Abstract
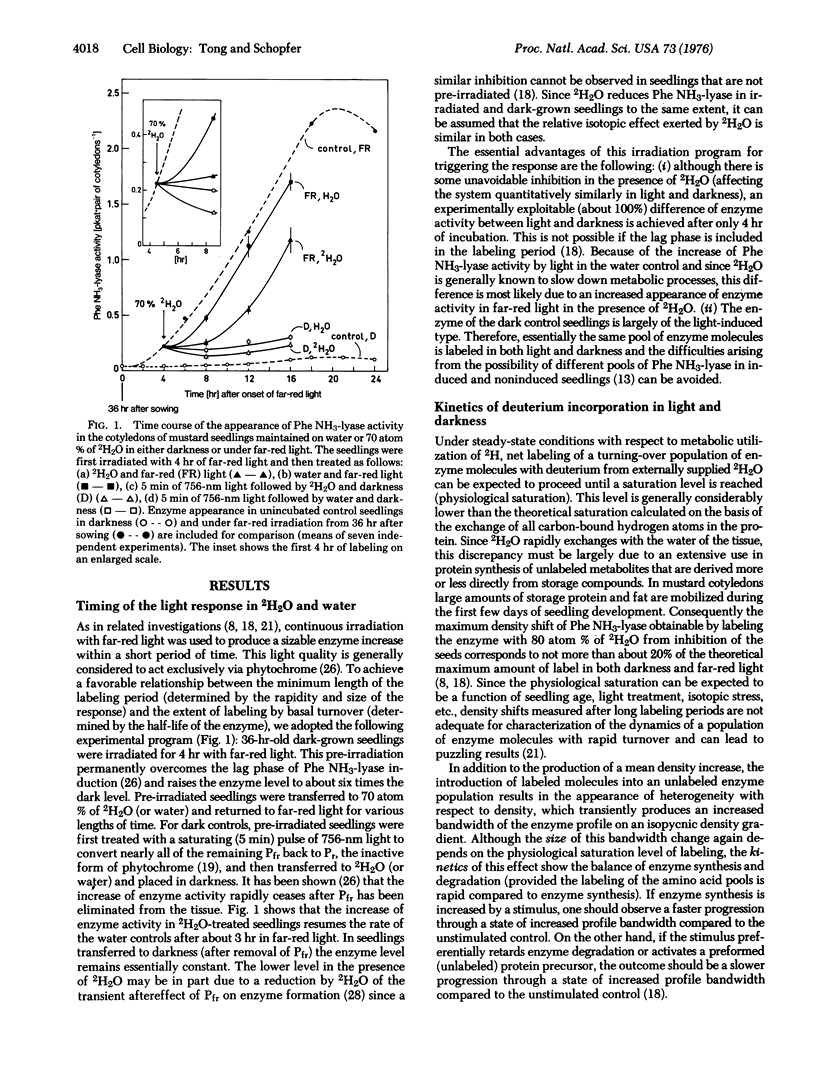
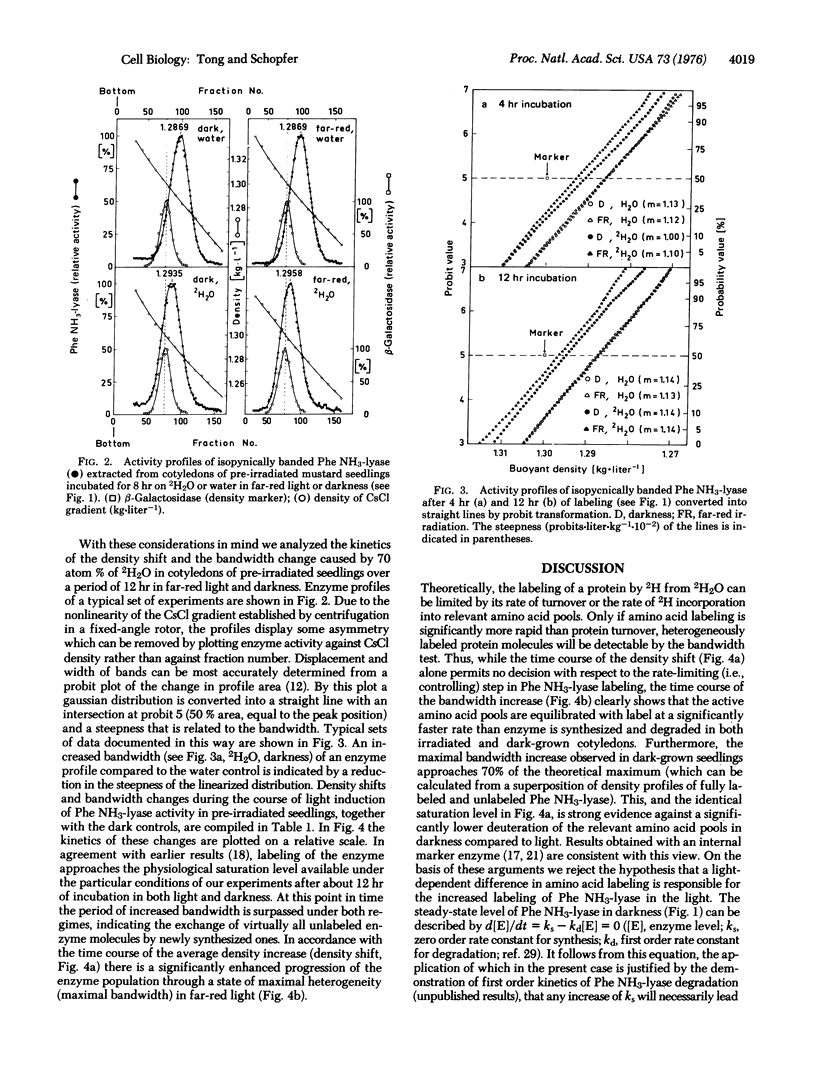
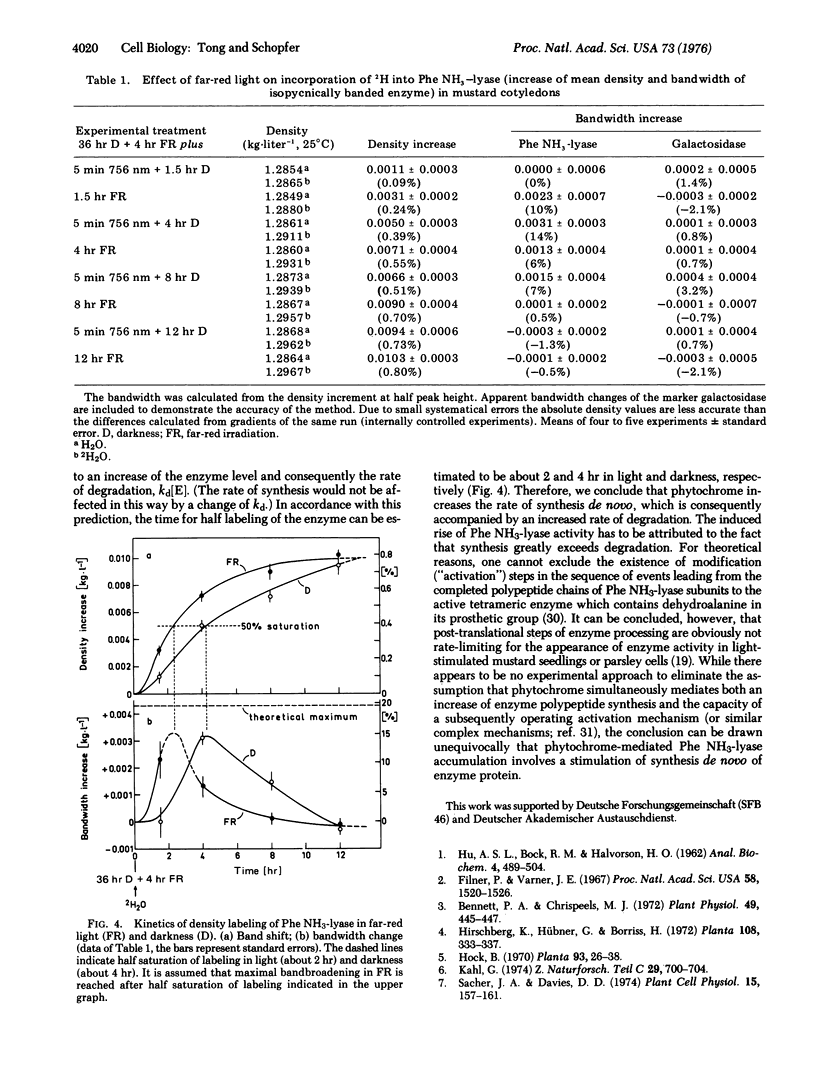
The molecular mechanism of enzyme (phenylalanine ammonia-lyase; EC 4.3.1.5) formation mediated by phytochrome in mustard seedlings was investigated by labeling the enzyme with deuterium followed by high resolution CsCl density gradient analysis. A favorable relationship between induced rise of activity and turnover of this short-lived enzyme (half-life 3-4 hr) was achieved by labeling pre-irradiated seedlings. The time course of deuterium incorporation during the light-mediated rise in enzyme activity that can be derived independently from density shifts and bandwidth changes demonstrates a stimulation of synthesis and degradation by phytochrome. When synthesis is faster than degradation, enzyme accumulates.
Keywords: enzyme synthesis versus activation, density labeling
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton G. J., Schopfer P. Control over activation or synthesis of phenylalanine ammonia-lyase by phytochrome in mustard (Sinapis alba L.)? A contribution to eliminate some misconceptions. Biochim Biophys Acta. 1975 Oct 9;404(2):231–242. doi: 10.1016/0304-4165(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Acton G. J., Schopfer P. Phytochrome-induced synthesis of ribonuclease de novo in lupin hypocotyl sections. Biochem J. 1974 Sep;142(3):449–455. doi: 10.1042/bj1420449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge T. H., Johnson C. B., Smith H. Density-labelling evidence for the phytochrome-mediated activation of phenylalanine ammonia-lyase in mustard cotyledons. Biochim Biophys Acta. 1974 May 24;343(3):440–451. doi: 10.1016/0304-4165(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Attridge T. H. Phytochrome-mediated synthesis of ascorbic acid oxidase in mustard cotyledons. Biochim Biophys Acta. 1974 Sep 5;362(2):258–265. doi: 10.1016/0304-4165(74)90218-9. [DOI] [PubMed] [Google Scholar]
- Bennett P. A., Chrispeels M. J. De Novo Synthesis of Ribonuclease and beta-1,3-Glucanase by Aleurone Cells of Barley. Plant Physiol. 1972 Mar;49(3):445–447. doi: 10.1104/pp.49.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU A. S., BOCK R. M., HALVORSON H. O. Separation of labeled from unlabeled proteins by equilibrium density gradient sedimentation. Anal Biochem. 1962 Dec;4:489–504. doi: 10.1016/0003-2697(62)90129-x. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase (maize and potato). Evidence that the enzyme is composed of four subunits. Biochemistry. 1973 Apr 10;12(8):1583–1591. doi: 10.1021/bi00732a019. [DOI] [PubMed] [Google Scholar]
- Kahl G. De novo synthesis of glucose-6-phosphate- (E.C. 1.1.1.49) and 6-phosphogluconate dehydrogenase (E.C. 1.1.1.44) in plant storage tissue slices. Z Naturforsch C. 1974 Nov-Dec;29(11-12):700–704. doi: 10.1515/znc-1974-11-1209. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Rubery P. H. Interpretation of the rate of density labelling of enzymes with 2H2O. Possible implications for the mode of action of phytochrome. Biochim Biophys Acta. 1976 Feb 24;421(2):308–318. doi: 10.1016/0304-4165(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Scandalios J. G. Turnover of genetically defined catalase isozymes in maize. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1402–1406. doi: 10.1073/pnas.68.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Schäfer E., Lassig T. U., Schopfer P. Photocontrol of phytochrome destruction in grass seedlings. The influence of wavelength and irradiance. Photochem Photobiol. 1975 Nov;22(5):193–202. doi: 10.1111/j.1751-1097.1975.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Wellmann E., Schopfer P. Phytochrome-mediated de Novo Synthesis of Phenylalanine Ammonia-Lyase in Cell Suspension Cultures of Parsley. Plant Physiol. 1975 May;55(5):822–827. doi: 10.1104/pp.55.5.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]


