected as the comparison group for the HIV-control subjects.
2.3 Randomized placebo controlled trial (RCT)
Fifty-eight subjects were recruited from eight regional centers and randomized to either placebo or supplementation with 7,000 IU/day vitamin D3 7000 IU capsules (Life Extension, Fort Lauderdale, FL) in a 1:1 ratio, as described previously [30]. Those unable to swallow capsules took 0.49 mL of 400 IU/drop vitamin D drops (J.R. Carlson Laboratories, Inc. Arlington Heights, IL) or placebo drops. Subjects were enrolled throughout the year, balanced by season, and participants and study staff were blinded. Serum samples were obtained at baseline, and 12, 24 and 52 wks. This trial was registered with ClinicalTrials.gov, number NCT01475890.
2.4 HIV disease status
For subjects with HIV at each visit, HIV-1 RNA plasma quantitative assay for viral load (copies/mL) was performed (CHOP Laboratory). For CD4%, an HIV specific multicolor flow cytometry immunophenotyping was performed with a Becton Dickinson LSR II flow cytometer and BD FACSDiva software (Becton Dickinson, San Diego, CA) [31]Medical record reviews documented HIV disease status based upon CDC immunity classification [32, 33], and whether or not subjects were receiving highly active antiretroviral therapy (HAART).
2.5 Quantification of serum vitamin D metabolites and serum CAMP protein
Serum concentrations of 25OHD were determined using liquid chromatography tandem mass spectrometry (Clinical Laboratory, CHOP) with intra and inter assay coefficients of variation (CV) below 8%. Serum concentrations of 1,25(OH)2D were quantified by radioimmunoassay (Heartland Assays, Ames, Iowa), with inter- and intra-assay CV of 12.6% and 9.8%, respectively. Vitamin D binding protein (DBP) was assessed by enzyme linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN) with inter- and intra-assay CV below 10%. Free 25OHD not bound to DBP or albumin was calculated using established equations [34]. Serum 25OHD status for health outcomes was defined as sufficient, ≥32 ng/mL; insufficient, 20-31.9 ng/mL; and deficient, <20 ng/mL). Serum concentrations of CAMP protein were analyzed using an ELISA kit (Hycult Biotech Inc., Plymouth Meeting, PA) as described by the manufacturer.
2.6 Cell culture
Ficoll isolated peripheral blood mononuclear cells (PBMCs) derived from anonymous healthy donors that were screened according to standard blood transfusion protocols were obtained from the Center for AIDS Research (CFAR) Virology Core (supported by the National Institutes of Health award AI-28697 and by the UCLA AIDS Institute and the UCLA Council of Bioscience Resources). PBMCs were cultured as described previously [35]. Briefly, monocytes were enriched by adherence by incubating 5 × 106 PBMCs per well in 12-well plates for 2 hrs in RPMI (Life Technologies, Grand Island, NY) with 1% fetal bovine serum (Omega Scientific, Tarzana, CA). Non-adherent cells were removed by washing with serum-free RPMI. Remaining cells were then cultured overnight in RPMI with 10% subject serum incubated with or without 100 ng/ml Pam3CSK4 (InVivoGen, San Diego, CA), a synthetic TLR2/1 ligand. After 24 hr incubation, media was aspirated and RNA isolated.
2.7 RNA isolation and quantitative real-time PCR
RNA was isolated by Trizol (Life Technologies, Grand Island, NY) extraction and cDNA was synthesized by Super Script III Reverse Transcriptase (Life Technologies, Grand Island, NY) according to manufacturer protocol utilizing random primers. qPCR analysis was performed on a MX-3005P (Agilent, Santa Clara, CA) instrument utilizing TaqMan system reagents (Life Technologies, Grand Island, NY) as described previously [35]. Primer/probe combinations used to amplify target cDNAs were as follows: CAMP (Hs00189038_m1), CYP27B1 (Hs00168017_m1), VDR (Hs00172113_m1), CYP24A1 (Hs00167999_m1) eukaryotic 18S rRNA probe/primer (part number 4319413E) as the internal calibrator, and TaqMan Master Mix (part number 4324020). All cDNAs were amplified under the following conditions: 50°C for 2 min; 95°C for 10 min followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. Data were initially generated as ΔCt values (cycle threshold for PCR amplification of target gene minus cycle threshold for amplification of housekeeping gene) for each target gene for vehicle (ΔCtV) and TLR2/1L (ΔCtT) treatments. Results for the comparison between HIV+ and HIV- subjects are shown as fold-change in mRNA expression for each target gene following treatment of monocytes with TLR2/1L, where fold-change was calculated according to the equation 2ΔCtV - δC tT (2ΔΔCtT). For the longitudinal analyses in each study, RT-PCR analyses were carried out in batches in which all RNA samples for a particular subject across the supplementation period were analyzed together to minimize intra-assay variation. For regression analyses of CAMP expression, unadjusted ΔΔCtT values for gene expression were used as these values were normally distributed.
2.8 Statistical analysis
Statistical analyses were performed using STATA 12.0 and 13.0 (STATA, Inc, College Station, TX). RNA (copies/mL) required transformation as log10. In descriptive statistics, continuous variables are presented as mean ± SD. For monocyte gene expression in serum from HIV+ versus HIV- participants data were analyzed by Mann-Whitney Rank Sum tests, with p values < 0.05 considered statistically significant. Spearman rank correlation coefficients were used to determine the relationship between serum 25OHD and other markers of vitamin D status (DBP, free 25OHD and 1,25(OH)2D) at each time point.
Generalized estimating equation (GEE) regression analysis was used to determine the change in vitamin D metabolites and CAMP mRNA expression over time for the two dose groups in the Dose Study and for the vitamin D supplementation and placebo groups in the RCT. We chose to perform GEE analysis to take advantage of our longitudinal data, to expand upon the significant changes found by the Wilcoxon signed-rank test, and to avoid multiple comparisons using the Wilcoxon signed-rank test repeatedly. The GEE regression is an expansion of the linear regression, assessing the correlation between outcome and predictors across time points and accounting for the correlation of repeated measures within participants. Multivariable GEE regression was used to assess the relationship between changes in CAMP mRNA expression and changes in 25OHD in the dose-finding study. For the RCT, multivariable linear regression was used to assess the relationship of changes in CAMP mRNA expression and changes in 25OHD at 52 weeks since CAMP expression was only increased at this time point. The change in 25OHD was expressed as a continuous variable of the percent change from baseline 25OHD. Covariates evaluated in these models included age, sex, race (black vs other), on/off HAART, and whether RNA viral load was detectable baseline. P values <0.05 were considered significant.
3. Results
3.1 Characteristics of HIV+ and HIV- patient cohorts
HIV+ subjects in the Dose Study (n=40) and the RCT (n=56) and healthy control subjects (n=20) were similar in age, sex and racial background to subjects with HIV+, however, a greater proportion of HIV+ subjects had 25OHD deficiency (Supplemental Table 1). Approximately half of HIV+ subjects had detectable RNA viral load and 75% were receiving highly active anti-retroviral therapy (HAART) at the time of the study.
3.2 Induction of 25OHD and 1,25(OH)2D metabolism (CYP27B1 and CYP24A1) and VDR expression in monocytes cultured with HIV+ and HIV- serum
Serum from HIV+ subjects at baseline and from age-matched HIV- control subjects was used in ex vivo cultures of human monocytes obtained from healthy HIV- donors. For this comparison, HIV+ subjects were restricted to those from the Dose Study with 25(OH)D ≥20 ng/ml (n=18) to match the vitamin D status of HIV- subjects. Data in Figure 1 indicate that monocyte exposure to TLR2/1L in HIV- serum culture, stimulated expression of mRNA for VDR (3.5-fold) and CYP27B1 (9.3-fold), but suppressed expression of CYP24A1 (0.5-fold). A similar pattern was observed for monocytes cultured in HIV+ serum, with expression of mRNA for VDR (3.5-fold) and CYP27B1 (4.1-fold) being enhanced, whilst CYP24A1 (0.6-fold) was suppressed. There was significantly higher expression of CYP27B1 in TLR2/1-treated cells cultured with HIV- serum relative to HIV+ serum (p=0.047), but no difference in TLR2/1L-induced VDR or CYP24A1. As previously reported [12], TLR2/1L suppressed monocyte expression of mRNA for the antibacterial protein CAMP. However, this effect was more pronounced with HIV+ serum relative to HIV- serum (p=0.004). Further analysis of TLR2/1L-induced monocyte CYP27B1 in the current study did not reveal any significant difference HIV+ subjects receiving or not receiving HAART (results not shown).
Figure 1. Expression of the vitamin D intracrine system and antibacterial cathelicidin in monocytes cultured in serum from HIV+ and HIV- patients.
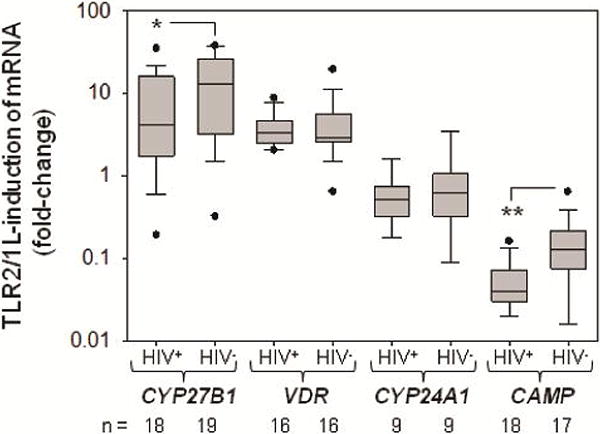
Human monocytes from normal healthy donors were cultured in serum (10%) from age-matched HIV+ or HIV- donors in the presence or absence of a toll-like receptor 2/1 ligand (TLR2/1L), PAM3CysK for 24 hrs. Total RNA from these cultures was then used to assess expression of: mRNA for CYP27B1, VDR, CYP24A1, and CAMP. Data are shown as fold-change in TLR2/1L-treated cells relative to vehicle-treated controls cells for each gene ± SE. * = HIV+ statistically different from HIV- subjects, p<0.05; ** p<0.01.
3.3 Effect of vitamin D supplementation in vivo on serum concentrations of 25OHD, free 25OHD, and 1,25(OH)2D in HIV+ subjects (Dose Study)
As previously reported [29], the Dose Study resulted in a significant increase in HIV+ subject serum concentrations of 25OHD following supplementation with either 4,000 IU/day or 7,000 IU/day vitamin D. As shown in Figure 2A, Baseline serum 25OHD concentrations of 17.3±8.0 ng/ml and 20.6±6.2 ng/ml (43 nM and 51 nM) respectively, increased by wk 6 of supplementation to 41.1±12.0 ng/ml (103 nM) (p<0.001) in subjects receiving 4,000 IU/day vitamin D, and 51.9±23.1 ng/ml (130 nM) (p<0.001) in subjects receiving 7,000 IU/day vitamin D. Neither supplementation group showed a further increase in serum 25OHD at 12 wks. Analysis of serum DBP concentrations showed no significant change in the level of this protein throughout the Dose Study (data not shown), and DBP concentrations did not correlate with serum concentrations of 25OHD (Supplemental Figure 1). As a consequence, estimated levels of free 25OHD also increased following vitamin D supplementation (Figure 2B) and correlated with serum levels of total 25OHD (Supplemental Figure 1). At wk 6, subjects supplemented with 7,000 IU/day vitamin D showed higher free 25OHD relative to subjects supplemented with 4,000 IU/day vitamin D, but this difference was not observed at wk 12 (Figure 2B).
Figure 2. Effect of vitamin D supplementation in HIV+ participants on serum concentrations of vitamin D metabolites and ex vivo CAMP mRNA expression (dose study).
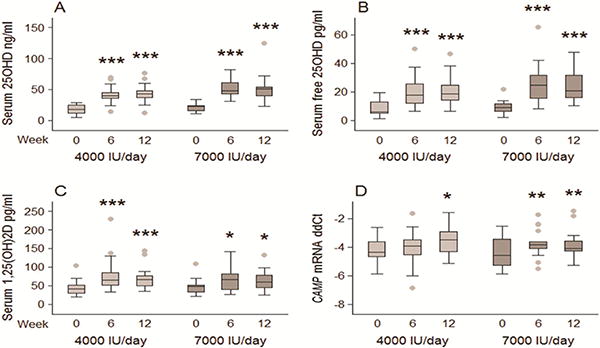
2A) serum concentrations of 25OHD in HIV+ participants receiving 4,000 IU/day (n = 21) or 7,000 IU/day (n = 19) vitamin D supplement; 2B) serum concentrations of calculated free 25OHD; 2C) serum concentrations of 1,25(OH)2D; 2D) CAMP mRNA expression. Statistically different, *p <0.05 compared to baseline, **p <0.01 compared to baseline, ***p <0.001 compared to baseline.
In the Dose Study, baseline 1,25(OH)2D concentrations of 44.4±20.6 and 45.9±20.1 pg/ml (107 pM and 100 pM) in the 4,000 and 7,000 IU/day supplementation groups, respectively increased to 77.3±45.4 pg/ml and 66.4±33.5 pg/ml (186 pM and 160 pM) by wk 6 (Figure 2C). As with 25OHD, there was no further increase in serum 1,25(OH)2D after 12 wks of supplementation. Serum concentrations of 1,25(OH)2D correlated with serum concentrations of 25OHD at baseline (wk 0) and wk 12 (Supplemental Figure 1).
3.4 Effect of vitamin D supplementation in vivo on expression of CAMP in monocytes cultured with HIV+ serum (Dose Study)
Data in Figure 1 indicated that serum from HIV+ subjects can support a functional system for activation of 25OHD, and 1,25(OH)2D-VDR-mediated induction of CAMP in monocytes. At wk 6 and wk 12 of the Dose Study, serum from HIV+ subjects supplemented with 7,000 IU vitamin D/day supported higher levels of TLR2/1L-induced CAMP mRNA relative to baseline sera (Figure 2D). A similar response was also observed for serum from subjects receiving 4,000 IU vitamin D/day, but this effect was only significant after 12 wks of vitamin D supplementation. GEE regression analysis showed that the change in CAMP expression from baseline was positively associated with the percent change in 25OHD from baseline, adjusted for baseline CAMP expression (p = 0.009, Table 1A). Other covariates tested (age, HIV acquisition group, gender, black race, HIV RNA detection at baseline, and therapeutic use of anti-retroviral drugs) were not significantly associated with the change from baseline CAMP expression.
Table 1. Regression analysis of the effect of change from baseline 25OHD on CAMP mRNA expression.
| a) GEE Regression Analysis in the Dose Study | |||
|---|---|---|---|
|
| |||
| Beta | 95% CI | P value | |
| Baseline CAMP (δδCt) | -0.469 | [-0.716,-0.223] | <0.001 |
| % change in 25OHD | 0.001 | [0.000,0.002] | 0.009 |
| b) Multivariable Linear Regression at 52 weeks in the Randomized Controlled Trial | |||
|---|---|---|---|
|
| |||
| Supplemented + Placebo (n = 48) Model R2 = 0.41 | Beta | 95% CI | P value |
| Baseline age (years) | 0.066 | [0.003,0.129] | 0.042 |
| Baseline CAMP (δδCt) | -0.379 | [-0.546,-0.213] | <0.001 |
| % change in 25OHD | 0.003 | [0.000,0.007] | 0.029 |
|
| |||
| Supplemented Only (n = 25) Model R2 = 0.69 | |||
|
| |||
| Baseline age (years) | 0.097 | [0.035,0.160] | 0.004 |
| Baseline CAMP (δδCt) | -0.419 | [-0.593,-0.245] | <0.001 |
| % change in 25OHD | 0.005 | [0.002,0.007] | 0.002 |
3.5 Effect of vitamin D supplementation in vivo on serum concentrations of 25OHD, free 25OHD and 1,25(OH)2D in HIV+ subjects (RCT)
As previously reported [30], baseline serum 25OHD concentrations of 18.0±8.6 ng/ml (45 nM) in the RCT showed no significant change in the placebo group across the 52 wks of the study (Figure 3A). By contrast, supplementation with 7,000 IU vitamin D/day significantly increased serum 25OHD to 32.7 ± 13.8 ng/ml (82 nM) after 12 wks (p<0.001), and 27.4±19.3 ng/ml (68 nM) after 52 wks (p<0.01) (Figure 3A). Following supplementation, baseline 1,25(OH)2D concentrations of 32.1 ± 11.3 pg/ml (77 pM) increased to 43.1±15.9 pg/ml (104 pM) (wk 12) and 41.8±14.3 pg/ml (100 pM) (wk 24) (both p<0.001), but levels at wk 52 (32.4±10.5 pg/ml, 78 pM) were not significantly different from either baseline or wk 52 placebo-treated subjects (Figure 3C).
Figure 3. Effect of vitamin D supplementation in HIV+ participants on serum concentrations of vitamin D metabolites and ex vivo CAMP mRNA expression (RCT).
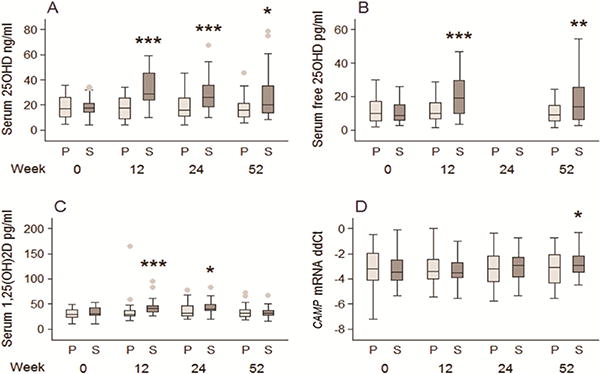
3A) serum concentrations of 25OHD in HIV+ participants receiving 7,000 IU/day vitamin D supplement (S; n = 29) or placebo (P; n = 27); 3B) serum concentrations of calculated free 25OHD; 3C) serum concentrations of 1,25(OH)2D; 2D) CAMP mRNA expression. *p <0.05 compared to baseline, **p <0.01 compared to baseline, ** p<0.001 compared to baseline.
3.6 Effect of vitamin D supplementation in vivo on expression of CAMP in monocytes cultured with HIV+ Serum (Randomized Control Study [RCT])
Induction of monocyte CAMP expression by TLR2/1L using serum from the RCT was not significantly different from wk 0 relative to wk 52 of the study when using serum from placebo-treated subjects (Figure 3D). There was also no significant change in CAMP when using serum from vitamin D-supplemented subjects at wk 12 and wk 24. However, serum from vitamin D supplemented subjects at wk 52 showed significantly higher induction of CAMP mRNA relative to baseline (p < 0.05). Linear regression analysis showed that CAMP expression at this time point was significantly associated with the percent change from baseline 25OHD, independent of baseline CAMP expression and age in all participants (p = 0.029, R2 = 0.41) and in analyses limited to vitamin D-supplemented participants (p = 0.002, R2 = 0.69). Use/non-use of HAART did not predict CAMP mRNA outcomes in either the Dose Study or the RCT (results not shown).
4. Discussion
Vitamin D-deficiency is common to populations across the globe [36], but appears to be particularly prevalent in subjects with HIV [37-39]. The underlying cause of this has yet to be fully defined and likely includes general factors commonly associated with vitamin D-deficiency such as seasonal and skin pigmentation variations that affect epidermal synthesis of vitamin D by UV light. However, additional HIV-specific factors may further compromise vitamin D status in HIV+ subjects, notably possible effects of the anti-retroviral drugs that are routinely used as therapy in HIV+ subjects [38-41]. Whilst this has immediate implications for the management of classical physiological responses to vitamin D such as the regulation of bone and mineral metabolism [41-43], increased awareness of non-classical, extra-skeletal effects of vitamin D suggests that vitamin D-deficiency has a much wider impact on the health of those infected with HIV.
Recent reports have described association between vitamin D-deficiency and circulating markers of inflammation in HIV+ subjects [44], but in other HIV+ cohorts this effect was not observed [45]. In the current study, we investigated the impact of serum 25OHD concentrations, before and after vitamin D supplementation, on innate antibacterial immune responses in children and young adults with HIV. An initial strategy was to assess the effect of vitamin D supplementation on circulating levels of the antibacterial protein CAMP in HIV+ patients. Data in Supplemental Figure 2 showed that even after 12 weeks of vitamin D supplementation, which elevated serum 25OHD concentrations by more than 2-fold, there was no change in serum concentrations of CAMP. These data were not entirely unexpected given that neutrophils are the major source of circulating CAMP in humans [46]. As neutrophils do not appear to express CYP27B1, it is unlikely that these cells will respond to changes in serum 25OHD concentrations, and thus measurement of this parameter may have little value in defining the immune impact of vitamin D supplementation. Instead, we hypothesized that serum 25OHD is more important for localized synthesis of 1,25(OH)2D by cells such as macrophages which encounter and internalize key pathogens such as M. tb. In the current study, we therefore utilized an ex vivo human monocyte cell culture model to assess the potential for enhanced antibacterial responses following in vivo supplementation with vitamin D.
The ability of vitamin D to promote monocyte antibacterial responses to a pathogenic challenge such as infection with M. tb or exposure to PAMPs such as the TLR2/1L ligand PAM3CysK is dependent, in part, on the induction of an intracrine vitamin D system [3, 7]. Monocytes cultured in serum from HIV+ and HIV- subjects showed similar patterns of TLR2/1L-induced VDR, CYP27B1, CYP24A1, and CAMP mRNA expression, but it was notable that the TLR2/1L effects on CYP27B1 and CAMP were enhanced in HIV- subjects relative to HIV+ subjects of similar serum 25OHD status. This effect does not appear to be a generalized insensitivity to the TLR2/1L as VDR and CYP24A1 expression was similar in the HIV+ and HIV- monocyte cultures. Rather these data suggest a specific impairment of CYP27B1 induction, and resulting intracrine induction of CAMP. In this setting, the ability of HIV+ serum to support optimal antibacterial responses may be further compromised by low serum levels of 25OHD that are frequently observed in HIV+ subjects [26].
One possible explanation for the impaired induction of CYP27B1 in HIV+ subjects is that anti-retroviral use by these subjects may inhibit monocyte responses to vitamin D [47]. In previous reports the effect of anti-retroviral drugs was only studied in the context of 1,25(OH)2D production, whereas data presented in the current study indicate that TLR2/1L-induction of the CYP27B1 transcript is impaired in HIV+ serum. Not all of the HIV+ subjects in our study were undergoing anti-retroviral therapy, and further analysis of TLR2/1L-induced monocyte CYP27B1 in the current study did not reveal any significant difference in TLR2/1L-induction of monocyte CYP27B1 in HIV+ subjects receiving or not receiving anti-retroviral therapy. There was also no consistent effect of anti-retroviral therapy on TLR2/1L-mediated regulation of CAMP in either the Dose Study or the RCT, although in both cases this may be due to the relatively low numbers of subjects not receiving anti-retroviral therapy.
Apart from the presence of anti-retroviral drugs, the impaired induction of CYP27B1 and associated CAMP in monocytes cultured in HIV+ serum may also be due to alterations in endogenous circulating factors. Studies by our group and others have demonstrated that factors associated with adaptive immune response to infection act to fine-tune TLR2/1L-induced CYP27B1 expression and activity in monocytes. These include inflammatory cytokines such as interleukin-15 [48], interferon γ [49, 50], and IL-4 [49]. It is possible that changes in T cell populations associated with HIV infection will lead to alterations in some of the circulating factors detailed above. This, in turn, may lead to dysregulation of monocyte intracrine vitamin D function in HIV+ patients, further exacerbating the underlying effects of vitamin D-deficiency associated with HIV [27].
In addition to activation of intracrine CYP27B1 and VDR, induction of monocyte antibacterial responses by vitamin D requires optimal availability of substrate 25OHD for the CYP27B1 enzyme. Subjects for HIV+ vs HIV- comparison were chosen to have similar serum 25OHD concentrations at baseline (data not shown), further emphasizing the link between impaired TLR2/1L-induction of CYP27B1 in monocytes with HIV+ serum, and expression of CAMP in these cells. We therefore postulated that this defect in antibacterial response could be abrogated through increased circulating levels of 25OHD in the HIV+ subjects. As previously reported [29, 30], both the Dose Study and the RCT were successful in elevating serum 25OHD concentrations by approximately 2-fold to 30-50 ng/ml (approximately 75-125 nM). In the Dose Study enhanced TLR2/1L-induced CAMP expression relative to baseline was demonstrated at 12 weeks for the 4000 IU/day group and at both 6 and 12 weeks for the 7000 IU/day group. Percent change from baseline in 25OHD was significantly related to the change from baseline CAMP expression. In the RCT, enhanced CAMP expression was only demonstrated at 52 weeks in the D3 supplemented group, and the change from baseline expression at this time was significantly related to the percent change in 25OHD from baseline. The difference between the CAMP responsiveness in the two studies may be attributable to the more robust 25OHD response in the Dose Study. In both studies, the rise in 25OHD appeared to precede that in CAMP expression, particularly in the RCT. The over-arching conclusion from these data is that enhanced monocyte responses to TLR2/1 challenge following vitamin D-supplementation are related to, but not exclusively dependent on serum concentrations of 25OHD.
It is possible that relatively small increases in serum 25OHD may be sufficient to promote antibacterial responses provided there is sufficient induction of the monocyte intracrine vitamin D system. Analysis of TLR2/1L-induced CYP27B1 mRNA showed no significant difference in expression at wk 12 and wk 52 of the RCT (data not shown). Thus differential induction of CAMP at these time points, despite similar serum 25OHD, is not due to temporal differences in the ability of TLR2/1L to induce activation of 25OHD. Another possible explanation is that supplementation at different time points is associated with different levels of free 25OHD. Systemic transport of 25OHD is mediated primarily via serum DBP, but in vitro DBP acts to attenuate 25OHD bioavailability for cells such as monocytes [35], with cellular uptake of 25OHD occurring via the so-called Free-Hormone Hypothesis [51]. In the current study it was interesting to note that serum concentrations of DBP did not vary significantly with vitamin D supplementation, leading to a concomitant rise in concentrations of free serum 25OHD (Figure 2B and 3B) that correlated with total serum 25OHD concentrations (Supplemental Figure 1).
Serum concentrations of 1,25(OH)2D were also enhanced following vitamin D supplementation, with one exception, wk 52 of the RCT. The reason for this remains unclear but the data nevertheless underline the potential for ‘uncoupling’ of serum concentrations of 25OHD and 1,25(OH)2D, despite the close correlation between these two metabolites in baseline serum (Supplemental Figure 1). Moreover, the fact that serum from vitamin D-supplemented HIV+ subjects at wk 52 supported enhanced TLR2/1L-induced monocyte CAMP with levels of 1,25(OH)2D that were no different from the placebo, suggests that circulating levels of this vitamin D metabolite are not essential for vitamin D-induced antibacterial immunity. This endorses the central hypothesis that immune responses to vitamin D are driven by localized intracrine synthesis of 1,25(OH)2D, with the expression of CYP27B1 and availability of substrate 25OHD being the key determinant of this mechanism [5].
Data presented in this study confirm that in vivo supplementation with vitamin D has the potential to enhance antibacterial immune responses in HIV+ subjects despite apparent impairment of intracrine CYP27B1 expression by circulating factors in these subjects. Similar vitamin D supplementation trials in HIV- subjects will help to clarify whether elevated serum 25OHD is more effective in supporting antibacterial responses in the absence of viral infection. To better define the impact of HIV on vitamin D-mediated antibacterial responses, it will also be important to assess monocytes isolated from HIV+ subjects before and after vitamin D supplementation. Finally, in future studies it will be interesting to assess other antibacterial targets for vitamin D such as β-defensin 2 [8] or hepcidin [11]. The over-arching objective will be to provide a clearer rationale for routine monitoring of vitamin D status in HIV+ patients, and maintenance of adequate serum concentrations of 25OHD through vitamin D supplementation.
Supplementary Material
Highlights.
Vitamin D-deficiency is common in patients with HIV infection
Vitamin D-deficiency may compromise antibacterial responses in patients with HIV
HIV sera support monocyte intracrine vitamin D system but less than healthy controls
Raised serum 25OHD in HIV patients enhances antibacterial cathelicidin ex vivo
Raise serum 25OHD is more effective when maintained for longer periods
Acknowledgments
We are grateful to subjects and families for participation. We thank Julia Samuel, Savannah Knell, Susan Ellenberg, PhD, Steven Douglas, MD, Kelly Dougherty, PhD, Florin Tuluc, PhD, Eric Riedel, Jennifer Murray, Clinical Translational Research Center, the Special Immunology Family Care Clinic, Adolescent Initiative Program at CHOP, Jonathan Lax Treatment Center, Cooper University Hospital, Alfred I DuPont Hospital for Children, Hospital of the University of Pennsylvania, Temple University Hospital, and Drexel University Hospital.
This work was supported by the NIH/National Center for Complementary and Alternative Medicine, Grant R01AT005531, the National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. Ex vivo analyses were supported by NIH/NIAMS grant AR063910. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This publication was made possible through core services and support from the University of Pennsylvania Center for AIDS Research (CFAR), an NIH-funded program (P30AI045008). Additional support was from the Jean A. Cortner Endowed Chair, Nutrition Center and the Research Institute at the Children's Hospital of Philadelphia. Life Extension (Ft. Lauderdale, FL) and J.R. Carlson Laboratories, Inc. (Arlington Heights, IL) donated the vitamin D3 supplements and placebo capsules and drops, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams JS, Hewison M. Update in vitamin D. The Journal of clinical endocrinology and metabolism. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewison M. An update on vitamin D and human immunity. Clinical endocrinology. 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 3.Hewison M. Antibacterial effects of vitamin D, Nature reviews. Endocrinology. 2011;7:337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 4.Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, Jurutka PW. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutrition reviews. 2008;66:S98–112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 5.Hewison M. Vitamin D and the intracrinology of innate immunity. Molecular and cellular endocrinology. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewison M. Vitamin D and innate and adaptive immunity. Vitamins and hormones. 2011;86:23–62. doi: 10.1016/B978-0-12-386960-9.00002-2. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 8.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PloS one. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell host & microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cellular microbiology. 2011;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, Nayak A, Wesseling-Perry K, Westerman M, Hollis BW, Salusky IB, Hewison M. Suppression of iron-regulatory hepcidin by vitamin d. J Am Soc Nephrol. 2014;25:564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. Journal of immunology. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting Edge: Vitamin D-Mediated Human Antimicrobial Activity against Mycobacterium tuberculosis Is Dependent on the Induction of Cathelicidin. Journal of immunology. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. Journal of immunology. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 16.Grad R. Cod and the consumptive: a brief history of cod-liver oil in the treatment of pulmonary tuberculosis. Pharmacy in history. 2004;46:106–120. [PubMed] [Google Scholar]
- 17.Moller KI, Kongshoj B, Philipsen PA, Thomsen VO, Wulf HC. How Finsen's light cured lupus vulgaris. Photodermatol Photoimmunol Photomed. 2005;21:118–124. doi: 10.1111/j.1600-0781.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 19.Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. The Journal of infection. 2005;50:432–437. doi: 10.1016/j.jinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J. 2008;27:941–942. doi: 10.1097/INF.0b013e31817525df. [DOI] [PubMed] [Google Scholar]
- 21.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. American journal of respiratory and critical care medicine. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 22.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. A single dose of vitamin d enhances immunity to mycobacteria. American journal of respiratory and critical care medicine. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 23.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta medica Indonesiana. 2006;38:3–5. [PubMed] [Google Scholar]
- 24.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes PF, Bloch AB, Davidson PT, Snider DE., Jr Tuberculosis in patients with human immunodeficiency virus infection. The New England journal of medicine. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 26.Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, Tsekela R, Bashe L, de Azevedo V, Caldwell J, Venton TR, Timms PM, Wilkinson KA, Wilkinson RJ. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A. 2011;108:19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Realegeno S, Modlin RL. Shedding light on the vitamin D-tuberculosis-HIV connection. Proc Natl Acad Sci U S A. 2011;108:18861–18862. doi: 10.1073/pnas.1116513108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8:1523–1525. doi: 10.4161/auto.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oda Y, Chalkley RJ, Burlingame AL, Bikle DD. The transcriptional coactivator DRIP/mediator complex is involved in vitamin D receptor function and regulates keratinocyte proliferation and differentiation. The Journal of investigative dermatology. 2010;130:2377–2388. doi: 10.1038/jid.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochiai E, Kitagawa H, Takada I, Fujiyama S, Sawatsubashi S, Kim MS, Mezaki Y, Tsushima Y, Takagi K, Azuma Y, Takeyama K, Yamaoka K, Kato S, Kamimura T. CDP/cut is an osteoblastic coactivator of the vitamin D receptor (VDR) Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:1157–1166. doi: 10.1359/jbmr.091105. [DOI] [PubMed] [Google Scholar]
- 31.Savkur RS, Bramlett KS, Stayrook KR, Nagpal S, Burris TP. Coactivation of the human vitamin D receptor by the peroxisome proliferator-activated receptor gamma coactivator-1 alpha. Molecular pharmacology. 2005;68:511–517. doi: 10.1124/mol.105.012708. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Cui J, Nakamura K, Ribeiro RC, West BL, Gardner DG. Coactivator-vitamin D receptor interactions mediate inhibition of the atrial natriuretic peptide promoter. The Journal of biological chemistry. 2000;275:15039–15048. doi: 10.1074/jbc.275.20.15039. [DOI] [PubMed] [Google Scholar]
- 33.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 34.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. The Journal of clinical endocrinology and metabolism. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 37.Pinzone MR, Di Rosa M, Malaguarnera M, Madeddu G, Foca E, Ceccarelli G, d'Ettorre G, Vullo V, Fisichella R, Cacopardo B, Nunnari G. Vitamin D deficiency in HIV infection: an underestimated and undertreated epidemic. European review for medical and pharmacological sciences. 2013;17:1218–1232. [PubMed] [Google Scholar]
- 38.Mueller NJ, Fux CA, Ledergerber B, Elzi L, Schmid P, Dang T, Magenta L, Calmy A, Vergopoulos A, Bischoff-Ferrari HA, Swiss HIVCS. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. Aids. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 39.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, Bush T, Brooks JT. H.I.V. Study to Understand the Natural History of, A.i.t.E.o.E.T. Investigators, Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 40.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, Sweep FC, Hermus AR, Bosch ME, Burger DM, Bravenboer B, Koopmans PP, Van Der Ven AJ. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS research and human retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 41.Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, Moniz CF, Post FA. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. Aids. 2010;24:1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 42.Rosenvinge MM, Gedela K, Copas AJ, Wilkinson A, Sheehy CA, Bano G, Hay PE, Pakianathan MR, Sadiq ST. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. Journal of acquired immune deficiency syndromes. 2010;54:496–499. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- 43.Wanner DP, Tyndall A, Walker UA. Tenofovir-induced osteomalacia. Clinical and experimental rheumatology. 2009;27:1001–1003. [PubMed] [Google Scholar]
- 44.Ansemant T, Mahy S, Piroth C, Ornetti P, Ewing S, Guilland JC, Croisier D, Duvillard L, Chavanet P, Maillefert JF, Piroth L. Severe hypovitaminosis D correlates with increased inflammatory markers in HIV infected patients. BMC infectious diseases. 2013;13:7. doi: 10.1186/1471-2334-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckard AR, Judd SE, Ziegler TR, Camacho-Gonzalez AF, Fitzpatrick AM, Hadley GR, Grossmann RE, Seaton L, Seydafkan S, Mulligan MJ, Rimann N, Tangpricha V, McComsey GA. Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antiviral therapy. 2012;17:1069–1078. doi: 10.3851/IMP2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS letters. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 47.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. Aids. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 48.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. Journal of immunology. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, Modlin RL. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klug-Micu GM, Stenger S, Sommer A, Liu PT, Krutzik SR, Modlin RL, Fabri M. CD40 ligand and interferon-gamma induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology. 2013;139:121–128. doi: 10.1111/imm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. The Journal of steroid biochemistry and molecular biology. doi: 10.1016/j.jsbmb.2013.09.012. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


