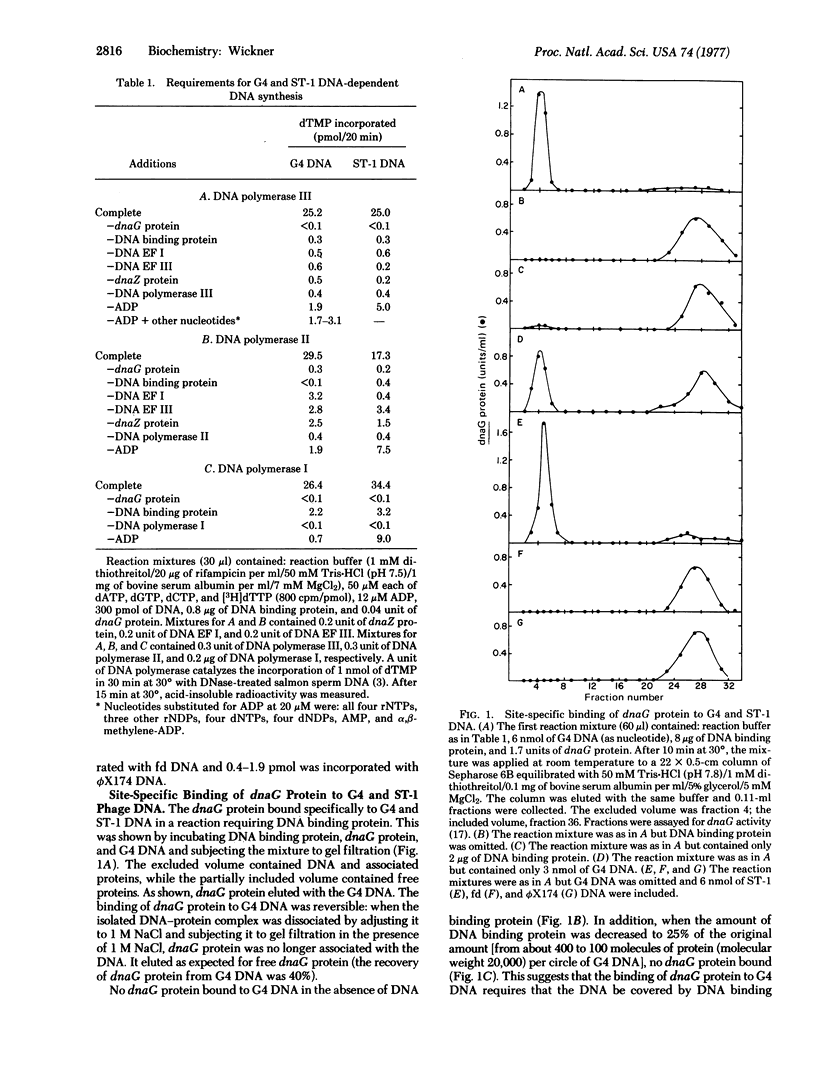
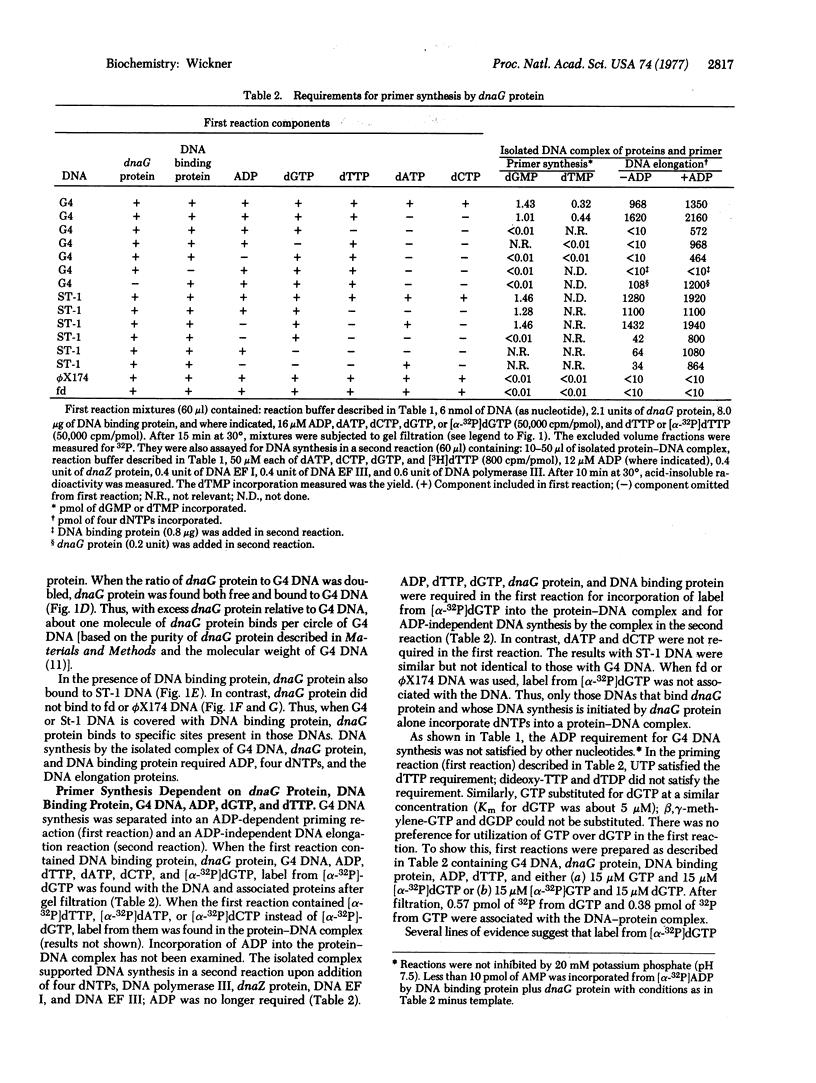
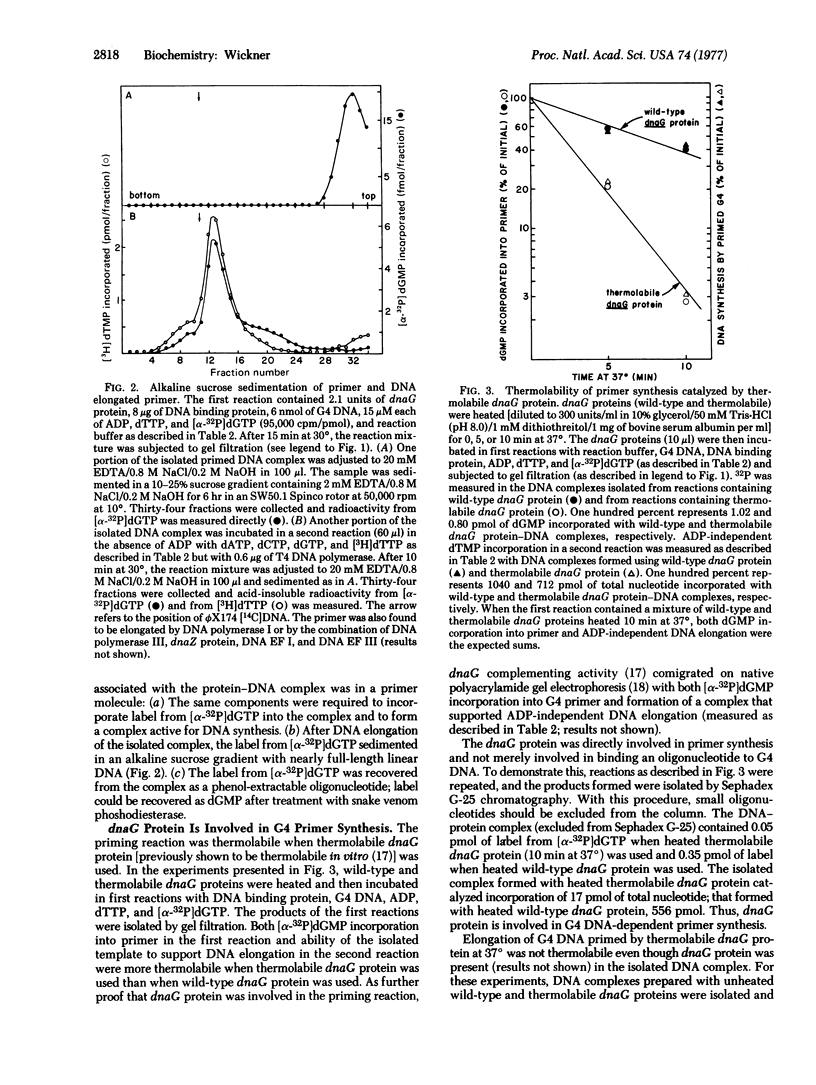
Abstract
Escherichia coli dnaG protein is involved in the initiation of DNA synthesis dependent on G4 or ST-1 single-stranded phage DNAs [Bouche, J.-P., Zechel, K & Kornberg, A. (1975) J. Biol. Chem. 250, 5995-6001]. The reaction occurs by the following mechanism: dnaG protein binds to specific sites on the DNA in a reaction requiring E. coli DNA binding protein. An oligonucleotide is synthesized in a reaction involving dnaG protein, DNA binding protein, and DNA. With G4 DNA this reaction requires ADP, dTTP (or UTP), and dGTP (or GTP). Elongation of the oligonucleotide can be catalyzed by DNA polymerase II or III in combination with dnaZ protein and DNA elongation factors I and III, presumably by the mechanism previously reported [Wickner, S. (1976) Proc. Natl. Acad. Sci. USA 73, 3511-3515] or by DNA polymerase I.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Bowes J. M., Dowell C. E. Purification and some properties of bacteriophage ST-1. J Virol. 1974 Jan;13(1):53–61. doi: 10.1128/jvi.13.1.53-61.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Fate of parental phi X174-DNA upon infection of starved thymine-requiring host cells. Virology. 1971 Apr;44(1):168–187. doi: 10.1016/0042-6822(71)90163-2. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Evolution of phi-chi 174. Isolation of four new phi-chi-like phages and comparison with phi-chi 174. Virology. 1974 Mar;58(1):272–289. doi: 10.1016/0042-6822(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Wickner S., Wright M. Studies on in vitro DNA synthesis. II. Isolation of a protein which stimulates deoxynucleotide incorporation catalyzed by DNA polymerase of E. coli. Biochem Biophys Res Commun. 1973 Mar 17;51(2):257–267. doi: 10.1016/0006-291x(73)91251-5. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Sadowski P. D., Hurwitz J. Enzymatic breakage of deoxyribonucleic acid. II. Purification and properties of endonuclease IV from T4 phage-infected Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6192–6198. [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Ginsberg B., Berkower I., Hurwitz J. Deoxyribonucleic acid plymerase II. of Escherichia coli. I. The purification and characterization of the enzyme. J Biol Chem. 1972 Jan 25;247(2):489–497. [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S. Mechanism of DNA elongation catalyzed by Escherichia coli DNA polymerase III, dnaZ protein, and DNA elongation factors I and III. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3511–3515. doi: 10.1073/pnas.73.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Wright M., Hurwitz J. Studies on in vitro DNA synthesis. Purification of the dna G gene product from Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1613–1618. doi: 10.1073/pnas.70.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Bouché J. P., Kornberg A. Replication of phage G4. A novel and simple system for the initiation of deoxyribonucleic acid synthesis. J Biol Chem. 1975 Jun 25;250(12):4684–4689. [PubMed] [Google Scholar]


