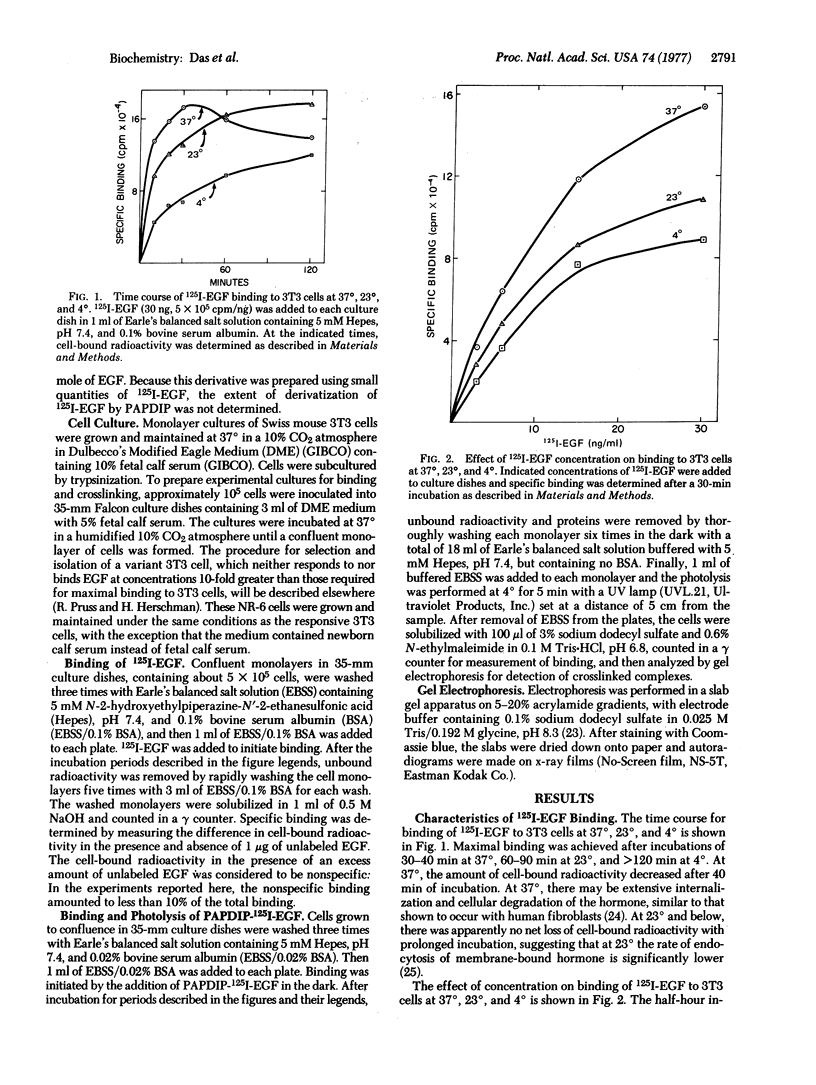
Abstract
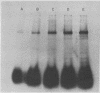
A photoreactive derivative of epidermal growth factor (EGF) has been used to identify and specifically label a membrane receptor for EGF on mouse 3T3 cells. Photoactivable EGF, labeled with 125I, was incubated with 3T3 cells and then photolyzed in situ to generate a nitrene capable of reacting with a wide variety of chemical bonds. Analysis of the system by sodium dodecyl sulfate/polyacrylamide gel electrophoresis revealed, besides the band of EGF, only one other major radioactive band, at a position indicating an apparent molecular weight of 190,000. This band was absent when a nonresponsive and nonbinding variant of 3T3 was used. A direct proportionality between binding activity and crosslinked complex formation was demonstrated using a variety of binding conditions. "Down regulated" cells, in which EGF binding activity was greatly reduced by prolonged incubation with an appropriate concentration of EGF, also had a decrease in covalent complex formation proportional to the decrease in EGF binding activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne D. T., Hixson S. S., Westheimer F. H. A diazo compound for the photochemical labeling of yeast alcohol dehydrogenase. J Biol Chem. 1971 Jul 25;246(14):4477–4484. [PubMed] [Google Scholar]
- COHEN S. ISOLATION AND BIOLOGICAL EFFECTS OF AN EPIDERMAL GROWTH-STIMULATING PROTEIN. Natl Cancer Inst Monogr. 1964 Apr;13:13–37. [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Epidermal growth factor. J Invest Dermatol. 1972 Jul;59(1):13–16. doi: 10.1111/1523-1747.ep12625690. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet G. W., Knowles J. R., Porter R. R. The antibody binding site. Labelling of a specific antibody against the photo-precursor of an aryl nitrene. Biochem J. 1972 Jul;128(3):499–508. doi: 10.1042/bj1280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Jamieson J. D., Printz M. P. Photoaffinity labeling of peptide hormone binding sites. J Biol Chem. 1974 Jun 10;249(11):3510–3518. [PubMed] [Google Scholar]
- Ginsberg B. H., Kahn C. R., Roth J., De Meyts P. Insulin-induced dissociation of its receptor into subunits: possible molecular concomitant of negative cooperativity. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1068–1074. doi: 10.1016/0006-291x(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Ji T. H. A novel approach to the identification of surface receptors. The use of photosensitive hetero-bifunctional cross-linking reagent. J Biol Chem. 1977 Mar 10;252(5):1566–1570. [PubMed] [Google Scholar]
- Katzenellenbogen J. A., Johnson H. J., Jr, Myers H. N. Photoaffinity labels for estrogen binding proteins of rat uterus. Biochemistry. 1973 Oct 9;12(21):4085–4092. doi: 10.1021/bi00745a010. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E., O'Hara D. Identification of the cardiac beta-adrenergic receptor protein: solubilization and purification by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2828–2832. doi: 10.1073/pnas.69.10.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver D., Chiu W., Wolff M. E., Edelman I. S. Photoaffinity site-specific covalent labeling of human corticosteroid-binding globulin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4462–4466. doi: 10.1073/pnas.73.12.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E., Hollenberg M. D., Cuatrecasas P. Epidermal growth factor. Characteristics of specific binding in membranes from liver, placenta, and other target tissues. Arch Biochem Biophys. 1974 Oct;164(2):518–526. doi: 10.1016/0003-9861(74)90062-9. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Meunier J. C., Changeux J. P. Progress in the purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 1972 Nov 15;28(1):96–100. doi: 10.1016/0014-5793(72)80686-0. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Hudgin R. L., Ashwell G., Stockert R. J., Morell A. G. A membrane receptor protein for asialoglycoproteins. Methods Enzymol. 1974;34:688–691. doi: 10.1016/s0076-6879(74)34090-6. [DOI] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Ruoho A. E., Kiefer H., Roeder P. E., Singer S. J. The mechanism of photoaffinity labeling. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2567–2571. doi: 10.1073/pnas.70.9.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Taylor J. M., Mitchell W. M., Cohen S. Epidermal growth factor. Physical and chemical properties. J Biol Chem. 1972 Sep 25;247(18):5928–5934. [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Westermark B. Density dependent proliferation of human glia cells stimulated by epidermal growth factor. Biochem Biophys Res Commun. 1976 Mar 22;69(2):304–310. doi: 10.1016/0006-291x(76)90522-2. [DOI] [PubMed] [Google Scholar]