Abstract
BDNF and its gene polymorphism may be important in synaptic plasticity and neuron survival, and may become a key target in the physiopathology of long-term heroin use. Thus, we investigated the relationships between brain-derived neurotrophic factor (BDNF) plasma concentrations and the BDNF Val66Met nucleotide polymorphism (SNP) in heroin-dependent patients. The pretreatment expression levels of plasma BDNF and the BDNF Val66Met SNP in 172 heroin-dependent patients and 102 healthy controls were checked. BDNF levels were significantly lower in patients (F = 52.28, p < 0.0001), but the distribution of the SNP was not significantly different. Nor were plasma BDNF levels significantly different between Met/Met, Met/Val, and Val/Val carriers in each group, which indicated that the BDNF Val66Met SNP did not affect plasma BDNF levels in our participants. In heroin-dependent patients, plasma BDNF levels were negatively correlated with the length of heroin dependency. Long-term (>15 years) users had significantly lower plasma BDNF levels than did short-term (<5 years) users. We conclude that plasma BDNF concentration in habitual heroin users are not affected by BDNF Val66Met gene variants, but by the length of the heroin dependency.
Opioid dependence, both physiological and psychological, is a complex disorder and a severe public health problem. The development of opioid dependence and the tendency to relapse into dependency are caused by a combination of environmental, biological, and genetic factors. Opioids impair cognitive function, sustained attention1,2, and long-term memory3. The functional brain impairment caused by long-term opioid use might intensify dependence and contribute to a relapse. Therefore, it is necessary to clarify the biological mechanism of neuronal dysfunction in opioid-dependent patients.
Neurotrophins in the brain increase the growth and maintenance of several neuronal systems, modulate neurotransmission, and affect neuronal function4. Brain-derived neurotrophic factor (BDNF) is a member of the nerve growth factor-related family of neurotrophins5. Because BDNF acts on the primary sensory and cholinergic neurons6 of the basal forebrain, its dysfunction might relate to mood disorders, schizophrenia, eating disorders, and substance use disorders. We previously reported7 that plasma BDNF levels were significantly lower in patients with bipolar disorder and schizophrenia, and, in our animal study8, that chronic administration of opioids significantly reduced the BDNF level in the drug-addiction-related area of the rat brain. Moreover, Angelucci et al.9 reported that in chronic heroin users had lower serum levels of nerve growth factor and BDNF. Thus, we hypothesize that the downregulation of brain and circulatory BDNF is highly correlated with the progression of opioid dependence. However, Heberlein et al. (2011)10 reported that serum BDNF levels were significantly higher in opioid-dependent patients. The increase of serum BDNF was found in during heroin withdrawal11. In rats, BDNF in the ventral tegmental area induces an opioid-dependent-like reward state12. Because of the dearth of published reports and small sample sizes, the role of BDNF in long-term heroin users requires additional study.
In addition, a number of genetic association studies13,14,15 have shown that a single nucleotide polymorphism (SNP) in the promoter of the BDNF gene at codon 66 (G196A, rs6265) is associated with the drug-seeking phenotypes in heroin-dependent people. The Met or Val allele has been associated with substance-use disorder, bipolar disorder, and schizophrenia13,14,16. In Chinese subjects, 66Val-allele carriers had a later onset of heroin abuse13. Met carriers reported more time- and cost-intensive heroin-seeking and more cigarette use than did carriers of the homozygous Val SNP (n = 34)15. Thus, the BDNF Val66Met SNP polymorphism might be involved in human heroin addiction.
To clarify the plasma BDNF profile and BDNF Val66Met SNP polymorphism in heroin-dependent humans, we analyzed and compared the plasma BDNF levels of healthy controls and heroin-dependent patients in the present study. The BDNF Val66Met SNP variant in these participants was also studied to determine its association within these groups. Because correlations between the BDNF Val/Met SNP and plasma BDNF levels in heroin-dependent patients have not been reported, we also studied the BDNF Val66Met variant's effect on plasma BDNF protein levels in all participants.
Results
There were no significant differences in gender or body weight between the controls and patients (Table 1). Heroin-dependent patients were significantly (<0.0001) older than healthy controls; thus, we controlled for age, and analyses of covariance (ANCOVA) were used to analyze the data. A general linear model was used and age was controlled as a covariate to analyze plasma BDNF levels between groups. Patients had significantly lower plasma BDNF levels than did controls (10.1 ± 7.7 ng/mL vs. 18.6 ± 9.4 ng/mL, respectively) [F (2, 274) = 51.69, p < 0.0001] (Table 1) when controlled for age (group × age, p = 0.115). All heroin-dependent patients were undergoing methadone maintenance therapy (MMT) during the study; their mean oral methadone dose was 35.6 ± 2.3 mg and mean plasma methadone level was 149.1 ± 167.9 ng/mL. The urine tests of 124 patients were positive for morphine and the test of 28 were positive for amphetamine (Table 1).
Table 1. Demographic data and plasma brain-derived neurotrophic factor baseline levels of heroin-dependent patients and healthy controls.
| Healthy Controls | Heroin-Dependent Patients | |||
|---|---|---|---|---|
| Participant Characteristics | n = 102 | n = 172 | χ2/t/F | p |
| Gender (M/F) | 78/24 | 141/31 | χ2 = 1.21 | 0.279a |
| Age (years) | 32.1 ± 7.0 | 37.0 ± 7.3 | t = −5.495 | <0.0001b |
| Body weight (kg) | 67.2 ± 13.2 | 66.6 ± 12.6 | t = 0.437 | 0.662b |
| Plasma BDNF (ng/mL) | 18.6 ± 9.4 | 10.1 ± 7.7 | F = 52.28 | <0.0001c |
| Heroin-dependency duration (years) | − | 8.2 ± 7.0 | ||
| Methadone oral dose (mg) | − | 35.6 ± 21.3 | ||
| Plasma methadone (ng/mL) | − | 149.1 ± 167.9 | ||
| Urine test morphine-positive (n) | − | 124 | ||
| Urine test methamphetamine-positive(n) | − | 28 |
Data are means ± standard deviation (SD). All patient data were compared with data from healthy controls.
BDNF, brain-derived neurotrophic factor.
aχ2 test;
bindependent samples t test;
canalysis of covariance (ANCOVA): covariate = age.
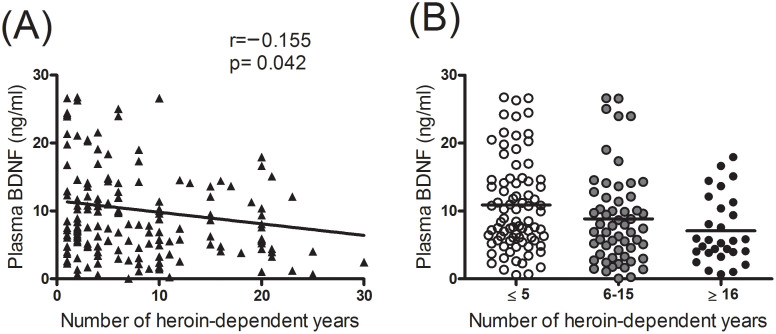
In the genotype analysis, there was no significant difference in the distribution of the BDNF Val66Met variant between groups (χ2 = 3.109, p = 0.211), nor was there a significant difference in the distribution of the BDNF Met and Val allele frequency between groups (χ2 = 2.058, p = 0.151) (Table 2). A comparison of carriers of the Met/Met, Met/Val, and Val/Val variants of the BDNF Val66Met gene showed significantly lower plasma BDNF levels in heroin-dependent patients than in healthy controls (Table 3). A multivariate linear regression analysis of plasma BDNF levels with related factors showed that in healthy controls they were not correlated with gender, age, body weight, or the Met/Val variant. In long-term heroin-dependent patients, plasma BDNF levels were significantly correlated with body weight (β = 0.204, p = 0.013), blood platelet counts (β = 0.379, p < 0.0001), and the duration of the heroin dependency (β = −0.18, p = 0.046), but not with gender, age, the BDNF Val66Met variant, the oral methadone dose, or the plasma methadone level (Table 4). Patients who had been heroin-dependent for more than 15 years had significantly lower plasma BDNF levels (7.8 ± 6.4 ng/mL, n = 30) than did those who had been heroin-dependent for less than 5 years (10.9 ± 7.0, n = 82, t = 2.072, p = 0.041) (Figure 1).
Table 2. BDNF Val66Met gene distribution in heroin-dependent patients and healthy controls.
| Group | Healthy Controls | Heroin-Dependent Patients | ||
|---|---|---|---|---|
| (n = 102) | (n = 172) | χ2 | p | |
| Genotype | 3.109 | 0.211a | ||
| Met/Met [n (%)] | 26 (25.5) | 49 (28.5) | ||
| Met/Val [n (%)] | 43 (42.2) | 84 (48.8) | ||
| Val/Val [n (%)] | 33 (32.4) | 39 (22.7) | ||
| Met/Val allele frequency | 2.058 | 0.151a | ||
| Met [n (%)] | 95 (46.6) | 182 (52.9) | ||
| Val [n (%)] | 109 (53.4) | 162 (47.1) |
BDNF, brain-derived neurotrophic factor.
aCompared with healthy controls (χ2 test).
Table 3. Plasma brain-derived neurotrophic factor level based on BDNF Val66Met gene variant in heroin-dependent patients and healthy controls.
| BDNF Val66Met | Plasma BDNF (ng/mL) | |||
|---|---|---|---|---|
| Gene Variant | Healthy Controls | Heroin-Dependent Patients | t | p |
| Met/Met | 19.5 ± 9.2 (n = 26) | 10.8 ± 7.0 (n = 49) | 4.587 | <0.0001a |
| Met/Val | 18.2 ± 9.7 (n = 43) | 9.3 ± 7.3 (n = 84) | 5.343 | <0.0001a |
| Val/Val | 18.5 ± 9.3 (n = 33) | 11.1 ± 9.2 (n = 39) | 3.362 | 0.001a |
Data are means ± SD.
BDNF, brain-derived neurotrophic factor.
aCompared with healthy controls with the same genotype (Independent samples t test).
Table 4. Multivariate linear regression of plasma brain-derived neurotrophic factor concentration with related factors.
| Healthy Controls | Heroin-Dependent Patients | |||
|---|---|---|---|---|
| Related Factors | β | p | β | p |
| Gender | 0.124 | 0.299 | −0.166 | 0.054 |
| Age | 0.105 | 0.313 | −0.049 | 0.624 |
| Body weight | 0.171 | 0.149 | 0.204 | 0.013 |
| BDNF Met/Val gene | −0.035 | 0.735 | 0.035 | 0.645 |
| Oral methadone dose at visit | − | − | −0.004 | 0.969 |
| Plasma methadone concentration | − | − | 0.038 | 0.697 |
| Blood platelet counts | − | − | 0.379 | <0.0001 |
| Heroin-dependency duration (years) | − | − | −0.180 | 0.046 |
Dependent Variable: Plasma BDNF.
BDNF, brain-derived neurotrophic factor.
Figure 1. Scatter plot of plasma BDNF levels with the number of years of heroin use.
(A) Representative linear regression of plasma BDNF levels with the number of years of heroin use. (B) Representative plasma BDNF levels with the number of years of heroin use. Patients were divided into 3 three groups: ≤5 years, 6–15 years, ≥16 years.
In addition, the duration of the heroin dependency was significantly correlated with blood platelet counts (Pearson correlation coefficient = −0.168, p = 0.03) and plasma methadone levels (Pearson correlation coefficient = 0.162, p = 0.035).
The study had a power of approximately 0.30 to detect a small effect, and 0.99–1 to detect medium and large effects in genotype frequencies (N = 274) for patient and control groups. For allele frequency, the study had a power of 0.38 to detect small effect, and 0.99–1.00 to detect medium and large effects. For independent samples t tests, the study had a power of 0.36 to detect a small effect, and 0.98–1.00 to detect medium and large effect when comparing heroin dependent patients with controls. For linear regression analysis, the study had a power of 0.17 to detect a small effect, 0.88 to detect medium effect, and 0.99 to detect large effect in the control group; while a power of 0.19 to detect a small effect, 0.97 to detect medium effect, and 0.99 to detect large effect in the heroin dependent group. In this power analysis, the effect-size conventions were determined according to Bunchner et al. (1996)17 as follows: small effect size = 0.10, medium effect size = 0.30, large effect size = 0.50 for the χ2 test; small effect size = 0.20, medium effect size = 0.50, large effect size = 0.80 for the independent t test; and small effect size = 0.02, medium effect size = 0.15, large effect size = 0.35 for the linear regression model (alpha = 0.05).
Discussion
We found lower plasma BDNF levels in heroin-dependent patients than in healthy controls. In long-term heroin-dependent patients, plasma BDNF levels were negatively correlated with the number of years they had used heroin. Serum BDNF is stored in human platelets and released by the stimulation of its agonists thrombin, collagen, the Ca2+ ionophore, and shear stress18. In humans, plasma levels represent free circulating BDNF18; however, the cellular sources of BDNF found in human plasma are not clearly defined. Possible sources of plasma BDNF are vascular endothelial and smooth muscle cells19. Activated macrophages or lymphocytes could be the sources of plasma BDNF20. Since BDNF is known to cross the blood–brain barrier, a substantial part of circulating BDNF might originate from neurons and glia cells of the central nervous system21,22. Thus, differences in BDNF levels in plasma may partially represent the variable BDNF secretion in the human brain. In the present study, we found that plasma BDNF was significantly negatively correlated with the duration of heroin dependency and significantly positively correlated with blood platelet counts. Thus, long-term heroin use might affect peripheral and neuronal cell activity. The neuronal cell or peripheral platelets affected by long-term heroin use might contribute to the downregulation of plasma BDNF.
BDNF can cross the blood-brain barrier21 and is involved in neuron survival, differentiation23, synaptogenesis, and maintenance24. Lower serum or plasma BDNF protein levels in adult humans might reflect the age-related white-matter atrophy25 and the degree of their neuronal degeneration in Alzheimer's disease26,27,28. In addition, plasma BDNF levels are significantly positively correlated with cerebral spinal fluid (CSF) BDNF levels in depressed patients29, which indicates that plasma BDNF levels might represent the central nervous system's BDNF expression profile. Although Heberlein et al. (2011)10 reported that serum BDNF levels were significantly higher in “opiate-dependent” patients, higher levels of serum BDNF were found in patients during heroin withdrawal11. However, our results were similar to those of studies which reported that chronic opioid dependency was associated with reduced serum concentrations of BDNF in human heroin users9. In rodents, chronic treatment with morphine reduced both peripheral and brain BDNF levels8. Moreover, prenatal exposure to opioids reduced BDNF precursor levels in the rat brain and impaired radial-arm-maze performance30. Thus, long-term opioid use compromises the expression of peripheral and central BDNF proteins and is involved in neural plasticity. These findings also indicate that peripheral and central BDNF regulates some functions of the adult brain: in particular, responses to opioid dependence. The downregulation of peripheral BDNF levels in long-term heroin-dependent patients represents the possible downregulation of central nervous system BDNF and its effect on opioid dependency.
Substance-use disorder had been postulated to be highly associated with dysfunction of the prefrontal cortex, which is involved with self-control and drug addiction31. Moreover, chronic opioid treatment substantially reduces the number of dopaminergic neurons in the ventral tegmental area of rats32. In humans, postmortem analysis has shown a certain degree of neuronal degeneration in the central nervous systems of heroin-dependent individuals33; thus, chronic opioid use may damage neurons that are associated with drug-seeking behavior or that exaggerate drug-seeking behavior. Thus, neuronal degeneration is important when studying substance use, abuse, and dependence. The downregulation of plasma BDNF after long-term heroin use might indicate a lesser neuronal protective effect and a certain degree of neuronal degeneration in heroin-dependent patients. However, the role of plasma BDNF in opioid dependence and neuronal protection requires further study.
We also found that, in addition to the duration of heroin use, body weight (β = 0.204, p = 0.013) was significantly correlated with the plasma BDNF levels in heroin-dependent patients but not in healthy controls. Pillai et al.29 reported that changes in BDNF levels were significantly positively correlated with body weight. In the present study, however, the healthy control and heroin-dependent patients had similar body weight profiles (67.2 ± 13.2 vs. 66.6 ± 12.6 kg). Therefore, the duration of heroin use might be more important for determining the level of plasma BDNF expression.
All heroin-dependent patients were undergoing MMT before the present study, but there was no significant correlation between plasma BDNF their oral methadone dose or plasma methadone level. However, there was a significant correlation between the duration of heroin use and the plasma methadone level (Pearson correlation coefficient = 0.162, p = 0.035). Our data represent the possible tolerance effect of long-term opioid use. Because most of the heroin-dependent patients were on MMT before the study began, the effect of long-term MMT on plasma BDNF levels needs to be examined in the future study.
In the genotype analysis, there was no significant difference in the distribution of the BDNF Val66Met (G196A) variant between the healthy controls and the heroin-dependent patients, nor was there a significant difference in the distribution of the BDNF Met and Val allele frequency between groups. However, other studies have associated the BDNF Val66Met variants with heroin-use disorder13,14,16,34. A recent meta-analysis demonstrates that Val alley was a risk factor for heroin dependence16. In Han Chinese, higher Val carrier frequency was found in heroin abuser13,34. The Met-alley confers a 21% protective effect compared with the Val/Val genotype in substance-related disorders14. Val carriers had an early onset of heroin uses34. However, Cheng et al. (2005) showed that the Val carrier had a later onset of heroin abuse compared with the Met allele carriers13. Because of the controversial results of BDNF Val66Met variants in heroin dependence, the correlation of BDNF Val66Met gene variants in heroin-dependent patients requires additional study.
To the best of our knowledge, this is the first investigation that compares plasma BDNF levels with the BDNF Val66Met SNP in heroin-dependent patients. The human BDNF gene is located on chromosome 11p14.1 and encodes a 247-amino acid proprotein that is proteolytically cleaved to form the 120-amino acid mature protein, which is 100% conserved between mice, rats, pigs, and humans35. The SNP in the BDNF gene leads to a G-to-A substitution at nucleotide 196, which results in a substitution of valine (Val) to methionine (Met) (Val66Met, rs6265) at codon 66 of the pro domain of BDNF. Although this BDNF polymorphism does not affect mature BDNF protein function, it does dramatically alter the intracellular trafficking and packaging of pro-BDNF, and reduces the trafficking and secretion of mature BDNF protein36. However, in this study, we found no significant differences in or correlations between different BDNF Val66Met SNP genotypes and mature BDNF levels in the plasma of our patients and healthy controls, which indicated that the BDNF Val66Met gene polymorphism did not affect their plasma BDNF levels. This finding supported our previous finding7 of no significant differences in or correlations between the plasma BDNF levels of healthy controls, patients with bipolar disorder, and patients with schizophrenia and carrying different BDNF Val66Met SNP genotypes. The downregulation of plasma BDNF levels in heroin-dependent patients might be due to the effects of long-term heroin use. Taking these findings together, we hypothesized that the downregulation of plasma BDNF in heroin-dependent patients was not associated with the BDNF Val66Met SNP genotype but with how long the patient had been using heroin.
Our study has some limitations. Although plasma BDNF might cross the blood-brain barrier and might correlate with brain BDNF expression, actual brain BDNF expression and its effect on heroin-dependent patients require additional study to draw stronger conclusions. BDNF levels in CSF might be required to confirm our findings. However, collecting CSF for this purpose presents an ethical problem. Noninvasive methods of measuring brain BDNF and brain function need to be developed.
In conclusion, we found different plasma BDNF expression profiles in healthy controls and heroin-dependent patients. The level of plasma BDNF was not affected by the BDNF Val66Met variants, but by how long the patient had been using heroin. The downregulation of plasma BDNF might indicate less neuronal protection and a certain degree of neuronal degeneration in long-term heroin users.
Methods
Participants
The research protocol was approved by the Institutional Review Board for the Protection of Human Subjects at National Cheng Kung University Hospital, and the methods were carried out in accordance with the approved guidelines. All study procedures were fully explained to the participants, and each provided a signed informed consent before the study began. To minimize the effect of ethnic differences on gene frequencies, only participants who were unrelated Han Chinese residing in Taiwan were recruited.
From the Department of Psychiatry at National Cheng Kung University Hospital, we enrolled 274 Taiwanese participants: 102 healthy controls and 172 long-term heroin users. The Chinese version of the Mini International Neuropsychiatric Interview (MINI), which has good reliability and validity37, was used to screen their psychiatric conditions to confirm that they met the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV (American Psychiatric Association 2000) diagnostic criteria for opioid dependence, and to rule out other major and minor mental illnesses, including alcohol abuse/dependency disorder, and illicit substance (other than heroin) use disorders. All healthy controls were free of any major and minor mental illness.
The following were the exclusion criteria for heroin-dependent patients: [1] pregnancy; [2] any major mental illness other than heroin dependency; and [3] any clinically significant and poorly controlled comorbidity, e.g., diabetes mellitus, hypertension, chronic kidney disease, etc.
The healthy control group included 102 volunteers recruited from the hospital staff and community. They were first screened by telephone, and those with a sleep disorder, a substance abuse disorder, mood swings, or possible symptoms of psychosis were excluded. The remaining volunteers were then carefully screened in face-to-face interviews with one of our research team's psychiatrists. The Chinese version of the Schedule for Affective Disorders and Schizophrenia Lifetime version (SADS-L) was used to screen the psychiatric conditions of the controls. All of them were free of present and past major and minor mental illnesses (schizophrenia; affective, anxiety personality; alcohol abuse/dependence; and illegal substance use disorders). Additionally, none of the first-degree relatives of the healthy controls had a history of psychiatric disorder.
Collecting and assessing blood samples
Ten milliliters of whole blood was drawn from the antecubital vein of each study participant and collected in Vacutainer blood collection tubes (Becton, Dickinson, Franklin Lakes, NJ, USA) with K2EDTA as the anticoagulant. Plasma was isolated from the whole blood after it had been centrifuged at 1000 × g for 15 min at 4°C and then immediately stored at −80°C. Plasma BDNF levels were quantified using enzyme-linked immunosorbent assays (ELISA). A kit (Quantikine Human Cytokine Kit; R&D Systems, Minneapolis, MN, USA) and an ELISA reader (SpectraMax-M2; Molecular Devices, Sunnyvale, CA, USA) were used to analyze plasma BDNF levels.
BDNF Genotyping
DNA was extracted from venous blood using a salting method. The G196A (Val66Met) BDNF gene polymorphism was examined using a polymerase chain reaction (PCR) plus restriction fragment length polymorphism analysis. The following primers were used:
Forward: 5′-AGA GGC TTG ACA TCA TTG GCT-3′;
Reverse: 5′-CGT GTA CAA GTC TGC GTC CT-3′.
DNA fragments were amplified in an Eppendorf thermal cycler and then digested overnight by PmlI restriction endonuclease (Eco72I; MBI Fermentas, Thermo Fisher Scientific, Waltham, MA, USA). The digestion products were separated on a 4% agarose gel and visualized using ethidium bromide staining. Band size was determined using a DNA ladder (O'Range Ruler 50 bp; MBI Fermentas). The undigested PCR product was 113 bp (allele A). Allele G consisted of digested bands of 78 and 35 bp.
Statistical analysis
A χ2 test was used for categorical variables. Independent samples t tests and analyses of covariance (ANCOVA) were used for statistical evaluations. Data are means ± standard deviation (SD). To determine whether the plasma BDNF levels were significantly correlated with the genotype and related factors, linear regression models were used. SPSS 17.0 for Windows was used for statistical computations. Significance was set at p < 0.05.
Author Contributions
S.L.C. and R.B.L. wrote the first draft. S.L.C., S.H.C. and C.H.C. managed the laboratory work and statistics. S.Y.L., Y.H.C., T.Y.W., P.S.C. and Y.K.Y. managed participant recruitment. J.S.H. supervised this work and edited the manuscript. All authors reviewed the manuscript.
Acknowledgments
This study was supported in part by grants NSC98-2314-B-006-022-MY3 (to RBL) and NSC101-2314-B-006-063-MY3 (to RBL) from the Taiwan National Science Council, and by a grant from the National Cheng Kung University Project to Promote Academic Excellence and Develop a World Class Research Center.
References
- Mintzer M. Z. & Stitzer M. L. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend 67, 41–51 (2002). [DOI] [PubMed] [Google Scholar]
- Prosser J., London E. D. & Galynker I. I. Sustained attention in patients receiving and abstinent following methadone maintenance treatment for opiate dependence: performance and neuroimaging results. Drug Alcohol Depend 104, 228–240, 10.1016/j.drugalcdep.2009.04.022 (2009). [DOI] [PubMed] [Google Scholar]
- Lu G. et al. Chronic morphine treatment impaired hippocampal long-term potentiation and spatial memory via accumulation of extracellular adenosine acting on adenosine A1 receptors. J Neurosci 30, 5058–5070, 10.1523/JNEUROSCI.0148-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4, 299–309, 10.1038/nrn1078 (2003). [DOI] [PubMed] [Google Scholar]
- Thoenen H., Zafra F., Hengerer B. & Lindholm D. The synthesis of nerve growth factor and brain-derived neurotrophic factor in hippocampal and cortical neurons is regulated by specific transmitter systems. Ann N Y Acad Sci 640, 86–90 (1991). [DOI] [PubMed] [Google Scholar]
- Alderson R. F., Alterman A. L., Barde Y. A. & Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron 5, 297–306 (1990). [DOI] [PubMed] [Google Scholar]
- Chen S. L. et al. The BDNF Val66Met polymorphism and plasma brain-derived neurotrophic factor levels in Han Chinese patients with bipolar disorder and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 51, 99–104, 10.1016/j.pnpbp.2014.01.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L. et al. Low-dose memantine attenuated morphine addictive behavior through its anti-inflammation and neurotrophic effects in rats. J Neuroimmune Pharmacol 7, 444–453, 10.1007/s11481-011-9337-9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F. et al. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J Psychopharmacol 21, 820–825 (2007). [DOI] [PubMed] [Google Scholar]
- Heberlein A. et al. Serum levels of BDNF are associated with craving in opiate-dependent patients. J Psychopharmacol 25, 1480–1484, 10.1177/0269881111411332 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Increased serum brain-derived neurotrophic factor levels during opiate withdrawal. Neurosci Lett 571, 61–65, 10.1016/j.neulet.2014.04.048 (2014). [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H. et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science 324, 1732–1734, 10.1126/science.1168501 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y. et al. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Brain Res Mol Brain Res 140, 86–90 (2005). [DOI] [PubMed] [Google Scholar]
- Gratacos M. et al. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry 61, 911–922 (2007). [DOI] [PubMed] [Google Scholar]
- Greenwald M. K., Steinmiller C. L., Sliwerska E., Lundahl L. & Burmeister M. BDNF Val(66)Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addict Biol 18, 836–845, 10.1111/j.1369-1600.2011.00431.x (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerian B. S. BDNF rs6265 polymorphism and drug addiction: a systematic review and meta-analysis. Pharmacogenomics 14, 2055–2065, 10.2217/pgs.13.217 (2013). [DOI] [PubMed] [Google Scholar]
- G-power: A Priori, Post Hoc, and Compromise Power Analyses for the Macintosh, v. Version 2.1.1. (University of Trier, Trier, Germany, 1996).
- Fujimura H. et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 87, 728–734 (2002). [PubMed] [Google Scholar]
- Nakahashi T. et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett 470, 113–117 (2000). [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M. et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189, 865–870 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Banks W. A., Fasold M. B., Bluth J. & Kastin A. J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37, 1553–1561 (1998). [DOI] [PubMed] [Google Scholar]
- Karege F., Schwald M. & Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 328, 261–264 (2002). [DOI] [PubMed] [Google Scholar]
- Mizuno K., Carnahan J. & Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 165, 243–256 (1994). [DOI] [PubMed] [Google Scholar]
- Ohira K. & Hayashi M. A New Aspect of the TrkB Signaling Pathway in Neural Plasticity. Curr Neuropharmacol 7, 276–285 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I. et al. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One 7, e35217, 10.1371/journal.pone.0035217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C. et al. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm 113, 1217–1224 (2006). [DOI] [PubMed] [Google Scholar]
- Diniz B. S. & Teixeira A. L. Brain-derived neurotrophic factor and Alzheimer's disease: physiopathology and beyond. Neuromolecular Med 13, 217–222, 10.1007/s12017-011-8154-x (2011). [DOI] [PubMed] [Google Scholar]
- Platenik J. et al. GSK3beta, CREB, and BDNF in peripheral blood of patients with Alzheimer's disease and depression. Prog Neuropsychopharmacol Biol Psychiatry 50, 83–93, 10.1016/j.pnpbp.2013.12.001 (2014). [DOI] [PubMed] [Google Scholar]
- Pillai A. et al. Plasma BDNF levels vary in relation to body weight in females. PLoS One 7, e39358, 10.1371/journal.pone.0039358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott L. M., Franklin L. M. & Serrano P. A. Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training. Brain Res 1198, 132–140 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. Z. & Volkow N. D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12, 652–669, 10.1038/nrn3119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklair-Tavron L. et al. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A 93, 11202–11207 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. et al. Postmortem changes in the central nervous system and adrenal medulla of the heroin addicts. Int J Neurosci 115, 1443–1449, 10.1080/00207450590956549 (2005). [DOI] [PubMed] [Google Scholar]
- Jia W. et al. Polymorphisms of brain-derived neurotrophic factor associated with heroin dependence. Neurosci Lett 495, 221–224, 10.1016/j.neulet.2011.03.072 (2011). [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C. et al. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics 10, 558–568 (1991). [DOI] [PubMed] [Google Scholar]
- Egan M. F. et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 (2003). [DOI] [PubMed] [Google Scholar]
- Huang S. Y. et al. Possible interaction of alcohol dehydrogenase and aldehyde dehydrogenase genes with the dopamine D2 receptor gene in anxiety-depressive alcohol dependence. Alcohol Clin Exp Res 28, 374–384 (2004). [DOI] [PubMed] [Google Scholar]