Abstract
Stripe smut of grasses, Ustilago striiformis s.l., is a complex of smut fungi widely distributed over temperate and subtropical regions. The disease results in the shredding and death of leaf tissue following the rupture of elongated sori. Nearly 100 different grass species in more than 30 genera are infected by stripe smut. During the last two centuries more than 30 smut taxa have been described from members of this complex. The present study attempts to clarify the taxonomy and phylogeny of stripe smuts on grasses by analysing both morphological and molecular data. More than 200 specimens from different continents and host plants were examined. DNA was extracted from teliospores of 23 specimens from different hosts collected in Europe, Asia, and North America. The ITS and LSU regions of ribosomal DNA were amplified and used in phylogenetic analyses. The results of Maximum Parsimony and Bayesian analyses demonstrated that there are several lineages of stripe smut fungi. Analyses of morphological characters assessed with light and scanning electron microscopy showed high support for the differentiation of two clades as distinct from U. striiformis s.l., i.e., U. nunavutica sp. nov. and U. bromina. Two additional clades, U. striiformis s.str. on Holcus and a clade containing specimens from Elymus, were identified with molecular data although morphological differences were not apparent. Descriptions are given for each species.
Keywords: fungi, ITS, LSU, phylogeny, plant pathogens, Ustilaginaceae
INTRODUCTION
The genus Ustilago contains about 170 described species (Vánky 2012). It belongs to the family Ustilaginaceae, which mostly infects grasses (Stoll et al. 2003, 2005, Begerow et al. 2006, McTaggart et al. 2012, Vánky 2012). During recent phylogenetic studies, Ustilago was shown to be polyphyletic with species distributed in three main clades (Stoll et al. 2005, Begerow et al. 2006, McTaggart et al. 2012). One of these clades included the type species U. hordei, and other species that destroy the inflorescences of pooid grasses. Stripe smut fungi U. striiformis s.l. and U. calamagrostidis were sister to this group.
Ustilago striiformis s.l. is a complex of cryptic smut fungi widely distributed over temperate and subtropical regions of the world (Fischer 1940, 1953, Zundel 1953, Vánky 1994, 2012). These fungi cause stripe smut diseases of numerous grasses, which result in the shredding and death of leaf tissue following the rupture of elongated sori (Fischer 1940). Since the beginning of the 20th century this disease has been documented as an important factor affecting the cultivation of grasses for hay, lawns, or pastures in the United States (Fischer 1940, 1953). The most substantial losses occurred in fields of timothy (Phleum pratense), Kentucky bluegrass (Poa pratensis), orchard grass (Dactylis glomerata), and redtop (Agrostis alba) (Pammel et al. 1901, Clinton 1902, Osner 1916, Davis 1924, Wellhausen et al. 1943, Krietlow & Myers 1944, Fischer 1953). In spite of wide applications of different fungicides, the economic losses from stripe smut are still significant. For instance, in a disease survey of orchard grass seed production fields in Oregon, during a three year study period, stripe smut occurred in about one third of the fields, with severity as high as 11 % of infected plants (Alderman et al. 2007).
Taxonomically, U. striiformis s.l. is one of the most difficult species in smut systematics. Nearly 100 different grass species in more than 30 genera of mostly pooid grasses are parasitized (Vánky 2012, Farr & Rossman 2013). Based on minor morphological differences, different hosts, or cultural characters, more than 30 taxa have been described for stripe smuts of grasses. Thus, seven taxa were described on various Poa hosts (U. kairamoi, U. poae, U. poae-annuae, U. poae-bulbosae, U. poae-nemoralis, U. poae-pratensis, and U. poarum), three on Hierochloë (U. hierochloes-odoratae, U. jaczevskyana var. jaczevskyana, and U. jaczevskyana var. sibirica), two on Holcus (U. striiformis s.str. and Tilletia debaryana), and one on each of Agrostis (U. agrostidis-palustris), Alopecurus (U. alopecurivora), Anthoxanthum (U. anthoxanthi), Arrhenatherum (U. denotarisii), Briza (U. brizae), Bromus (U. bromina), Calamagrostis (U. corcontica), Dactylis (U. clintoniana), Deschampsia (U. airae-caespitosae), Elymus and Agropyron (U. striiformis f. hordei), Festuca (U. festucarum), Helictotrichon (U. scaura), Hystrix (U. johnstonii), Lolium (U. loliicola), Milium (U. milii), Setaria (U. taenia), and Trisetum (U. triseti) (Fischer 1953, Vánky 2012). Additionally, two species were described from incorrectly identified hosts (U. salweyi and U. duriusculae) (Vánky 2012).
Several studies have focused on host specificity in U. striiformis s.l. Liro (1924), Davis (1930, 1935), and Fischer (1940) conducted several cross-inoculation experiments with different hosts and reported specialised forms confined to certain host species or genera. Davis (1924, 1935), Fischer (1940), Krietlow (1943a, b), Leach & Ryan (1946), and Thirumalachar & Dickson (1953) conducted germination experiments. As a result, several patterns of spore germination, also restricted to specimens from certain hosts, were reported. Those experiments included only a small proportion of known stripe smut hosts. Molecular phylogenetic methods opened a new era in smut systematics, but thus far only one specimen of U. striiformis has been included in such studies (Stoll et al. 2005, Begerow et al. 2006, McTaggart et al. 2012). Ustilago striiformis and U. calamagrostidis, another stripe smut fungus distinguished from the former species by spore ornamentation only, were shown to be closely related and considered as possibly conspecific by Stoll et al. (2005). The lack of studies comparing material from different hosts and geographic origin, as well as variability in morphological characters such as spore shape, size, and height of ornamentation between specimens from different host taxa (Mäkinen 1963, Vánky 1985, 1994) has resulted in inconsistent treatment of species in smut monographs and check-lists (Liro 1924, Kochman 1936, Gutner 1941, Ainsworth & Sampson 1950, Moesz 1950, Fischer 1953, Viennot-Bourgin 1956, Săvulescu 1957, Lindeberg 1959, Schwarzman 1960, Uljanishchev 1968, Kochman & Majewski 1973, Vánky 1985, 1994, 2012, Zogg 1985, Scholz & Scholz 1988, Ignatavičiute 2000, Denchev 2001, Vánky & McKenzie 2002, Zwetko & Blanz 2004, Vánky & Shivas 2008).
The aim of this study was to infer the phylogeny of U. striiformis species complex using partial sequence data of the Internal Transcribed Spacer (ITS) and Large Subunit (LSU) rDNA, and to provide a backbone phylogenetic tree for future studies on stripe smuts of grasses.Our analysis was based on morphological and molecular characters examined from recently collected material, as well as other relevant herbarium specimens that were available from different regions of the world.
MATERIALS AND METHODS
Specimen sampling and documentation
Studied specimens were from herbaria BPI, BR, BRNM, DAOM, ILLS, ISC, H, HAI, KR, KRAM, KW, MA, MIN, MICH, NY, PDD, S, TUR, UC, UPS, VPRI, and WSP, in addition to material sent to us from several private collections and field-collected by the authors. Specimens examined represented different host genera and different geographic regions. A total of 215 specimens of stripe smuts of grasses from 26 countries from Europe, Asia, Africa, North and South America, and Oceania were examined. The complete list of specimens included in this study is given below. Nomenclatural novelties are registered in MycoBank (Crous et al. 2004). The hologenetype concept follows the proposal of Chakrabarty (2010).
Specimens examined.
On Agropyron cf. kufuim. ARGENTINA, Tierra del Fuego, Ushuaia, 24 July 1970, H. Roivainen, DAOM 172732.
On Agropyron tenerum. CANADA, British Columbia, Sidney, July 1941, W. Jones, DAOM 7646. – USA, South Dakota, Northville, 23 June 1927, J.F. Brenckle, BPI 166921; Utah, Salt Lake City, 8 Nov. 1922, A.O. Garrett, BPI 166923.
On Agrostis alba. JAPAN, Sapporo, prov. Isikari, 5 June 1928, Anonymous,BPI 166949; Sapporo, prov. Isikari, 23 May 1929, Y. Tokunaga, BPI 166951. – USA, Illinois, Effingham Co., Mason, 11 June 1936, G.H. Boewe, ILLS 25493; Illinois, Crawford Co., Oblong, 14 June 1940, G.H. Boewe, ILLS 27985; Illinois, Clay Co., Flora, 9 June 1949, G.H. Boewe, ILLS 32429.
On Agrostis canina. BULGARIA, 7 km W ab urbe Khaskovo, alt. 250 m, 8 July 1983, T. & K. Vánky,K. Vánky Ustilaginales 495, BPI 870880.
On Agrostis gigantea. CANADA, Ontario, Dunwich Tp., Dutton Swamp, c. 5 km SW of Dutton PO, 29 June 1989, M.J. Oldham, DAOM 212869. – SWEDEN, Dalarna, pag. Gagnef, alt. 165 m, 13 July 1975, T. & K. Vánky, K. Vánky Ustilaginales 246, BPI 870631. – SWITZERLAND, Gadmen, Nähe Ortslage, schattiger Bachrand, 9 July 2004, V. Kummer, HAI 4640. – UKRAINE, Kyiv region, Lisnyky, 18 Aug. 2010, K.G. Savchenko, HAI 2871.
On Agrostis rupestris. SWITZERLAND, Graubünden, alt. 2100 m, 2 Aug 2009, J. Kruse, HAI 4618; near the Hospice de La Bernina, Alpes Grisonnes, 16 Aug. 1916, P. Cruchet, BPI 167053.
On Agrostis stolonifera. CANADA, Quebec, Senneville, 24 July 1942, I.H. Crowell, DAOM 18991. – FINLAND, Alandia, Kökar par., Idö, 24 July 1938, J.I. Liro & H. Roivainen, UPS 439273; Nyland, Bredvik, 25 July 1915, J.I. Liro & E. Kitunen, TUR 170627. – POLAND, Małopolska Province, Kraków, at T. Ptaszyckiego Street, 13 June 2010, J. & M. Piątek, KRAM F-49099. – SWEDEN, Skåne, Lomma par., Alnarp, June 1935, H. Christoffersson, UPS 439271. – USA, Utah, Weber County, moist seepage, along upper portion of Wheeler Creek, Snow Basin, Wasatch Mountains, alt. 1700 m, 25 July 1985, C.T. Rogerson, NY.
On Agrostis tenuis. FINLAND, Alandia, Jomala par, Djurvik, Nylunds, 14 July 1938, J.I. Liro & H. Roivainen, UPS 439274; Ab. Turku, Ruissalo 114, Torikauppiaat, 12 July 1957, K.E. Saloranta, TUR 158660. – UKRAINE, Volyn reg., Stara Vyzhivka distr., Stara Vyzhivka, 16 Aug. 2009, K.G. Savchenko, KW 36868.
On Agrostis sp. NEW ZEALAND, North Canterbury, Andrews Shelter, 1 Feb. 1990, E.H.C. McKenzie & K. Vánky, PDD 70959.
On Alopecurus geniculatus. GERMANY, Hessen, Wetteraukreis, Friedberg, alt. 130 m, 22 May 2010, J. Kruse, HAI 4651. – USA, Texas, College Station, 10 Apr. 1890, S.H. Jennings, BPI 167060.
On Alopecurus pratensis. CZECH REPUBLIC, Okres Vyskov, 10 May 2009, P. Kokeš, BRNM 02823. – FINLAND, Turku, Ruissalo, 22 July 1956, L.E. Kari, NY. – GERMANY, Hessen, Wetteraukreis, Friedberg, 22 May 2010, V. Kummer, HAI 4653. – UKRAINE, Kyiv, 13 June 1973, Z.G. Lavitska, KW 55576.
On Ammophila arenaria. USA, Connecticut, Fairfield Beach, 9 Oct. 1923, G.P. Clinton, BPI 167066; Connecticut, Momouguin, 13 July 1905, G.P. Clinton, BPI 167074; Massachusetts, Woods Hole, 8 June 1910, A.B. Seymour, DAOM 6189; Massachusetts, Woods Hole, 18 June 1910, A.B. Seymour, BPI 167064; Rhode Island, Watch Hill, 8 Sept. 1908, J.L. Sheldon, BPI 198892.
On Ammophila arundinacea. USA, Connecticut, South Norwalk, Roton Point, 10 June 1906, G.P. Clinton, BPI 167067; Maine, Old Orchard, 23 Aug. 1904, G.P. Clinton, MIN 13662; Massachusetts, Woods Hole, 3 July 1890, W.G. Farlow, BPI 198893.
On Anthoxanthum odoratum. NORWAY, Kaofjorde in Alta, 1 Aug. 1924, I. Jørstad, BPI 167082. – RUSSIAN FEDERATION, Leningrad obl., Vyborg distr., Saarenpaa (= Krasnyi Ostrov), 18 July 1936, H. Roivainen & J.I. Liro, H 6025893.
On Arrhenatherum elatius. BELGIUM, West-Vlaanderen, Zuidschote, 10 July 1997, H. Rugsseveldt, BR 67064-37. – BULGARIA, Montium Rodopi, 5 km SW Narecenski bani, alt. 1000 m, 7 July 1983, K. Imre & K. Vánky, K. Vánky Ustilaginales 497, BPI 870882. – GERMANY, Lebus, Hakengrund, c. 1 km N der Altstadt, 8 Sept. 2007, V. Kummer, HAI 4654; Knoblauch, Schanze (= Mittelalterl. Burgwall), 22 May 2007, V. Kummer, HAI 4655; Thuringia, Hildburghausen, 20 June 2010, J. Kruse, HAI 4656. – SWEDEN, Gotland, Visby par., Palissaderna, 6 July 1913, T. Vestergren, UPS 439275; Södermanland, Nacka par., Järla, June 1909, G. Lagerheim, UPS 439277. – UK, England, Wheatfen Broad, Surlingham, Norfolk, 8 June 1941, E.A. Ellis, BPI 167086; England, Wheatfen Broad, Norfolk, 8 June 1941, E.A. Ellis, BPI 167087.
On Avena pubescens. FINLAND, Ab. Turku, Ruissalo, 18 Aug. 1922, L.E. Kari, TUR 033400. – GERMANY, Brandenburg, Hirengraben bei Iandschloß Stern, Kreis Teltow, 18 June 1935, E. Fahrendorf, S F163772; Brandenburg, Kaehnsdorf, 25 July 1937, E. Fahrendorf, BPI 167089; Wendisch, Wilmersdorf, 30 May 1935, E. Fahrendorf, BPI 167090.
On Beckmannia cruciformis. CANADA, Manitoba, Dauphin, 5 Aug. 1917, W.P. Fraser, DAOM 13761; Manitoba, Winnipeg, 23 June 1921, I.L. Conners & H. Groh, BPI 167093; Manitoba, Winnipeg, 23 July 1921, I.L. Conners & H. Groh, BPI 167092.
On Beckmannia syzigachne. CANADA, Manitoba, Winnipeg, Manitoba Agr. Col., 23 July 1921, I.L. Conners & H. Groh, DAOM 204172. – USA, California, Pini Creek, 28 July 1950, G.W. Fischer & R. Sprague, BPI 167095; Colorado, Hebron, 7 Aug. 1950, G.W. Fischer & R. Sprague, BPI 167096; Oregon, Silvies, 28 July 1950, G.W. Fischer & R. Sprague, BPI 167094.
On Brachypodium sylvaticum. DENMARK, Jylland, Viborg, Hald Ege, alt. 50 m, 9 June 1988, P. Alanko, K. Vánky Ustilaginales 1183, BPI 844224.
On Briza media. FINLAND, Alandia, Lemland par., Norrby, 1 July 1919, T. Putkonen, UPS 439280. – GERMANY, Coburg, Rogener Berg, July 1879, E. Ule, S F37590, isolectotype of Tilletia brizae; Hessen-Nassau, auf dem Eube-Berg bei Gersfeld im Rhongebirge, 6 July 1907, H. Sydow, S F163743. – HUNGARY, Pr. pad. Csakvar, in siivis montium ‘Vertes hegyseg’, alt. 200 m, 24 June 1965, S. Toth, K. Vánky Ustilaginales 99, BPI 870484. – POLAND, Lublin Province, Gliniska Reserve, c. 30 km NE of Zamość, 7 June 2005, J. & M. Piątek, KRAM F-49097.
On Bromus erectus. GERMANY, Rheinland-Pfalz, Mainz, Mainz-Gonsenheim, sandy slope, alt. 100 m, 23 May 2010, J. Kruse, HAI 4660.
On Bromus inermis. GERMANY, Berlin, Lichterfelde, ad canalem Teltowiensem, alt. 36 m, May 1983, H. Scholz, K. Vánky Ustilaginales 498, BPI 870883; Berlin, Wilmersdorfer Wiesen, Aug. 1898, P. Sydow, S F163740, isolectotype of Ustilago bromina; Mallnow, Oderhänge NW des Ortes Nähe Huderberg, 19 May 2007, V. Kummer, HAI 4663. – ISRAEL, Golan Heights, Majdal Shams, 22 Apr. 2011, K.G. Savchenko, HAI 4600. – POLAND, Małopolska Province, Hebdów, c. 35 km E of Kraków, xerothermic grassland, 18 June 2004, M. Piątek, KRAM F-49096; Małopolska Province, Kraków, Wanda’s Mound (at Ujastek Mogilski Street), 10 Sept. 2006, M. Piątek, KRAM F-49095; Świętokrzyskie Province, Skorocice Reserve, c. 6.5 km S of Busko-Zdrój, 18 June 2004, J. & M. Piątek, KRAM F-49093; Świętokrzyskie Province, Górki, c. 14 km S of Busko-Zdrój, 19 May 2006, M. Piątek, KRAM F-49094; Lublin Province, Czumów, c. 50 km E of ZamoŚć, 13 June 2006, J. & M. Piątek, KRAM F-49098. – SWEDEN, Dalarna, Leksand par., Tällberg, Aug. 1925, O. Juel, UPS 439279. – USA, Iowa, Dickinson Co., Iowa Lakeside lab., Lake West Okoboji, 16 July 2000, L.H. Tiffany, ISC 27821; Iowa, Monona, Loess Hills Forest, 3 June 2001, L.H. Tiffany, ISC 27222.
On Bromus secalinus. USA, Illinois, Knox Co., Abingdon, 7 Aug. 1922, O.A. Plunkett, ILLS 16707; Illinois, Knox Co., Oneida, 9 Aug. 1922, O.A. Plunkett, ILLS 13810; Illinois, Stark Co., Wyoming, 11 Aug. 1922, O.A. Plunkett, ILLS 14784.
On Calamagrostis canadensis. CANADA, Quebec, Gatineau Co., Cantely, Ginn’s Farm, 29 May 1982, J. Ginns, DAOM 183336. – USA, Alaska, Horner, 6 Aug. 1948, C.L. Lefebvre, BPI 167126; Colorado, Fraser, 6 Aug. 1950, G.W. Fischer & R. Sprague, BPI 167125; Colorado, Skyway, 6 Aug. 1948, G.W. Fischer, R. Sprague & J.P. Meiners, BPI 167132; Iowa, Howard Co., Hayden Prairie State preserve, 17 June 1998, L.H. Tiffany, ISC 13766; Montana, Logan Pass, Glacier Park, 8 Aug. 1952, G.W. Fischer, BPI 167128.
On Calamagrostis villosa. GERMANY, Sachsen, Mts Erzgebirge, alt. 690 m, between Fichtelberg & Klinovec, 22 Aug. 1987, W. Dietrich, K. Vánky Ustilaginales 794, BPI 1113401. – SLOVAKIA, Vysoke Tatry, 24 Aug. 1979, S. Toth & K. Vánky, K. Vánky Ustilaginales 312, HAI 4665. – SWITZERLAND, Graubunden, Bever, 23 Aug. 1961, E. Muller & R.A. Shoemaker, DAOM 92855.
On Cynosurus cristatus. FINLAND, Alandia, Lemland par., Natö, 11 July 1938, H. Roivainen, UPS 439281.
On Dactylis glomerata. CANADA, Ontario, Elara, 31 May 1942, I.H. Crowell & J.D. McLachlan, DAOM 18992. – FINLAND, Alandia, Lemland par., Natö, 12 July 1938, H. Roivainen, UPS 439288. – GERMANY, Baden-Württemberg, Mainfränkische Platten u. Tauberland, Ks. Main-Tauber-Kreis, Tauberbischotsheim, 1.5 km WSW Werbach, 4 June 2010, M. Scholler & V. Hemm, KR-0026884; Bavaria, Habberge, 20 June 2010, J. Kruse, HAI 2999. – HUNGARY, in mte. Kopasz oldar. pr. pag. Nagykovacsi, 1 July 1965, S. Toth, K. Vánky Ustilaginales 114, BPI 870499. – ISRAEL, Carmel Coast, Atlit, 15 Apr. 2011, K.G. Savchenko, HAI 4601; Golan Heights, Majdal Shams, 11 June 2011, K.G. Savchenko, HAI 4667. – JAPAN, Sapporo, Hokkaido, 28 May 1914, T. Hemmi, BPI 167201. – NEW ZEALAND, Auckland, Mt Albert, Oct. 1953, J.M. Dingley, DAOM 62184. – ROMANIA, Transilvania, urbs Tirgu-Mures, alt. 333 m, 15 May 1957, K. Vánky, K. Vánky Ustilaginales 247, KR-0016646. – SWEDEN, Gästrikland, Gävle par., Lövudden, 22 June 1947, J.Ax. Nannfeldt, UPS 439287; Skåne, Lund par, Tuna park, 9 June 1936, N. Hylander, UPS 439283. – USA, Utah, Weber Co., upper reaches of Wheeler Creek, Snow Basin, Wasatch Mountains, 25 July 1985, C.T. Rogerson, NY; Utah, Weber Co., SE of Eagles Camp, along South Fork of Ogden River, 3 miles east of Huntsville, 10 May 1987, C.T. Rogerson, NY.
On Deschampsia cespitosa. FINLAND, Nylandia, Helsingfors, Hagasund, 10 Aug. 1902, I.I. Liro, S F163723, isolectotype of Tilletia airae-ceaspitosae. – SWEDEN, Jylland, Gaardbo Sö., July 1889, O. Rostrup, UPS 439289. – USA, Utah, Gupers Pass, La Sal National Forest, 1 Aug. 1950, G.W. Fischer &R. Sprague, BPI 167273; Wyoming, Medicine Bow National Forest, 17 Aug. 1948, G.W. Fischer, R. Sprague & J.P. Meiners, BPI 167270.
On Elymus canadensis. USA, Kansas, Wichita, 9 June 1936, A.G. Johnson, BPI 167281; Minnesota, St. Paul, Fair Grounds, 19 July 1917, F.J. Piemeisel, MIN 465384.
On Elymus glaucus. USA, Oregon, 29 June 1999, P. Gray & L.M. Carris, LMC 379, HAI 4602; California, Siskiyou Co., MacBride Springs Public Camp Mt Shasta, 27 June 1947, W.B. & V.G. Cooke, UC 788761; Utah, Salt Lake City, Lindsay’s Garden, 12 Nov. 1922, A.O. Garrett, BPI 167301.
On Elymus macounii. CANADA, Saskatchewan, Saskatoon, July 1935, W.P. Fraser, DAOM 18952. – USA, New Mexico, Agua Fria, 4 Aug. 1950, G.W. Fischer & R. Sprague, BPI 167321.
On Elymus robustus. USA, Iowa, Altoona, 28 June 1916, A.G. Johnson,BPI 167329; Utah, Salt Lake Co., Parley’s Canyon, 23 June 1906, A.O. Garrett, MICH 00073559.
On Elymus virginicus. USA, Kansas, Manhattan, 4 June 1935, W.G. Johnson,BPI 167347; Utah, Weber Co., foothills east of Ogden, alt. 1524 m, 22 June 1976, C.T. Rogerson, NY.
On Festuca arundinacea. FINLAND, Uusikaupunki, Melsa, 3 Aug. 1956, L.E. Kari, NY.
On Festuca elatior. FINLAND, Nyland, Vantaa, Tikkurila, 19 Aug. 1915, E. Kitunen, TUR 033391.
On Festuca idahoensis. CANADA, British Columbia, Okanagan Valley, Summerland Exp. Station, 28 July 1953, J.A. Calder & D.B.O. Savile, DAOM 145095. – USA, Idaho, 26 June 2004, L.A. Castlebury & L.M. Carris, LMC 409, HAI 4612.
On Festuca ovina. FINLAND, Lapponia Kemensis, Muonio par., Utkujärvi, 8 July 1939, H. Roivainen, UPS 439290. – GERMANY, Berlin, in einem Kiefernwaldchen bei Hohen-Schonbausen, Aug. 1877, E. Ule, S F163778. – USA, North Dakota, Kulm, June 1921, J.F. Brenckle, NY 6693.
On Festuca rubra. NORWAY, Tromsø, date unknown, G. Lagerheim, UPS 439931.
On Festuca sulcata. ROMANIA, Transilvania, ad balneas Borsec ‘Bükkhavas’, alt. 1250 m, 14 June 1964, K. Vánky, K. Vánky Ustilaginales 62, BPI 870447.
On Festuca valesiaca. ROMANIA, Banat, Eşelniţa, pr. oppid. Orsova, 30 Apr. 1967, K. Vánky, HUV 4656; Transilvania, oppid. Topilita, July 1965, K. Vánky, HUV 4655; Transilvania, pr. oppid. Cluj-Napoca, pag. Apahida, 3 June 1965, K. Vánky, HUV 4654.
On Helictotrichon turgidulum. SOUTH AFRICA, Mpumalanga Prov., near Piet Retrief, alt. 1255 m, 21 Dec. 2002, A. Witt, HUV 20181.
On Holcus lanatus. AUSTRALIA, Victoria, 24 Nov. 1994, I. Pascoe, VPRI 20419. – CZECH REPUBLIC, Okres Viskov, P. Kokeš, 10 May 2009, BRNM 02824. – GERMANY, Mecklenburg, Danneuwalde, Kreis Stargard, 11 June 1939, H. Sydow, S F163771; Lower Saxony, Papenburg, Aschendorf, 24 Sept. 2009, J. Kruse, HAI 4605; Lower Saxony, Hannover, Herenhansen, 29 May 2010, J. Kruse, HAI 4667; Hessen, Wetteraukreis, Friedberg, 22 May 2010, J. Kruse, HAI 4668. – NEW ZEALAND, Mangonui, Parapara, 21 Sept. 1977, E.H.C. McKenzie, PDD 37375. – UK, Wales, Gwaelod-y-Brithdir, alt. 270 m, 25 Apr. 2011, P.A. & K. Smith, HAI 2784. – USA, California, Contra Costa Co., Tilden park, 6 June 1990, E.C. Swann, MIN 927605.
On Holcus mollis. GERMANY, Freiburg in Breisgau, June, A.A. Fischer v. Waldheim, S F37593, isolectotype of Tilletia debaryana; Falkensee-Finkenkrug; wenig NW der Gr. Moosbruchwiese, Station 4 des Lehrpfades, 20 May 2010, V. Kummer, HAI 4609; Schlamau, magerer frischwiesenrand wenig SSE des Ortes, 1 June 2005, V. Kummer, HAI 4670. – ISRAEL, Haifa, Carmel National Park, 2 km N of Haifa University campus, 27 Feb. 2010, T. Pavlichek, HAI 4608. – NEW ZEALAND, Mt Cook, ‘The Hermitage’, 3 Feb. 1990, E.H.C. McKenzie & K. Vánky, PDD 81559. – SWEDEN, Västergötland, Hagellberg par., Klagstorp, 1 July 1973, S. Kilander, UPS 000302. – UK, Wales, Powys, Llangynidr, 4 July 1999, G.S. Motley, HAI 2782; Wales, Perllwyn grasslands, 20 July 2011, P.A. Smith, HAI 2783; Wales, Ceredigion, Cors Fochno, alt. 2 m, 30 May 2011, A.O. Chater, HAI 2785; Wales, Ceredigion, Llyn Fanod, alt. 320 m, 14 June 2011, A.O. Chater, HAI 2789; Wales, Llwyncelyn, alt. 20 m, 8 May 2011, A.O. Chater, HAI 2790; Wales, Blaen Pennal, alt. 230 m, 18 May 2011, A.O. Chater, HAI 2791; Wales, 2 km ESE of Tregaron, alt. 260 m, 21 May 2011, A.O. Chater, HAI 2792; Wales, Cwmenion, alt. 165 m, 4 July 2010, A.O. Chater, HAI 2793; Wales, Ceredigion, Llanafan, 9 Apr. 2011, A.O. Chater, HAI 2788.
On Leucopoa kingii. USA, Utah, Mt Timpanogos, Wasatch Mountains, 28 July 1927, C.T. Rogerson, NY.
On Lolium perenne. GERMANY, Berlin, auf einem Feld-wege zwischen Weissensee und der Verbindungsbahn, Sept. 1877, P. Ule, S F163741, isolectotype of Ustilago loliicola. – USA, Oregon, Corvallis, 18 May 1933, R. Sprague, BPI 167472.
On Lolium sp. AUSTRALIA, Victoria, Sandy Point, 16 Nov. 1994, I. Pascoe, VPRI 20418.
On Melica spectabilis. USA, Wyoming, Shashom National Forest, Logwater Pass, G.W. Fischer, 12 Aug. 1948, BPI 167475.
On Milium effusum. DENMARK, Fyn, Ringe, 24 June 1897, J. Lind, UPS 439303. – FINLAND, Turku, Ruissalo, 14 Aug. 1956, L.E. Kari, NY. – GERMANY, Rabenkopf Mt near Oestrich, 1894, L. Fuckel, S F163736, isolectotype of Tilletia milii; Westfalen, Dillkreis, Haiger, July 1933, A. Ludwig, S F41016. – SWEDEN, Skåne, Hyby par., Bökebergsslätt, near Nyhus, 15 June 1977, S. Ryman, UPS 439300. – UKRAINE, Dnipropetrovsk reg., Dnipropetrovsk, Gagarin distr., 9 July 2009, K.G. Savchenko, HAI 4610; Kyiv, Trukhaniv Island, 2 June 1975, Z.G. Lavitska, KW 5671.
On Phleum pratense. CANADA, Ontario, Ottawa, C.E.F., 28 May 1934, M. Timotin, DAOM 3587; Nova Scotia, Truro, 10 June 1983, G. Sampson, DAOM 192976. – GERMANY, Berlin, Charlottenburg, Park ‘Jungfernheide’, alt. 50 m, 5 June 1988, H. & I. Scholz, K. Vánky Ustilaginales 718, BPI 1113296; Saxonia, Schmilka pr. Schandau, 23 July 1901, P. Sydow, S F163761. – JAPAN, Garuwaga, prov. Ishikari, 24 Aug. 1929, Y. Tokunaga, BPI 167567. – ROMANIA, Suceava, Clit, Valea Saca, alt. 425 m, 26 Aug. 1982, G. Negrean, MA Fungi 27705. – UKRAINE, Cherkasy reg., Trakhtemyriv Reg. Landscape Park, 22 May 2010, K.G. Savchenko, HAI 4611. – USA, Illinois, Jefferson Co., Mt Vernon, 11 June 1941, G.H. Boewe, ILLS 28584.
On Poa annua. AUSTRALIA, Victoria, Ardmona, 4 Oct. 1893, G.H. Robinson, VPRI 3131, holotype of Ustilago poarum.
On Poa bulbosa. ROMANIA, Transilvania, pag. Apahida pr. oppid, Cluj, 3 June 1965, K. Vánky, K. Vánky Ustilaginales 79, BR 26855-83.
On Poa caespitosa. NEW ZEALAND, Waiau, Mt Highfield, 9 Dec. 1954, A.J. Healy, PDD 14198.
On Poa chaixii. ROMANIA, Transilvania, balneas Borsec, ‘Bukkhavas’, alt. 1300 m, 4 June 1964, K. Vánky, K. Vánky Ustilaginales 59, BR 26835-23.
On Poa compressa. CANADA, Quebec, St. Geneviève, 3 June 1941, I.H.C. & A. Ludwig, DAOM 19000. – USA, Illinois, Edwards Co., West Salem, 20 May 1937, G.H. Boewe, ILLS 35458.
On Poa nemoralis. ISRAEL, Golan Heights, near Odem forest, 22 May 2011, K.G. Savchenko, HAI 4613. – RUSSIAN FEDERATION, Lapponia ponojensis, Orlow, near the rivulet Gubnoi, 1 Aug. 1889, A.O. Kairamo, H, holotype of Ustilago kairamoi.
On Poa pratensis. ARGENTINA, Chubut Prov., 215 km urbe Bariloche, pr. Chokila, alt. 590 m, 6 Dec. 1999, C. & K. Vánky, K. Vánky Ustilaginales 1112, BPI 841724. – CANADA, Quebec, St. Geneviève, 3 June 1941, I.H. Crowell, DAOM 220004. – GERMANY, Berlin, June 1888, P. Sydow, S F163782; Potsdam, Park Sanssouci, Marlygarten, 10 May 2005, V. Kummer, HAI 4676. – UKRAINE, Kyiv, Truchaniv island, 18 June 2010, K.G. Savchenko, HAI 4675. – USA, Illinois, Massac Co., Metropolis, 23 Apr. 1941, G.H. Boewe, ILLS 28208; Idaho, Cottonwood, 27 June 1945, G.W. Fischer & A.G. Law, MICH 00073562; Utah, Wever Co., lawn, 833 Kershaw Ave., Ogden, 12 Aug. 1980, C.T. Rogerson, NY; Michigan, Washtenaw Co., Ann Arbor, Streinbach Road, 17 May 1914, M.E. Elder, MICH 00073557.
On Poa secunda. USA, Oregon, 29 June 1999, P. Gray & L.M. Carris, LMC 382, HAI 4677.
On Poa timoleontis. GREECE, Euboea, Eparchia Chalkidas, NE Eritrea, alt. 100 m, 26 Mar. 1991, H. & I. Scholz, K. Vánky Ustilaginales 895, BPI 1113505.
On Poa trivialis. FINLAND, Alandia, Lemland par, Natö, 11 July 1938, J.I. Liro & H. Roivainen, UPS 439306. – GERMANY, Lower Saxony, Poggenhagen, 11 June 2010, J. Kruse, HAI 4678. – IRAN, Khosaran, 45 km W Mashad, Abarden-Olia, alt. 1470 m, 12 May 1990, D. Ershad, T. & K. Vánky, K. Vánky Ustilaginales 795, BPI 1113402. – NEW ZEALAND, Wellington, Palmerston North, Massey, 23 Nov. 1962, G.C.M. Latch, PDD 33798. – UKRAINE, Kherson reg., Black Sea Biosphere Reserve, 12 May 2009, O.Yu. Umanets, HAI 4614.
On Puccinellia angustata. CANADA, Northwest Territories (now Nunavut), Franklin Distr., Ellesmere Island, Trace, 1 mile north of Hazen Camp, 19 July 1962, D.B.O. Savile, DAOM 91211.
On Puccinellia distans. USA, Montana, Nairada, 30 June 1955, G.B. Cummins, BPI 167924.
On Puccinellia nuttaliana. USA, Oregon, Union, 29 June 1945, G.W. Fischer & A.G. Law, NY.
On Sesleria coerulea. SWITZERLAND, Valley of Margius, Canton of Valais, Pastures near Green Lakes, 25 Aug. 1912, Anonymous, BPI 167925.
On Sitanion hystrix. USA, California, Mono Co., 2 miles E of Sonora Pass, alt. 2750 m, 21 Aug. 1988, M. Berbee & C. Cruhn, K. Vánky Ustilaginales 719, BPI 1113297; California, Lassen Volcanic National Park, Parking turnoff, foot of Broke-off Mountain Trail, 14 Aug. 1962, W.B. & V.G. Cooke, UC 318021; California, Lassen Volcanic National Park, Broke-off Mountain Trail, 14 Aug. 1962, W.B. & V.G. Cooke, UC 318041; Colorado, Mesa Verde National Park, 2 Aug. 1950, G.W. Fischer & R. Sprague, BPI 167929; Idaho, Ferdinand, 27 June 1945, G.W. Fischer & A.G. Law, BPI 167930.
On Trisetum spicatum. RUSSIAN FEDERATION, Leningrad obl., NW-Enontekiö, Guonjarvaarri, alt. 900–950 m, 3 Aug. 1936, H. Roivainen & J.I. Liro, H 6025898; Leningrad obl., NW-Enontekiö, Guonjarvaarri, alt. 900–950 m, 2 Aug. 1936, H. Roivainen & J.I. Liro,H 6025897.
Morphological examination
Sorus and spore characteristics were studied from dried herbarium material. Specimens were examined by light microscopy (LM) and scanning electron microscopy (SEM). Pictures of sori were taken with a Canon Power Shot G10 camera. For LM, spores were mounted in 90 % lactic acid on a microscope slide, covered with a cover glass, gently heated to boiling point and cooled, and then examined under a Carl Zeiss Axiostar microscope at 1 000× magnification. LM photographs were taken with a Canon Power Shot G10 camera. At least 30 spores were measured from each collection, and the variation is presented as a range, with extreme values given in parentheses. In the descriptions, mean and standard deviations (SD) were calculated from spores measured in all specimens and provided after the spore size ranges. Hierarchical clustering analysis (www.wessa.net) was used to access the differences in spore length of U. bromina and U. striiformis s.str.
For SEM studies, spores were attached to metal stubs by doublesided adhesive tape and coated with gold. The surface ornamentation of spores was observed at 15 kV and photographed with a scanning electron microscope JEOL JSM-6700F with a working distance of c. 12–13 mm. At least eight spores were photographed from the each specimen used in SEM analysis to get reliable results on the character of spore ornamentation. The number of warts was counted on a 10 μm2 segment of a lateral side of the spores.
DNA extraction, PCR amplification and sequencing
The majority of sequences generated in this study were from specimens of stripe smut on grasses collected from 1962 to 2011 (Table 1). GenBank accession numbers are included in Table 1 and Fig. 1. Genomic DNA was isolated from spores removed from herbarium specimens using FastPrep 24 (MP Biomedicals, Irvine, California). Tubes were incubated in a water bath for 5 h at 55 °C, and DNA extracted using DNeasy Plant Mini Kit (Qiagen, Valencia, California) following the manufacturer’s instructions.
Table 1.
Specimens used in molecular analysis.
| Species | Voucher/Source | Host | Year of collection | Geographic origin of collection | Collector | Accession number |
|
|---|---|---|---|---|---|---|---|
| ITS | LSU | ||||||
| U. bromina | ISC 27821 | Bromus inermis | 2000 | USA, Iowa | L.H. Tiffany | KF381006 | KF381030 |
| U. bromina | ISC 27222 | Bromus inermis | 2001 | USA, Iowa | L.H. Tiffany | KF381007 | KF381031 |
| U. bromina | HAI 4600 | Bromus inermis | 2011 | Israel | K.G. Savchenko | KF381008 | KF381032 |
| U.striiformiss.l. | HAI 2871 | Agrostis gigantea | 2010 | Ukraine | K.G. Savchenko | KF381003 | KF381027 |
| U.striiformiss.l. | HAI 4651 | Alopecurus geniculatus | 2010 | Germany | J. Kruse | KF381004 | KF381028 |
| U.striiformiss.l. | BRNM 02823 | Alopecurus pratensis | 2009 | Czech Republic | P. Kokeš | KF381005 | KF381029 |
| U.striiformiss.l. | Stoll et al. 2005 | Alopecurus pratensis | – | Germany | – | AY740172 | AY740172 |
| U.striiformiss.l. | KRAM F 49097 | Briza media | 2005 | Poland | J. & M. Piątek | KF381009 | KF381033 |
| U.striiformiss.l. | ISC 13766 | Calamagrostis canadensis | 1998 | USA, Iowa | L.H. Tiffany | KF381024 | KF381034 |
| U.striiformiss.l. | DAOM-183336 | Calamagrostis canadensis | 1982 | Canada, Quebec | J. Ginns | KF381010 | KF381035 |
| U.striiformiss.l. | HAI 4601 | Dactylis glomerata | 2011 | Israel | K.G. Savchenko | KF381011 | KF381036 |
| U.striiformiss.l. | HAI 4602 | Elymus glaucus | 1999 | USA, Oregon | P. Gray & L.M. Carris | KF381012 | KF381037 |
| U.striiformiss.l. | NY | Elymus virginicus | 1976 | USA, Utah | C.T. Rogerson | KF381013 | KF381038 |
| U.striiformiss.l. | HAI 4612 | Festuca idahoensis | 2004 | USA, Idaho | L.A. Castlebury & L.M. Carris | KF381014 | KF381039 |
| U.striiformiss.str. | BRNM 02824 | Holcus lanatus | 2009 | Czech Republic | P. Kokeš | KF381015 | KF381040 |
| U.striiformiss.str. | HAI 2788 | Holcus mollis | 2011 | UK, Wales | A.O. Chater | KF381019 | KF381044 |
| U.striiformiss.str. | HAI 4609 | Holcus mollis | 2010 | Germany | V. Kummer | KF381020 | KF381045 |
| U.striiformiss.str. | HAI 2789 | Holcus mollis | 2011 | UK, Wales | A.O. Chater | KF381016 | KF381041 |
| U.striiformiss.str. | HAI 2790 | Holcus mollis | 2011 | UK, Wales | A.O. Chater | KF381017 | KF381042 |
| U.striiformiss.str. | HAI 2791 | Holcus mollis | 2011 | UK, Wales | A.O. Chater | KF381018 | KF381043 |
| U.striiformiss.l. | HAI 4610 | Milium effusum | 2009 | Ukraine | K.G. Savchenko | KF381021 | KF381046 |
| U.striiformiss.l. | HAI 4611 | Phleum pratense | 2010 | Ukraine | K.G. Savchenko | KF381022 | KF381047 |
| U.striiformiss.l. | DAOM 192976 | Phleum pratense | 1983 | Canada | G. Sampson | KF381023 | KF381048 |
| U. nunavutica | DAOM 91211 | Puccinellia angustata | 1962 | Canada, Nunavut | D.B.O. Savile | KF381025 | KF381049 |
| U.calamagrostidis | Stoll et al. 2005 | Calamagrostis epigeios | – | Bulgaria | – | AY740065 | AY740119 |
| U. cynodontis | Stoll et al. 2005 | Cynodon dactylon | – | Mexico | – | AY345000 | AF009881 |
| U. filiformis | HAI 4799 | Glyceria maxima | 2009 | Ukraine | K.G. Savchenko | KF381026 | KF381050 |
| U. hordei | Stoll et al. 2005 | Avena sativa | – | Spain | – | AY740068 | AY740122 |
| U. sparsa | Stoll et al. 2005 | Dactyloctenium aegyptium | – | India | – | AY345008 | DQ864974 |
| U. xerochloae | Stoll et al. 2005 | Xerochloa imberbis | – | Australia | – | AY345012 | AY740150 |
| Moesziomyces bullatus | Stoll et al. 2005 | Paspalum disticum | – | India | – | AY740153 | AY740153 |
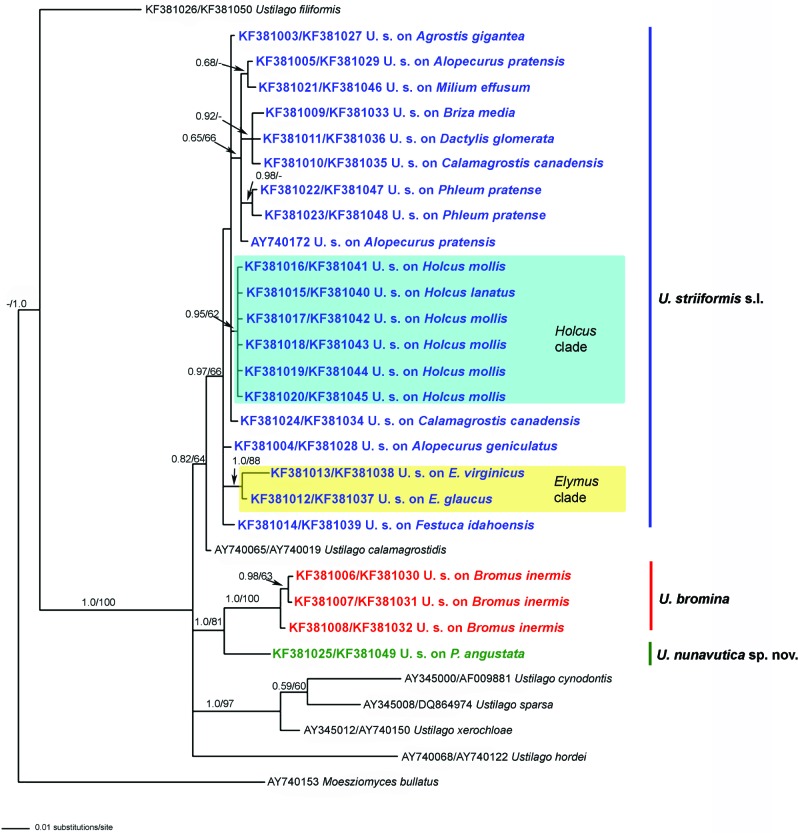
Fig. 1.
Bayesian inference of phylogenetic relationships resulting from the combined analysis of ITS and LSU nucleotide sequence data. Numbers on branches are estimates for PPs from Bayesian inference, MP bootstrap support values. The tree was rooted using Moesziomyces bullatus. E. = Elymus; P. = Puccinellia; U. s. = Ustilago striiformis.
All amplifications were performed in 20 μL aliquots on a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, California). ITS5 or ITS1 primers were used as the forward primer and ITS4 was used as the reverse primer for the ITS region (White et al. 1990), and NL1 and NL4 were used as the forward primer and the reverse primer, respectively, for the LSU region (O’Donnell 1993).
Standard cycling parameters with an annealing temperature of 57 °C for the ITS region and 60 °C for the LSU region were used for the amplification. PCR products were purified with ExoSAP-IT (USB, Cleveland, Ohio) according to the manufacturer’s instructions and amplified with respective forward and reverse PCR primers with the BigDye v. 3.1 terminator kit (Applied Biosystems, Foster City, California). Amplification products were sequenced on an ABI 3130xl automated DNA sequencer. Consensus sequences were assembled, aligned, and edited with Sequencher v. 4.5 for Windows (Gene Codes Corp., Ann Arbor, Michigan) and MAFFTv6 (Katoh et al. 2002, Katoh & Toh 2008), using the default settings and optimized manually when needed.
Sequence alignment and phylogenetic analysis
To ascertain the phylogenetic position of the analysed specimens, we included GenBank sequences of Ustilago calamagrostidis AY740065, AY740119, U. cynodontis AY345000, AF009881, U. sparsa AY345008, DQ864974, and U. xerochloae AY345012, AY740150, which were shown to be closely related to U. striiformis in previous studies (Stoll et al. 2003, 2005, McTaggart et al. 2012). We also added GenBank sequences of U. hordei AY740068, AY740122 – the type species of the genus Ustilago.
Ambiguously aligned regions were recorded using GBlocks (Castresana 2000) and excluded from the analyses. A partition homogeneity test (Farris et al. 1994) was applied as implemented in PAUP v. 4.0b10 (Swofford 2002) to evaluate the feasibility of combining datasets.
PAUP v. 4.0b10 was used to conduct the parsimony analysis. Trees were inferred using the heuristic search option with 1 000 random sequence additions. Maxtrees were unlimited, branches of zero length were collapsed, and all multiple parsimonious trees were saved. Descriptive tree statistics for parsimony (Tree Length (TL), Consistency Index (CI), Retention Index (RI), Relative Consistency Index (RC), and Homoplasy Index (HI) were calculated for each dataset. Kishino-Hasegawa tests (KHT) (Kishino & Hasegawa 1989) were performed in order to determine whether trees were significantly different.
Bayesian analysis using a Monte Carlo Markov chain (MCMC) technique was implemented in MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003). Models of nucleotide substitution for each gene were determined using MrModeltest (Nylander 2004). The models of evolution for each dataset were: i) ITS = k80 + G; ii) LSU = GTR + G; iii) ITS + LSU = GTR + I + G. The combined dataset was partitioned by gene, each of which used a GTR model with gamma-distributed rate variation across sites and a proportion of invariable sites (Nylander et al. 2004). Four incrementally heated MCMC chains were run simultaneously, starting from random trees, for 1 000 000 generations. Trees were sampled every 100th generation for a total of 10 000 trees. The first 2 000 trees were discarded as the burn-in phase of each analysis. Convergence criteria were further checked in AWTY (Nylander et al. 2008). Posterior probabilities (Rannala & Yang 1996) were determined from a majority-rule consensus tree generated with the remaining 8 000 trees. This analysis was done four times starting from different random trees to ensure that trees from the same tree space were being sampled during each analysis. Well-supported clades were inferred with a minimum of 95 % Bayesian posterior probabilities and 80 % bootstrap support.
Trees were rooted using Moesziomyces bullatus AY740153 as it was resolved to be basal to the species from Ustilago-Sporisorium-Macalpinomyces complex (Stoll et al. 2005, McTaggart et al. 2012). Ustilago filiformis KF381026, KF381050 was included in the analysis as a representative of another Ustilago clade. Alignments and analyses were deposited in TreeBase 14596.
RESULTS
Phylogenetic analysis
For the ITS region, the adjusted alignment included 31 sequences (24 sequences of U. striiformis complex and seven sequences of other species). The ITS data matrix contained 710 characters (including gaps). Of these characters 97 were excluded in the parsimony analysis. The resulting statistics for the parsimony analysis revealed that 470 characters were constant, 75 variable characters were parsimony-uninformative and 68 characters were parsimony informative. The parsimony analysis yielded six equally parsimonious trees, and the first tree was recognized as the best tree (TL = 206; CI = 0.791; RI = 0.750; RC = 0.593; HI = 0.209). For the LSU region, the adjusted alignment included 31 sequences (24 sequences of stripe smuts of grasses and seven sequences of other species). The LSU data matrix contained 570 characters (including gaps). All characters were used in the parsimony analysis. The resulting statistics for the parsimony analysis revealed that 519 characters were constant, 25 variable characters were parsimony-uninformative, and 24 characters were parsimony informative. The parsimony analysis yielded four equally parsimonious trees and the first tree was recognised as the best tree (TL = 62; CI = 0.790; RI = 0.840; RC = 0.663; HI = 0.210).
The partition homogeneity test showed that no significant conflict existed between the phylogenies of the rDNA ITS and LSU regions, and they were combined. The combined data matrix contained 31 specimens including the outgroup and an average of 1 223 characters (including gaps); 55 characters were excluded in parsimony analysis. The statistics for the parsimony analysis revealed that 984 characters were constant, 91 characters were parsimony-informative, while 93 variable characters were parsimony-uninformative. The parsimony analysis yielded 20 equally parsimonious trees and the first tree was recognized as the best tree (TL = 267; CI = 0.772; RI = 0.759; RC = 0.586; HI = 0.228).
For the combined ITS + LSU dataset, the different runs of Bayesian phylogenetic analyses yielded consistent topologies. We present the consensus tree of one run of Bayesian phylogenetic analyses to illustrate the results (Fig. 1). All sequences of U. striiformis s.l. used in this study clustered in two main clades. The first clade (PP = 100, BP = 81) contained all specimens from Bromus and Puccinellia and was subdivided into two phylogenetic lineages. The first lineage (PP = 100, BP = 100) included specimens from Bromus inermis (ISC 27821, ISC 27222, HAI 4600), and the second lineage (PP = 100, BP = 81) accommodated the specimen from Puccinellia angustata (DAOM 91211). Within the second clade of U. striiformis s.l. (PP = 82, BP = below 70), the analyses revealed several well-supported, distinct phylogenetic lineages. All specimens from Holcus clustered together (PP = 95, BP = below 70). Additionally, a strongly supported lineage included specimens from Elymus (PP = 100, BP = 88) and the one with lower support for the specimens from Phleum (PP = 98, BP = below 70). In all analyses the sequence of U. calamagrostidis was in a basal position to the second U. striiformis clade.
Morphological analysis
Morphological characters for U. striiformis s.l. on different grass genera are summarized in Table 2. The specimens used in this study did not differ significantly in spore width, spore wall thickness, colour of spores, and colour of spore mass. The phylogenetic analyses indicated that U. striiformis is a species complex comprising several distinct phylogenetic lineages. Therefore, the morphological analysis focused on a comparison of the specimens representing different clades and host genera with the U. striiformis s.str. clade on Holcus. Mean spore length of U. striiformis specimens from Bromus was larger than those of the specimens from Holcus (Fig. 2, 3), and from all of the other host genera (Table 2). No clear differences were found among spore lengths of the specimens from Puccinellia angustata, Elymus, and Holcus.
Table 2.
Morphological data of stripe smuts included in the analysis based on host taxonomy (ech = echinulate; verr = verruculose).
| Species | Host | Number of Specimens studied | Av. Length of spores | Av. Width of spores | Type of warts | Number of warts per 10 μm2 / number of specimens studied by SEM |
|---|---|---|---|---|---|---|
| Ustilago bromina | Bromus | 15 | 13.0 | 10.3 | ech | 6/4 |
| Ustilago nunavutica | Puccinellia | 1 | 11.7 | 10.1 | verr-ech | 25/1 |
| Ustilago striiformis s.str. | Holcus | 24 | 10.7 | 9.7 | ech | 8/5 |
| Ustilago striiformis s.l. | Agropyron | 4 | 11.9 | 9.6 | ech | 10/1 |
| Agrostis | 22 | 10.8 | 9.4 | ech | 8/6 | |
| Alopecurus | 6 | 10.6 | 9.8 | ech | 8/3 | |
| Ammophila | 8 | 12.1 | 10.4 | ech | 7/2 | |
| Anthoxanthum | 3 | 10.4 | 9.3 | ech | 6/1 | |
| Arrhenatherum | 9 | 11.7 | 10.5 | ech | 7/2 | |
| Avena | 4 | 11.9 | 11.0 | ech | 8/2 | |
| Beckmannia | 7 | 12.5 | 10.6 | ech | 8/2 | |
| Brachypodium | 1 | 9.8 | 9.1 | ech | 11/1 | |
| Briza | 5 | 12.7 | 10.9 | ech | 9/2 | |
| Calamagrostis | 9 | 12.1 | 10.4 | ech | 8/3 | |
| Cynosurus | 1 | 10.7 | 8.7 | ech | 14/1 | |
| Dactylis | 14 | 11.4 | 10.1 | ech | 9/3 | |
| Deschampsia | 4 | 11.1 | 9.8 | ech | 9/1 | |
| Elymus | 11 | 11.7 | 10.1 | ech | 11/3 | |
| Festuca | 12 | 11.5 | 10.3 | ech | 11/5 | |
| Helictotrichon | 1 | 11.5 | 10.3 | ech | 8/1 | |
| Leucopoa | 1 | 12.5 | 10.5 | ech | 13/1 | |
| Lolium | 3 | 11.8 | 10.6 | ech | 6/1 | |
| Melica | 1 | 11.5 | 9.9 | ech | 9/1 | |
| Milium | 7 | 10.8 | 8.9 | ech | 8/2 | |
| Phleum | 8 | 11.6 | 9.8 | ech | 7/3 | |
| Poa | 24 | 10.8 | 9.2 | ech | 8/9 | |
| Puccinellia | 2 | 11.7 | 10.3 | ech | 7/2 | |
| Sitanion | 5 | 10.7 | 10.3 | ech | 8/2 | |
| Sesleria | 1 | 11.0 | 9.7 | ech | 10/1 | |
| Trisetum | 2 | 11.8 | 10.1 | ech | 9/1 |
Fig. 2.
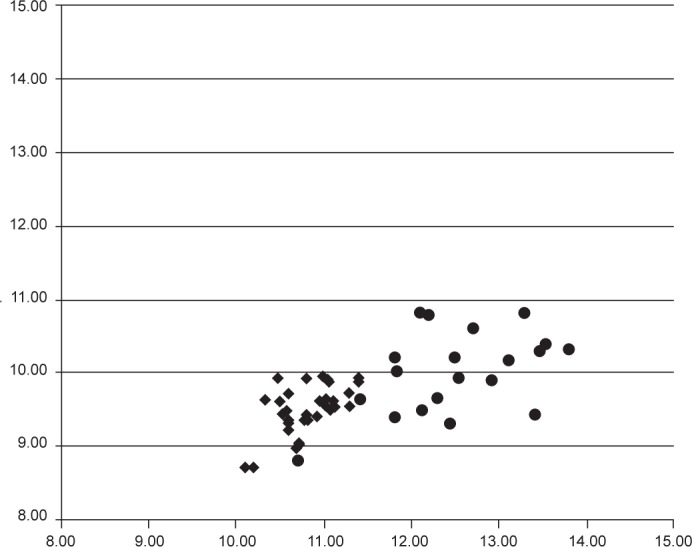
Scatter diagram showing teliospore mean length and width (μm) of different collections of Ustilago striiformis s.str. (◆) and U. bromina (●).
Fig. 3.
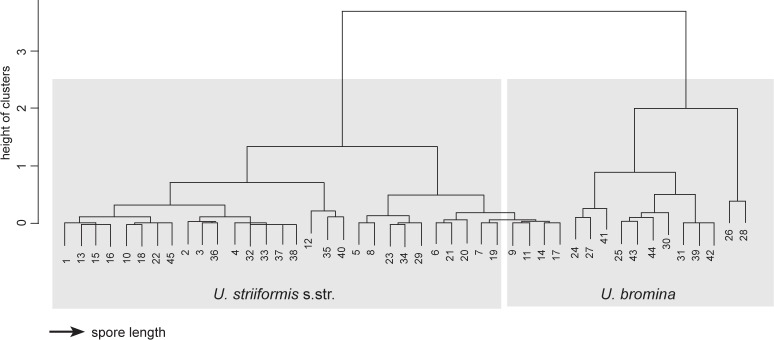
Dendrogram from the hierarchical clustering analysis, with complete method showing the distribution of mean spore lengths of different collections of Ustilago striiformis s.str. and U. bromina.
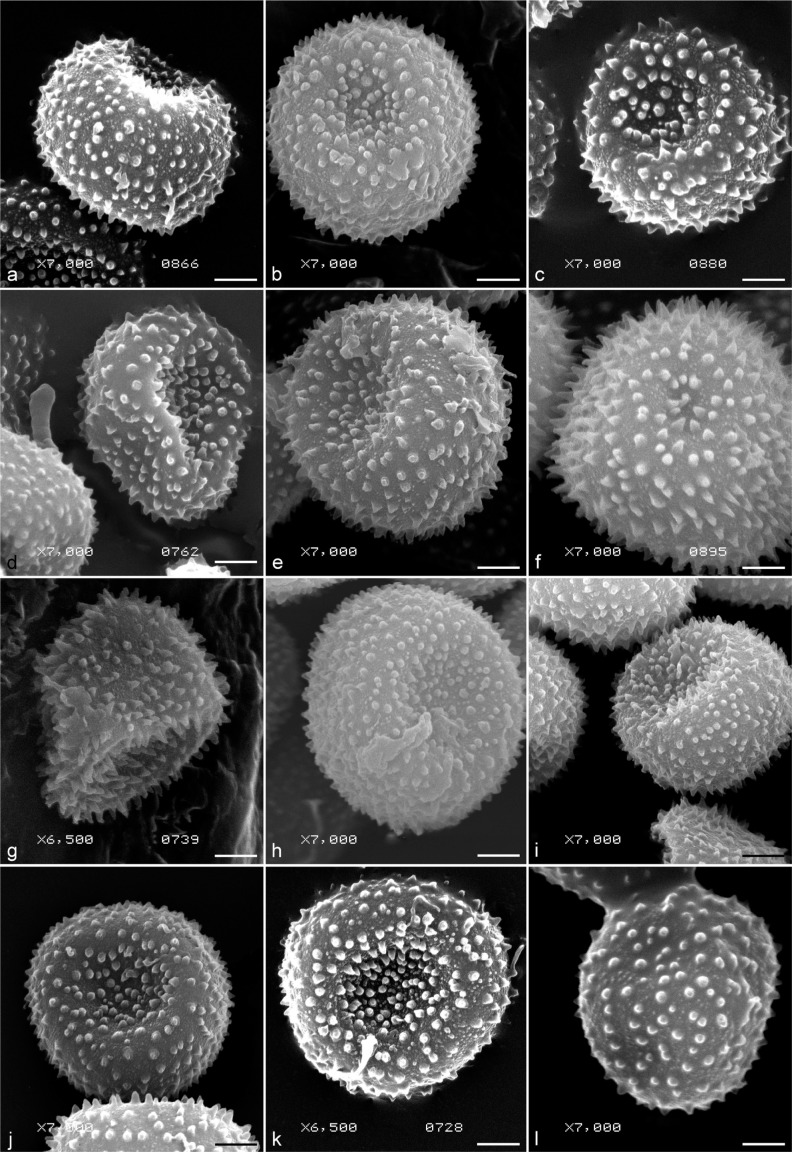
Spore ornamentation was evaluated by scanning electron microscopy. In total, 72 specimens were studied by SEM (Fig. 4). The density of spore warts per 10 μm2 did not differ remarkably between the specimens, except for the specimen from Puccinellia angustata, which had spores with 25 warts/10 μm2 (Fig. 6, Table 2). Spore ornamentation was echinulate in all specimens, except for the specimen from Puccinellia angustata with verruculose-echinulate spores. The height of warts varied markedly among all of the specimens, ranging from 0.1–0.2 μm in the specimen from Puccinellia angustata to 0.7–0.9 μm in the specimen from Agropyron tenerum.
Fig. 4.
SEM micrographs of selected specimens of Ustilago striiformis s.l. from different host plants. a. From Agrostis gigantea (HAI 2871); b. from Alopecurus geniculatus (HAI 4651); c. from Lolium perenne (S F163741 – isolectotype of U. loliicola); d. from Festuca ovina (S F163778); e. from Briza media (S F37590 – isolectotype of Tilletia brizae); f. from Deschampsia cespitosa (S F163723 – isolectotype of Tilletia airae-caespitosae); j. from Milium effusum (S F163736 – isolectotype of Tilletia milii); h. from Trisetum spicatum (H 6025898); i. from Elymus glaucus (HAI 4602); j. from Poa nemoralis (H – holotype of Ustilago kairamoi); k. from Poa annua (VPRI 3131 – holotype of U. poarum); l. from Anthoxanthum odoratum (H 6025893). — Scale bars = 2 μm.
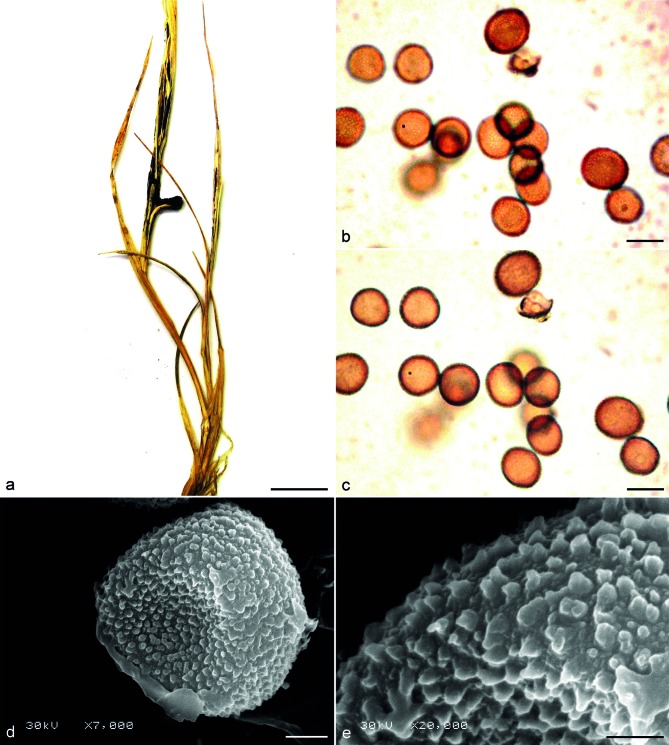
Fig. 6.
Sori and spores of Ustilago nunavutica sp. nov. (DAOM 91211). a. Infected plant with sori on leaves, leaf sheats and around stems of Puccinellia angustata; b, c. spores seen by LM, superficial, and median views, respectively; d. teliospore seen by SEM; e. verruculose-echinulate surface of teliospore seen by SEM. — Scale bars: a = 1 cm; b, c = 10 μm; d = 2 μm; e = 1 μm.
Taxonomy
Ustilago bromina Syd, Ann. Mycol. 22, 3/6: 277. 1924 — Fig. 5a–e
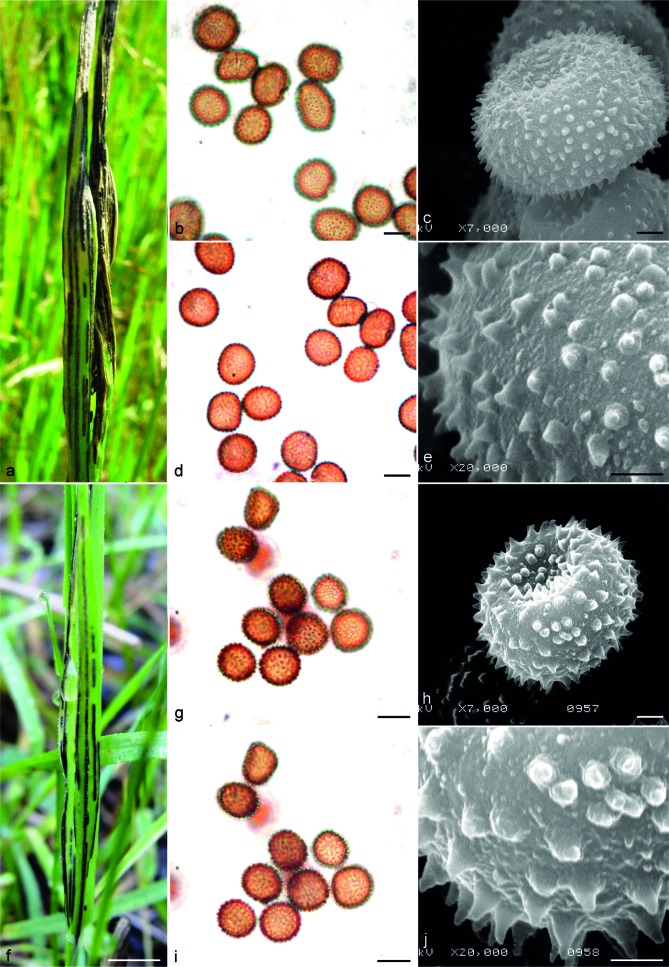
Fig. 5.
Sori and spores of Ustilago bromina (a–e) and U. striiformis s.str. (f–j). a. Sori of U. bromina (HAI 4600); b, d. teliospores seen by LM, superficial, and median views respectively (S F163740); c. teliospore seen by SEM (S F163740); e. surface of teliospore seen by SEM (S F163740); f. sori of U. striiformis s.str. (HAI 4608); g, i. teliospores seen by LM, superficial, and median views, respectively (S F163771); h. teliospore seen by SEM (S F163771); j. surface of teliospore seen by SEM (S F163771). — Scale bars: a, f = 1 cm; b, d, g, I = 10 μm; c, h = 2 μm; e, j = 1 μm.
Type. GERMANY, Berlin, Wilmersdorfer Wiesen, on Bromus inermis, Aug. 1892, P. Sydow (Sydow, Mycoth. march. no. 3508, as Tilletia striiformis, S F163740, isolectotype).
Sori in leaves and leaf sheaths, as streaks parallel with the veins, sometimes fused, initially covered by the epidermis which soon ruptures, often leading to the shredding of the leaf blades. Infection systemic. Spore mass black to dark brown, powdery. Spores subglobose, globose, elongated, ovoid, yellowish brown, (8–)8.5–12 × (10–)11–15(–16) μm (av. ± SD, 10.3 ± 1.4 × 13.0 ± 1.7 μm). Spore wall c. 0.5 μm thick, even, covered with 0.2–0.5 μm high spines, with small verrucae between them. The density of spines is 5–7/10 μm2.
Host — Bromus inermis (Poaceae-Bromeae).
Distribution — Asia, Europe, North America.
Notes — Morphologically, U. bromina differs from U. striiformis s.str. by larger spores (8–)8.5–12 × (10–)11–15(–16) μm vs 8–11 × (9–)9.5–12(–14) μm (Fig. 3). Phylogenetically, this species is more closely related to U. nunavutica than to other stripe smuts of grasses (Fig. 1).
Stripe smut of Bromus was described by Sydow (1924) based on the observation that Bromus spp. are not infected by the smut on Holcus. Sydow described U. bromina on B. erectus and B. inermis (type host) and it was later found on several other species in Europe, Asia, and North America (Gutner 1941, Fischer 1953, Zundel 1953). The assignment of collections on Bromus species different than Bromus inermis to Ustilago bromina should be confirmed by molecular phylogenetic analyses. To our knowledge, U. bromina has not been included in physiological or cross-inoculation studies. Although it could be distinguished from U. striiformis s.str. by larger spores, there are several smuts within Ustilago striiformis s.l. having spore dimensions similar to U. bromina, i.e., specimens from Beckmannia, Briza, and Leucopoa (Table 2). Spore ornamentation is unreliable as a criterion for the delimitation of this species. Nevertheless, phylogenetically, there is strong support to recognize U. bromina as a separate taxon (PP = 100, BP = 100). Within the U. bromina clade an additional bifurcation occurs (PP = 98, BP = below 70) with the specimen from Israel clustering separately from the specimens from the United States.
Ustilago nunavutica Savchenko, Carris, Castl., Heluta, Wasser & Nevo, sp. nov. — MycoBank MB807231; Fig. 6
Etymology. Named after Nunavut where the species was first found.
Type. CANADA, Northwest Territories (now Nunavut), Franklin Distr., Ellesmere Island, Trace, 1 mile north of Hazen Camp, N81°49' W71°21', on Puccinellia angustata, 19 July 1962, D.B.O. Savile (DAOM 91211, holotype).
Sori in leaves, inflorescences, stems, leaf sheaths, as short to long streaks, sometimes confluent, initially covered by the epidermis. Infection systemic. Spore mass black, semi-powdery. Spores subglobose, globose, ovoid, rarely irregular, yellowish brown, (8–)8.5–11 × (9.5–)10–13 μm, (av. ± SD, 10.1 ± 1.3 ×11.7 ± 1.5 μm). Spore wall verruculose-echinulate, c. 0.5 μm thick, even, finely covered with 0.1–0.2 μm high, sometimes confluent warts. The density of warts is 25/10 μm2. The surface between warts is additionally densely covered by minute verrucae. The ITS/LSU hologenetype sequences are deposited in GenBank as KF381025/KF381049, respectively.
Host — Puccinellia angustata (Poaceae-Poeae).
Distribution — North America (Canada).
Notes — Ustilago nunavutica can be distinguished from U. striiformis s.str. And U. bromina by the ornamentation of spores, which are verruculose-echinulate with 25 warts/10 μm2 in the former species and echinulate with 5–12 warts/10 μm2 in the latter species.
The holotype specimen of U. nunavutica was found in the far north of Canada, just 800 km from the North Pole, making U. nunavutica the most northerly found smut species. Thus far, U. striiformis s.l. was found on three other Puccinellia species: P. distans, P. maritima, and P. nuttaliana (Vánky 2012, Farr & Rossman 2013). We studied the collections on P. distans and P. nuttaliana, both from the United States. We were unable to obtain sequences from these specimens but morphologically, they were distinct from U. nunavutica. Spores from P. distans and P. nuttaliana are of the same size as the spores of U. nunavutica but differ in spore ornamentation, which is low, 0.1–0.2 μm high, verruculose-echinulate, with 25/10 μm2 warts in U. nunavutica, and 0.3–0.4 μm high, echinulate, with 6–8/10 μm2 warts in the two other specimens. Yunchangia puccinelliae is another species of smut fungi with sori as stripes in the leaves of Puccinellia (Guo & Xu 2013), but it can be easily distinguished from U. nunavutica by the presence of fungal hyphae in the sorus of the former. It is likely that at least three different stripe smut species occur on Puccinellia.
Ustilago striiformis s.str. (Westend.) Niessl, Hedwigia 15: 1. 1876 — Fig. 5f–j
Type. BELGIUM, environs de Courtrai, on Holcus lanatus, G.D. Westendorp (Westendorp, Herb. crypt. belge no. 677, HUV 9453, lectotype n.v.).
Sori in leaves, leaf sheaths, rarely in inflorescences, as streaks parallel with the veins, sometimes fused, initially covered by the epidermis which soon ruptures, leading to the shredding of the leaf blades. Infection systemic. Spore mass black to dark brown, powdery. Spores globose, subglobose, elongated, ovoid, irregular, yellowish brown, 8–11 × (9–)9.5–12(–14) μm, (av. ± SD, 9.7 ± 1.2 × 10.7 ± 1.5 μm). Spore wall c. 0.5 μm thick, even, covered with 0.2–0.7 μm high spines, with small verrucae between them. The density of spines is 6–12, av. 8/10 μm2.
Hosts — Holcus lanatus, Holcus mollis (Poaceae-Aveneae).
Distribution — Europe, Asia, North and South America, Oceania.
Notes — Type has not been examined and the description is collective, based on the specimens studied by the authors. Several stripe smuts have been described on Holcus (see discussion above) and most probably they are synonyms of U. striiformis s.str., as phylogenetic analyses showed strong monophyly for the specimens from Holcus.
Westendorp (1851) described Uredo ‘striaeformis’ as a pathogen of Holcus lanatus and Anthoxanthum odoratum. Later, this smut was transferred to the genus Ustilago with a creation of a new combination, U. striiformis. Zundel (1953) designated the lectotype from material on Holcus lanatus collected in Courtrai, Belgium, but without mentioning the specific herbarium specimen, and Vánky (1985) narrowed the lectotype to specimen preserved in his personal herbarium. In his works, Fischer (1940, 1953) presented sound arguments for the permanent establishment of the collective species U. striiformis. Since then all stripe smuts of grasses were united under this name. In our phylogenetic analyses, U. striiformis s.str. appeared to be essentially monophyletic, with specimens from H. lanatus and H. mollis clustered in one lineage (Fig. 1). However, morphologically, it is indistinguishable from the other forms of U. striiformis s.l.
DISCUSSION
Analyses of rDNA ITS and LSU sequence data coupled with morphology have been used in successful taxonomical revisions of different groups of smuts in recent molecular phylogenetic studies (Vánky & Lutz 2007, 2010, Bauer et al. 2008, Lutz et al. 2008, Kemler et al. 2009, Piątek et al. 2011, 2012, 2013a, b, Lutz et al. 2012a, b, Zhang et al. 2013). In this work specimens of U. striiformis from different hosts and regions of the world were studied in terms of morphology and phylogenetic position based on nucleotide sequence data from ITS and LSU loci. One species is described as new, while one existing name that was considered a synonym of U. striiformis is recognised as valid and distinct. Furthermore, the specimens from Holcus formed a monophyletic lineage that could be assigned to U. striiformis s.str.
Our phylogenetic analyses demonstrate the polyphyletic nature of this group, placing it in two independent phylogenetic lineages within the Ustilago s.str. clade (McTaggart et al. 2012) (Fig. 1). It should be noted, that clear morphological characters were distinctly present for the three separate species recognised in this work.
Interestingly, we did not find a strong correlation between the age of collections and the quality of sequences. One of the best sequences obtained was from U. nunavutica, which at the same time, was the earliest collection used for DNA extraction. Furthermore, we were unable to sequence several recent specimens, collected during the last five years. It is possible that the storage conditions are even more important than the age of specimens for the quality of DNA of U. striiformis.
In a number of smut genera, host phylogeny is correlated with the evolutionary history of the parasites (Begerow et al. 2004, Refrégier et al. 2007, Bauer et al. 2008, Lutz et al. 2008, Kemler et al. 2009, Piątek et al. 2012, 2013a, b). However, our results do not indicate that host evolution is correlated with the evolution of stripe smuts of grasses. Thus, U. nunavutica is a close relative of U. bromina, while their hosts – Puccinellia and Bromus, belong to different tribes of Pooideae, Poeae and Bromeae, respectively (Schneider et al. 2009). The other specimens of U. striiformis on Poeae clustered apart from the U. nunavutica-U. Bromina lineage, in the main clade of U. striiformis (Fig. 1). Begerow et al. (2004) reported the evolutionary history of the Ustilaginaceae involved extensive host jumps and shifts, and it may be also the case in the evolution of stripe smuts of grasses, but more material on diverse host species should be analysed phylogenetically to support this hypothesis.
Within the main clade of U. striiformis a vast majority of specimens from different hosts could not be attributed to any of the separate species of stripe smuts of grasses due to still limited sampling of multiple sequences on the same host species, morphological similarity and also weak support for some branches. Thus, a separate clade with weak support was recorded for two specimens from Phleum collected in different regions of the world. Despite the fact that a vast diversity of taxa has been described within the U. striiformis complex, no separate species exists for the stripe smut of Phleum. Phylogenetically, this host genus is a close relative of Poa and Milium (Schneider et al. 2009) and several fungal species have been described on each of the latter genera. However, morphologically, there is a difference in spore size, with spores from Phleum being larger than those from Poa and Milium (Table 2). Therefore, it is possible that the independent species occurs on Phleum. Another interesting discovery of this work is that some specimens from the same host species (Alopecurus pratensis and Calamagrostis canadensis) were not grouped together (Fig. 1). In both cases the samples from Alopecurus and Calamagrostis were from the same regions, i.e. Europe and North America, respectively and have no morphological differences between each other. Therefore, at present, it is impossible to speculate why they were clustered separately and more specimens from these hosts should be analysed in future studies. Our phylogenetic analyses showed strong support for the recognition of a separate lineage for the smut on Elymus, as two of its specimens cluster together in all of the analyses. Physiologically, stripe smuts of Triticeae (Agropyron and Elymus) differ from the other forms, as it was first revealed by Fischer (1940). It has a predominantly sporidial type of growth in culture, and the cross-inoculation experiments showed this smut was transmitted only between Agropyron and Elymus, with Agrostis, Bromus, Festuca, and Sitanion not susceptible. Fischer (1940) considered that this is a new form of U. striiformis, which parasitizes two genera of the tribe Hordeae (= Triticeae), and created a new name – U. striiformis f. hordei. There is a subtle difference in spore dimensions between U. striiformis f. hordei and U. striiformis s.str. Furthermore, there is a morphological variation between collections from different Agropyron and Elymus species. Thus, spores were 10.1 × 12.6 μm in size with 0.7–0.9 μm high spines in the smut from A. tenerum, and 9.9 × 10.9 μm in size with 0.4 μm high spines in the smut from E. glaucus. The current study clearly supports the view that U. striiformis on Triticeae differs phylogenetically from other forms of stripe smuts of grasses, but as it could not be morphologically distinguished from U. striiformis s.str., more material from the same host species should be analysed in the future with the inclusion of additional gene regions in order to confirm the necessity of the creation of a separate species.
Acknowledgments
We thank A.O. Chater (UK), P. Kokeš (Czech Republic), J. Kruse (Germany), V. Kummer (Germany), M. Lutz (Germany), M. Piątek (Poland), P.A. & K. Smith (UK), N. Stringer (UK), O.Yu. Umanets (Ukraine), and K. Vánky (Germany) for providing us with the specimens from their collections, the curators of herbaria BPI, BR, BRNM, DAOM, ILLS, ISC, H, HAI, KR, KRAM, KW, MA, MICH, MIN, NY, PDD, S, TUR, UC, UPS, VPRI, and WSP for the loan of specimens, anonymous reviewers for useful comments, and V. Sapsai (Ukraine) for help with the SEM pictures.
REFERENCES
- Ainsworth GC, Sampson K. 1950. The British smut fungi (Ustilaginales). C.M.I., Kew. [Google Scholar]
- Alderman SC, Ocamb CM, Mellbye ME, Sedegui SM. 2007. Occurrence of Ustilago striiformis in Dactylis glomerata seed production fields in Oregon. Plant Health Progress. Online. doi:10.1094/PHP-2007-1023-01-RS. [Google Scholar]
- Bauer R, Lutz M, Begerow D, et al. 2008. Anther smut fungi on monocots. Mycological Research 112: 1297–1306. [DOI] [PubMed] [Google Scholar]
- Begerow D, Göker M, Lutz M, Stoll M. 2004. On the evolution of smut fungi on their hosts. In: Agerer R, Peiepenbring M, Blanz P. (eds), Frontiers in Basidiomycete mycology: 81–98 IHW-Verlag, Eching. [Google Scholar]
- Begerow D, Stoll M, Bauer R. 2006. A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia 98: 906–916. [DOI] [PubMed] [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552. [DOI] [PubMed] [Google Scholar]
- Chakrabarty P. 2010. Genetypes: a concept to help integrate molecular phylogenetics and taxonomy. Zootaxa 2632: 67–68. [Google Scholar]
- Clinton GP. 1902. North American Ustilagineae. Journal of Mycology 8: 128–156. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Davis WH. 1924. Spore germination of Ustilago striaeformis. Phytopathology 14: 251–267. [Google Scholar]
- Davis WH. 1930. Two physiologic forms of Ustilago striaeformis (Westd.) Niessl. Phytopathology 20: 65–74. [Google Scholar]
- Davis WH. 1935. Summary of investigations with Ustilago striaeformis parasitizing some common grasses. Phytopathology 25: 810–817. [Google Scholar]
- Denchev CM. 2001. Class Ustomycetes (ordines Tilletiales, Usilaginales et Graphiolales). In: Fungi Bulgariae Vol. 4. Pensoft, Bulgaria. [Google Scholar]
- Farr DF, Rossman AY. 2013. Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved July 8, 2013, from http://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Farris JS, Kallersjo M, Kluge AG, Bult C. 1994. Testing significance of incongruence. Cladistics 10: 315–320. [Google Scholar]
- Fischer GW. 1940. Fundamental studies of the stripe smut of grasses (Ustilago striaeformis) in the Pacific Northwest. Phytopathology 30: 93–118. [Google Scholar]
- Fischer GW. 1953. Manual of the North American smut fungi. Ronald Press Company, USA. [Google Scholar]
- Guo L, Xu B. 2013. Yunchangia, a new genus of smut fungi (Ustilaginaceae) from China. Mycotaxon 123: 261–264. [Google Scholar]
- Gutner LS. 1941. Golovnevye griby (po materialam A.A. Jaczevskogo). OGIZ-Selhozgiz, USSR. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 817: 754–755. [DOI] [PubMed] [Google Scholar]
- Ignatavičiute M. 2000. Kûlieèiai (Ustilaginales). In: Lietuvos Grybai (Mycotha Lithuaniae) 4. UAB ‘Valstieèiø Laikraštis’, Lithuania. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program (outlines version 6). Briefings in Bioinformatics 9: 286–298. [DOI] [PubMed] [Google Scholar]
- Kemler M, Lutz M, Göker M, et al. 2009. Hidden diversity in the non-caryophyllaceous plant-parasitic members of Microbotryum (Pucciniomycotina: Microbotryales). Systematics and Biodiversity 7: 297–306. [Google Scholar]
- Kishino H, Hasegawa M. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the ranching order in Hominoidea. Journal of Molecular Evolution 29: 170–179. [DOI] [PubMed] [Google Scholar]
- Kochman J. 1936. Grzyby głowniowe Polski. Ustilaginales Poloniae. Planta Polonica 4: 1–161. [Google Scholar]
- Kochman J, Majewski T. 1973. Głowniowe (Ustilaginales). In Grzyby (Mycota). 5. Państwowe Wydawnictwo Naukowe, Poland. [Google Scholar]
- Krietlow KW. 1943a. Ustilago striaeformis I. Germination of chlamydospores and culture of forma agrostidis on artificial media. Phytopathology 33: 707–712. [Google Scholar]
- Krietlow KW. 1943b. Ustilago striaeformis II. Temperature as a factor influencing development of smutted plants of Poa pratensis L. and germination of fresh chlamydospores. Phytopathology 33: 1055–1063. [Google Scholar]
- Krietlow KW, Myers WM. 1944. Prevalence and distribution of stripe smut of Poa pratensis in some pastures of Pennsylvania. Phytopathology 34: 411–415. [Google Scholar]
- Leach JG, Ryan MA. 1946. The cytology of Ustilago striiformis forma poae-pratensis in artificial culture. Phytopathology 36: 876–886. [Google Scholar]
- Lindeberg B. 1959. Ustilaginales of Sweden (exclusive of the Cintractias on Caricoideae). Symbolae Botanicae Upsalinenses 16: 1–175. [Google Scholar]
- Liro JI. 1924. Die Ustilagineen Finnlands. Annales Academiae Scientiarum Fennicae, Series A 17: 1–636. [Google Scholar]
- Lutz M, Piąltek M, Kemler M, et al. 2008. Anther smuts of Caryophyllaceae: Molecular analyses reveal further new species. Mycological Research 112: 1280–1296. [DOI] [PubMed] [Google Scholar]
- Lutz M, Vánky K, Bauer R. 2012a. Melanoxa, a new genus in the Urocystidales (Ustilaginomycotina). Mycological Progress 11: 149–158. [Google Scholar]
- Lutz M, Vánky K, Piątek M. 2012b. Shivasia gen. Nov. for the Australasian smut Ustilago solida that historically shifted through five different genera. IMA Fungus 3: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen Y. 1963. On the smuts of the genus Ustilago on Calamagrostis species in Finland. Karstenia 6–7: 88–94.22422014 [Google Scholar]
- McTaggart AR, Shivas RG, Geering ADW, et al. 2012. Soral synapomorphies are significant for the systematics of the Ustilago-Sporisorium-Macalpinomyces complex (Ustilaginaceae). Persoonia 29: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesz G. 1950. A Kárpát-medence üszöggombái (Les Ustilaginales du basin des Carpathes). Egyetemi Könyvkiadó, Hungary. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Programme distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden. [Google Scholar]
- Nylander JAA, Ronquist AF, Huelsenbeck JP, Nieves-Aldrey JL. 2004. Bayesian phylogenetic analysis of combined data. Systematic Biology 53: 47–67. [DOI] [PubMed] [Google Scholar]
- Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenies. Bioinformatics 24: 581–583. [DOI] [PubMed] [Google Scholar]
- O’Donnell K. 1993. Fusarium and its near relatives. In: Reynolds DR, Taylor JW. (eds), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics: 225–233 CAB International, Wallingford. [Google Scholar]
- Osner GA. 1916. Leaf smut of timothy. New York Agricultural Experimental Station Bulletin 381, USA. [Google Scholar]
- Pammel LH, Weems JB, Lamson-Scribner F. 1901. Grasses of Iowa. Part I. Iowa Geological Survey Bulletin 1, USA. [Google Scholar]
- Piątek M, Lutz M, Chater AO. 2013a. Cryptic diversity in the Antherospora vaillantii complex on Muscari species. IMA Fungus 4: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piątek M, Lutz M, Kemler M. 2013b. Microbotryum silenes-saxifragae sp. Nov. Sporulating in the anthers of Silene saxifraga in southern European mountains. IMA Fungus 4: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piątek M, Lutz M, Ronikier A, Kemler M, Świderska-Burek U. 2012. Microbotryum heliospermae, a new anther smut fungus parasitic on Heliosperma pusillum in the mountains of the European Alpine System. Fungal Biology 116: 185–195. [DOI] [PubMed] [Google Scholar]
- Piątek M, Lutz M, Smith PA, Chater AO. 2011. A new species of Antherospora supports the systematic placement of its host plant. IMA Fungus 2: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannala B, Yang Z. 1996. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311. [DOI] [PubMed] [Google Scholar]
- Refrégier G, Le Gac M, Jabbour F, et al. 2007. Cophylogeny of the anther smut fungi and their caryophyllaceaous hosts: prevalence of host shifts and importance of delimiting parasite species for inferring cospeciation. BMC Evolutionary Biology 8: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Săvulescu T. 1957. Ustilaginalele din R.P. Romînă. I-II. Edit. Acad, Romania. [Google Scholar]
- Schneider J, Döring E, Hilu KW, Röser M. 2009. Phylogenetic structure of the grass subfamily Pooideae based on comparison of plastid matK gene-3′ trnK exon and nuclear ITS sequences. Taxon 58: 405–424. [Google Scholar]
- Scholz H, Scholz I. 1988Die Brandpilze Deutschlands (Ustilaginales). Englera 8: 1–691. [Google Scholar]
- Schwarzman SR. 1960. Golovniovye griby (Smut fungi). In: Flora Sporovych Rastenij Kazakhstana 2. USSR. [Google Scholar]
- Stoll M, Begerow D, Oberwinkler F. 2005. Molecular phylogeny of Ustilago, Sporisorium, and related taxa based on combined analyses of rDNA sequences. Mycological Research 109: 342–356. [DOI] [PubMed] [Google Scholar]
- Stoll M, Piepenbring M, Begerow D, Oberwinkler F. 2003. Molecular phylogeny of Ustilago and Sporisorium species (Basidiomycota, Ustilaginales) based on internal transcribed spacer (ITS) sequences. Canadian Journal of Botany 81: 976–984. [Google Scholar]
- Swofford DL. 2002. PAUP 4.0b10: phylogenetic analysis using parsimony. Sinauer Associates, Sunderland. [Google Scholar]
- Sydow H. 1924. Notizen über Ustilagineen. Annales Mycologici 22: 277–291. [Google Scholar]
- Thirumalachar MJ, Dickson JG. 1953. Spore germination, cultural characters, and cytology of varieties of Ustilago striiformis and the reaction of hosts. Phytopathology 43: 527–536. [Google Scholar]
- Uljanishchev VI. 1968. Opredelitel golovniovyh gribov SSSR (Key to the smut fungi of the USSR). Nauka, USSR. [Google Scholar]
- Vánky K. 1985. Carpathian Ustilaginales. Symbolae Botanicae Upsalinenses 24: 1–309. [Google Scholar]
- Vánky K. 1994. European smut fungi. Fischer Verlag, Stuttgart, Germany. [Google Scholar]
- Vánky K. 2012. Smut fungi of the world. APS Press, St. Paul, MN, USA. [Google Scholar]
- Vánky K, Lutz M. 2007. Revision of some Thecaphora species (Ustilaginomycotina) on Caryophyllaceae. Mycological Research 111: 1207–1219. [DOI] [PubMed] [Google Scholar]
- Vánky K, Lutz M. 2010. Entyloma majewskii sp. Nov. (Entylomataceae) on Ranunculus ficaria from Iran. Polish Botanical Journal 55: 271–279. [Google Scholar]
- Vánky K, Mckenzie EHC. 2002. Smut fungi of New Zealand. Fungal Diversity Press, Hong Kong. [Google Scholar]
- Vánky K, Shivas RG. 2008. Fungi of Australia. The smut fungi. ABRS Press, Australia. [Google Scholar]
- Viennot-Bourgin G. 1956. Mildious, oidiums, caries, charbons, rouilles des plantes de France. I–II. Encyclopédie Mycologique 26, France. [Google Scholar]
- Wellhausen EJ, Krietlow KW, Leach JG. 1943. Observations on the prevalence of stripe smut (Ustilago striaeformis) on blue grass. Plant Disease Reporter 27: 23–24. [Google Scholar]
- Westendorp GD. 1851. Notice sur quelques cryptogames inédites ou nouvelles pour la flore belge. Bulletins de l’Academie Royale des Sciences, des Lettres et des Beaux Arts de Belgique 18: 384–417. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA sequences for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, New York. [Google Scholar]
- Zhang JZ, Guan PG, Tao G, et al. 2013. Ultrastructure and phylogeny of Ustilago coicis. Journal of Zhejiang University-Science B 14: 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg H. 1985. Die Brandpilze Mitteleuropas unter besonderer Berücksichtigung der Schweiz. Cryptogamica Helvetica 16: 1–277. [Google Scholar]
- Zundel GL. 1953. The Ustilaginales of the World. Contributions of the Department of Botany, Pennsylvania State College of Agriculture 176: 1–410. [Google Scholar]
- Zwetko P, Blanz P. 2004. The smut fungi from Austria. Doassansiales, Entorrhizales, Entylomatales, Georgefischeriales, Microbotryales, Tilletiales, Urocystales, Ustilaginales. Catalogus Florae Austriae III/3. Österreichische Akademie der Wissenschaften, Austria. [Google Scholar]