Abstract
The emerging pathogens Candida palmioleophila, Candida fermentati, and Debaryomyces nepalensis are often misidentified as Candida guilliermondii or Candida famata in the clinical laboratory. Due to the significant differences in antifungal susceptibilities and epidemiologies among these closely related species, a lot of studies have focused on the identification of these emerging yeast species in clinical specimens. Nevertheless, limited tools are currently available for their discrimination. Here, two new molecular approaches were established to distinguish these closely related species. The first approach differentiates these species by use of restriction fragment length polymorphism analysis of partial internal transcribed spacer 2 (ITS2) and large subunit ribosomal DNA with the enzymes BsaHI and XbaI in a double digestion. The second method involves a multiplex PCR based on the intron size differences of RPL18, a gene coding for a protein component of the large (60S) ribosomal subunit, and species-specific amplification. These two methods worked well in differentiation of these closely related yeast species and have the potential to serve as effective molecular tools suitable for laboratory diagnoses and epidemiological studies.
INTRODUCTION
Newly emerging species that are closely related to the common Candida species pose a challenge to conventional methods performed in the clinical laboratory (1–4). These closely related species actually belong to diverse species complexes, as revealed by sequence and phylogenetic analyses. Recent advances in molecular techniques have allowed differentiation of these species complexes, such as the Candida albicans complex composed of C. albicans, Candida dubliniensis, Candida africana, and Candida stellatoidea type I, the Candida parapsilosis complex composed of C. parapsilosis, Candida orthopsilosis, Candida metapsilosis, and Lodderomyces elongisporus, and the Candida glabrata complex composed of C. glabrata, Candida nivariensis, and Candida bracarensis (1, 5–17).
Candida guilliermondii (the anamorph of Pichia guilliermondii), a species with decreased susceptibility to fluconazole and echinocandins, was reported as a common cause of candidiasis and sometimes even candidemia (18–21). Candida fermentati (the anamorph of Pichia caribbica), an emerging species that is very closely related to C. guilliermondii, has often been misidentified as C. guilliermondii using routine identification methods (22–26). Because of lower susceptibility to triazoles, Candida palmioleophila, which is often misidentified as Candida famata (the anamorph of Debaryomyces hansenii) or C. guilliermondii, has been emphasized in recent studies (24, 25, 27). Additionally, Debaryomyces nepalensis and Debaryomyces fabryi, two species that are closely related to C. famata, have been isolated from clinical samples, including blood (22, 28, 29), and also are potential pathogens. The above-mentioned yeast species are likely to be confused with one another by conventional identification methods. In particular, isolates confused with C. famata were frequently misidentified, and C. famata was, in fact, very rare in clinical specimens (22, 24, 25, 27).
Currently, limited tools are available for molecular differentiation of these closely related yeast species. Molecular methods such as PCR-restriction fragment length polymorphism (RFLP), real-time PCR, Luminex techniques, electrophoretic karyotyping, and PCR with type-specific primers have been developed to distinguish some of these species, but other species were not involved in those studies (30–33). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) is a promising technique that works well to distinguish yeast species, although a few closely related species (such as C. fermentati) may still be misidentified (34–36).
Because the internal transcribed spacer (ITS) regions (ITS1, 5.8S, and ITS2) and the D1 and D2 regions of the large ribosomal subunit were confirmed as the most useful targets for species-level identification of yeasts (37–39), these regions were sequenced for these closely related yeast species, for further RFLP analysis. In addition, PCR analyses based on intron size differences or intron loss were used to easily differentiate closely related yeast species in previous studies (9, 16, 40, 41). Because ribosomal protein-coding genes carry longer introns than nonribosomal protein-coding genes (16, 42), the RPL18 gene, coding for a protein component of the large (60S) ribosomal subunit, was chosen, and a long intron within this gene was characterized for D. hansenii type strain CBS767 (GenBank accession no. NC_006048; locus tag DEHA2F16566g). Differences in intron sizes among these closely related species were analyzed for further multiplex PCR assays. In this study, two PCR-based methods were established to distinguish C. guilliermondii complex, C. famata complex, and the closely related species C. palmioleophila; both of them are simple, inexpensive, and reliable molecular tools.
MATERIALS AND METHODS
Strains and identification.
One hundred six strains, including 46 C. guilliermondii isolates, 19 C. famata isolates, 17 C. palmioleophila isolates, 11 C. fermentati isolates, 8 D. nepalensis isolates, and 5 D. fabryi isolates, were included in this study. Among them, 29 were type strains from the CBS and NRRL culture collections and 77 were clinical isolates, of which 27 were kindly provided by other researchers (see Acknowledgments) and 50 were from our own collection. Genomic DNA was extracted with the MasterPure yeast DNA purification kit (Epicentre Biotechnologies, Madison, WI), according to the manufacturer's instructions. All isolates were identified and confirmed by sequencing of internal transcribed spacers 1 and 2, including the 5.8S ribosomal DNA (rDNA) (ITS) region, using primers ITS1 and ITS4 (Table 1), as described previously (40). In order to distinguish among D. fabryi, D. nepalensis, and C. famata, strains identified as C. famata with ITS sequencing were further analyzed by amplification and sequencing of the intergenic spacer 1 (IGS1) region using primes IGS1F and IGS1R (Table 1). Species identification was determined by comparison of the DNA sequences of PCR products with corresponding sequences of the type strains using the BLASTN tool online. Additionally, other common pathogenic Candida species, including C. albicans, C. glabrata, Candida tropicalis, C. parapsilosis, Candida krusei, and Candida lusitaniae, were tested as controls.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′ to 3′) | Position | Purpose |
|---|---|---|---|
| ITS1 | TCCGTAGGTGAACCTGCGG | 18S rDNA | ITS sequencing |
| ITS4 | TCCTCCGCTTATTGATATGC | 26S rDNA | ITS sequencing |
| IGS1F | TGTAAGCAGTAGAGTAGCCTTGTTG | 26S rDNA | IGS1 sequencing |
| IGS1R | AGACCGAGTAGTGTAGTGGGAGAC | 5S rDNA | IGS1 sequencing |
| ITS2F | GATGTATTAGGTTTATCCAACTCGT | ITS2 rDNA | PCR-RFLP assay |
| 26SR | TCATTTCAACCCCAATACCTC | 26S rDNA | PCR-RFLP assay |
| DG5F | GCCCTCCTTCTTAGCTCGTAWGTAT | RPL18 gene | Multiplex PCR assay |
| DG5R | GGCAGATGACCTTGTTGAATGG | RPL18 gene | Multiplex PCR assay |
| GuF | TGCTATATCTTTGGCTCAGCG | IGS1 rDNA | Multiplex PCR assay |
| GuR | GTCGTCTAGCATTGGTTTTGACT | IGS1 rDNA | Multiplex PCR assay |
| PalF | GCGGCGAATTGTTATTTAATACT | ITS1 rDNA | Multiplex PCR assay |
| PalR | GTGAATGCACTTCTCAGCGTC | ITS2 rDNA | Multiplex PCR assay |
Sequence and phylogenetic analyses of ITS region.
Sequence alignments were conducted using ClustalW2. Phylogenetic analysis based on the ITS sequences was performed by using MEGA 6.05 software to clarify the genetic relationships among these closely related species. A total of 76 selected sequences together with 10 sequences available from GenBank were included in the analysis. A dendrogram was produced by use of neighbor-joining analysis using a Kimura 2-parameter model. Gaps were treated as pairwise deletions. Statistical support for each clade was assessed using bootstrap analysis with 500 replicates. The sequence from Saccharomyces cerevisiae type strain ATCC MYA-4900 was used to root the tree.
PCR-RFLP analysis of partial ITS2 and 26S rDNA.
Primers specific for partial ITS2 and 26S rDNA, including the D1/D2 region, of the C. guilliermondii complex, the C. famata complex, and C. palmioleophila were designed based on the consensus nucleotide sequences of the ITS2 and 26S rDNA regions, respectively, of the reference strains. PCR was performed in a final volume of 50 μl containing 50 ng DNA, 1× PCR buffer with 2 mM MgSO4, 0.2 mM (each) dATP, dCTP, dGTP, and dTTP, 0.2 μM each primer, and 2.5 U of Taq polymerase. PCR was performed in a Bio-Rad thermal cycler, with initial denaturation at 94°C for 5 min, 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 50 s, and final extension at 72°C for 8 min. PCR products were separated on a 1.0% (wt/vol) agarose gel at 140 V for 20 min. Amplicons were doubly digested with FastDigest enzymes BsaHI and XbaI for RFLP analysis. Restriction digestions were performed according to the manufacturer's instructions (Fermentas, Vilnius, Lithuania), and the reaction mixtures were incubated at 37°C for 30 min before separation on a 2.0% (wt/vol) agarose gel at 90 V for 50 min.
Multiplex PCR assay based on RPL18 gene, ITS, and IGS1 region.
The homologous sequences of the RPL18 gene in the reference strains C. guilliermondii Y-324, C. fermentati Y-27403, C. palmioleophila Y-17323, D. fabryi CBS796, and D. nepalensis CBS2334 were cloned and sequenced. Then, intron lengths of the RPL18 gene were calculated as 182 bp for C. guilliermondii, 191 bp for C. fermentati, 391 bp for C. palmioleophila, 472 bp for C. famata, 473 bp for D. fabryi, and 401 bp for D. nepalensis. Specific primers for amplification of the RPL18 gene of the C. guilliermondii complex, the C. famata complex, and C. palmioleophila, DG5F and DG5R, were designed based on the consensus nucleotide sequences of the RPL18 gene orthologs of the reference strains. C. guilliermondii-specific primers GuF and GuR were designed based on the IGS1 region of rDNA, and the C. palmioleophila-specific primer pair of PalF and PalR was designed based on the ITS region of rDNA. A multiplex PCR with the six primers was established and performed in a final volume of 50 μl containing 50 ng DNA, 1× PCR buffer with 2 mM MgSO4, 0.2 mM (each) dATP, dCTP, dGTP, and dTTP, 0.2 μM each primer, and 2.5 U of Taq polymerase. PCR was performed in a Bio-Rad thermal cycler, with initial denaturation at 94°C for 5 min, 30 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s, and final extension at 72°C for 7 min. PCR products were separated on a 2.0% (wt/vol) agarose gel at 100 V for 1 h. All PCRs were conducted in duplicate. The primers used are listed in Table 1.
Nucleotide sequence accession numbers.
The sequences of the RPL18 gene fragments of the reference strains were deposited in GenBank under accession numbers KJ705079 to KJ705083. The ITS and partial 26S rDNA sequences obtained in this study were deposited in GenBank under accession numbers KJ705003 to KJ705078.
RESULTS
Sequence alignment and phylogenetic analysis based on ITS region.
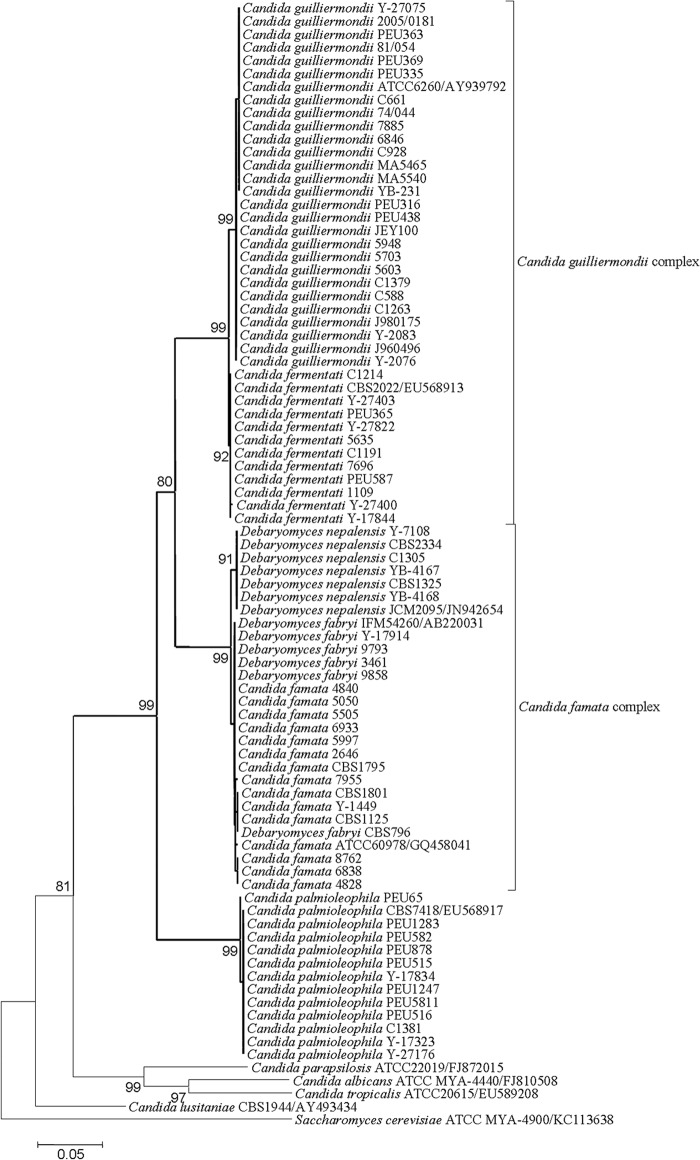
The ITS sequences of the C. guilliermondii group and the C. fermentati group were 99% identical, showing only four distinguishable nucleotide differences. The ITS sequences of the C. famata group and the D. nepalensis group were 99% identical, showing five distinguishable nucleotide differences. The ITS sequences of the C. famata group and the D. fabryi group were 99% to 100% identical, showing no discernible nucleotide differences. Phylogenetic analysis showed that C. famata, D. fabryi, and D. nepalensis constitute one cluster, which has a sibling relationship with the cluster composed of C. guilliermondii and C. fermentati. The C. palmioleophila clade clustered basal to the aforementioned species with strong bootstrap support (Fig. 1).
FIG 1.
Neighbor-joining tree for Candida strains determined through analysis of ITS sequences. The Saccharomyces cerevisiae type strain was used as an outgroup. Bootstrap values of >70% are indicated for the main branches. Clusters containing the C. famata complex, the C. guilliermondii complex, and C. palmioleophila are shown in bold type.
Molecular identification using PCR-RFLP analysis.
PCR using the primer set ITS2F-26SR yielded about 1.1-kb amplicons for strains of the C. guilliermondii complex, the C. famata complex, and C. palmioleophila, whereas no amplicon was produced from the other Candida species tested. In concordance with the in silico analysis, RFLP patterns of amplification products from the C. guilliermondii, C. fermentati, C. palmioleophila, C. famata, D. fabryi, and D. nepalensis strains showed 3 bands (822 bp, 212 bp, and 76 bp), 2 bands (822 bp and 288 bp), 2 bands (660 bp and 446 bp), 3 bands (447 bp, 375 bp, and 290 bp), 3 bands (447 bp, 375 bp, and 290 bp), and 4 bands (447 bp, 375 bp, 214 bp, and 76 bp), respectively (Fig. 2a). All included isolates belonging to the C. guilliermondii complex, the C. famata complex, or C. palmioleophila were correctly identified by using this analysis.
FIG 2.
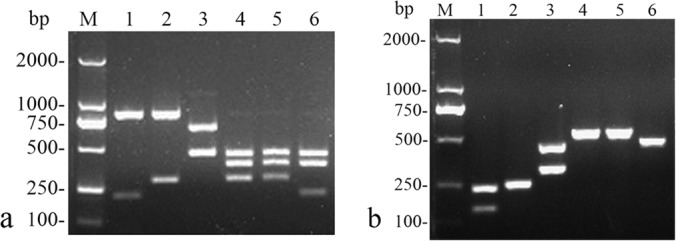
Agarose gel electrophoresis of enzyme-digested products (a) and amplicons from the multiplex PCR assay (b). Lanes M, DL2000 ladder; lanes 1, CBS2030 (C. guilliermondii); lanes 2, Y-27403 (C. fermentati); lanes 3, Y-17323 (C. palmioleophila); lanes 4, CBS1795 (C. famata); lanes 5, CBS796 (D. fabryi); lanes 6, CBS2334 (D. nepalensis). Bands of <100 bp in the RFLP analysis are not shown.
Molecular determination by multiplex PCR assay.
The multiplex PCR assay resulted in amplified products of approximately 235 bp and 151 bp for C. guilliermondii, 244 bp for C. fermentati, 444 bp and 317 bp for C. palmioleophila, 525 bp for C. famata, 526 bp for D. fabryi, and 454 bp for D. nepalensis (Fig. 2b). Negative results were obtained for the other Candida species tested. All isolates belonging to the C. guilliermondii complex, the C. famata complex, or C. palmioleophila that were included herein were also successfully identified by use of this assay.
DISCUSSION
Accurate identification of clinical isolates closely related to C. guilliermondii or C. famata has clinical importance because of the remarkably diverse susceptibility profiles of these species (22–24). Because these genetically related species are difficult to identify by conventional methods, molecular techniques are more suitable for their differentiation (24, 30). ITS sequence analysis has been widely used as the gold standard for yeast species identification and for phylogenetic analysis (24, 25, 38). In this study, D. nepalensis exhibits little divergence from D. hansenii based on ITS sequences, like the C. guilliermondii complex. So, we propose that D. nepalensis may be a member of the C. famata complex. D. fabryi was proposed as a species instead of D. hansenii var. fabryi in several studies (32, 42). In the present study, however, no distinguishable nucleotide difference within the ITS region was found to distinguish this species from D. hansenii. Similarly, the PCR-RFLP and multiplex PCR assays developed here did not distinguish between them and, in the latter analysis, no marked difference in intron lengths within the RPL18 gene was observed between the two species. According to the above-mentioned molecular evidence, we think that the nomination of D. fabryi needs to be reevaluated. C. guilliermondii and C. fermentati are very closely related species and constitute the C. guilliermondii complex; slight intron differences in the RPL18 gene were observed between them, similar to the observations for another two closely related Candida species, i.e., C. orthopsilosis and C. metapsilosis, published previously (9). Thus, a C. guilliermondii-specific primer pair targeting the IGS1 region, which shows extensive sequence divergence between C. guilliermondii and C. fermentati, was designed to further differentiate C. guilliermondii from C. fermentati in the multiplex PCR assay (32). Similarly, a C. palmioleophila-specific primer pair was designed to distinguish C. palmioleophila from the other species, because the ITS sequence of C. palmioleophila is highly divergent from those of the C. guilliermondii complex and the C. famata complex.
In the present study, PCR analysis based on intron length differences combined with species-specific amplification, in which fewer primers were used than in regular multiplex PCR, successfully identified these closely related species. PCR-RFLP analysis based on two hypervariable parts of the rDNA region that are most commonly used for yeast species identification also works well for differentiation. Results from the two molecular assays developed herein are exactly consistent with the results of ITS sequencing, thus providing two rapid reliable methods for laboratory identification of clinically relevant species closely related to C. guilliermondii and C. famata.
ACKNOWLEDGMENTS
We thank James Swezey (ARS Culture Collection, United States), Donna MacCallum (University of Aberdeen, Aberdeen, United Kingdom), Oliver Bader (University Medical Centre Göttingen, Göttingen, Germany), László Majoros (University of Debrecen, Debrecen, Hungary), Frederic Dalle (Laboratoire de Parasitologie-Mycologie, CHU Dijon, Dijon, France), Dominique Sanglard and Jamel Eddouzi (University Hospital Lausanne and University Hospital Center, Lausanne, Switzerland), Elisa Borghi (Università degli Studi di Milano, Milan, Italy), and Jozef Nosek (Comenius University, Bratislava, Slovak Republic) for generously contributing strains for this study.
This work was supported by grants from the National Key Basic Research Program of China (grants 2013CB531601 and 2013CB531606), the Major Infectious Disease Fund (grant 2013ZX10004612), the Shanghai Science and Technology Commission Fund (grant 10dz2220100), and the Shanghai Key Laboratory of Molecular Medical Mycology Fund (grant 14DZ2272900).
Footnotes
Published ahead of print 20 June 2014
REFERENCES
- 1.Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284–292. 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockhart SR, Messer SA, Gherna M, Bishop JA, Merz WG, Pfaller MA, Diekema DJ. 2009. Identification of Candida nivariensis and Candida bracarensis in a large global collection of Candida glabrata isolates: comparison to the literature. J. Clin. Microbiol. 47:1216–1217. 10.1128/JCM.02315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romeo O, Criseo G. 2009. Molecular epidemiology of Candida albicans and its closely related yeasts Candida dubliniensis and Candida africana. J. Clin. Microbiol. 47:212–214. 10.1128/JCM.01540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavanti A, Davidson AD, Fordyce MJ, Gow NA, Maiden MC, Odds FC. 2005. Population structure and properties of Candida albicans, as determined by multilocus sequence typing. J. Clin. Microbiol. 43:5601–5613. 10.1128/JCM.43.11.5601-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman AM, Linton CJ, Oliver D, Palmer MD, Szekely A, Odds FC, Johnson EM. 2009. Pyrosequencing analysis of 20 nucleotides of internal transcribed spacer 2 discriminates Candida parapsilosis, Candida metapsilosis, and Candida orthopsilosis. J. Clin. Microbiol. 47:2307–2310. 10.1128/JCM.00240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Effron G, Canton E, Peman J, Dilger A, Roma E, Perlin DS. 2011. Assessment of two new molecular methods for identification of Candida parapsilosis sensu lato species. J. Clin. Microbiol. 49:3257–3261. 10.1128/JCM.00508-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souza AC, Ferreira RC, Goncalves SS, Quindos G, Eraso E, Bizerra FC, Briones MR, Colombo AL. 2012. Accurate identification of Candida parapsilosis (sensu lato) by use of mitochondrial DNA and real-time PCR. J. Clin. Microbiol. 50:2310–2314. 10.1128/JCM.00303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prandini TH, Theodoro RC, Bruder-Nascimento AC, Scheel CM, Bagagli E. 2013. Analysis of inteins in the Candida parapsilosis complex for simple and accurate species identification. J. Clin. Microbiol. 51:2830–2836. 10.1128/JCM.00981-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng X, Wu Z, Ling B, Pan S, Liao W, Pan W, Yao Z. 2014. Identification and differentiation of Candida parapsilosis complex species by use of exon-primed intron-crossing PCR. J. Clin. Microbiol. 52:1758–1761. 10.1128/JCM.00105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borman AM, Szekely A, Linton CJ, Palmer MD, Brown P, Johnson EM. 2013. Epidemiology, antifungal susceptibility and pathogenicity of Candida africana isolates from the United Kingdom. J. Clin. Microbiol. 51:967–972. 10.1128/JCM.02816-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romeo O, Racco C, Criseo G. 2006. Amplification of the hyphal wall protein 1 gene to distinguish Candida albicans from Candida dubliniensis. J. Clin. Microbiol. 44:2590–2592. 10.1128/JCM.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeo O, Criseo G. 2011. Candida africana and its closest relatives. Mycoses 54:475–486. 10.1111/j.1439-0507.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 13.Romeo O, Criseo G. 2008. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagn. Microbiol. Infect. Dis. 62:230–233. 10.1016/j.diagmicrobio.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Paredes K, Sutton DA, Cano J, Fothergill AW, Lawhon SD, Zhang S, Watkins JP, Guarro J. 2012. Molecular identification and antifungal susceptibility testing of clinical isolates of the Candida rugosa species complex and proposal of the new species Candida neorugosa. J. Clin. Microbiol. 50:2397–2403. 10.1128/JCM.00688-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cendejas-Bueno E, Kolecka A, Alastruey-Izquierdo A, Theelen B, Groenewald M, Kostrzewa M, Cuenca-Estrella M, Gomez-Lopez A, Boekhout T. 2012. Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii group I), C. duobushaemulonii sp. nov. (C. haemulonii group II), and C. haemulonii var. vulnera var. nov.: three multiresistant human pathogenic yeasts. J. Clin. Microbiol. 50:3641–3651. 10.1128/JCM.02248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enache-Angoulvant A, Guitard J, Grenouillet F, Martin T, Durrens P, Fairhead C, Hennequin C. 2011. Rapid discrimination between Candida glabrata, Candida nivariensis, and Candida bracarensis by use of a singleplex PCR. J. Clin. Microbiol. 49:3375–3379. 10.1128/JCM.00688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romeo O, Scordino F, Pernice I, Lo PC, Criseo G. 2009. A multiplex PCR protocol for rapid identification of Candida glabrata and its phylogenetically related species Candida nivariensis and Candida bracarensis. J. Microbiol. Methods 79:117–120. 10.1016/j.mimet.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA. 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48:1366–1377. 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller MA, Diekema DJ, Mendez M, Kibbler C, Erzsebet P, Chang SC, Gibbs DL, Newell VA. 2006. Candida guilliermondii, an opportunistic fungal pathogen with decreased susceptibility to fluconazole: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J. Clin. Microbiol. 44:3551–3556. 10.1128/JCM.00865-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang TP, Ho MW, Yang YL, Lo PC, Lin PS, Wang AH, Lo HJ. 2013. Distribution and drug susceptibilities of Candida species causing candidemia from a medical center in central Taiwan. J. Infect. Chemother. 19:1065–1071. 10.1007/s10156-013-0623-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Liu Y, Feng X, Liu Y, Wang S, Zhu X, Chen Q, Pan S. 2014. Candidemia: incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. Int. J. Infect. Dis. 22:4–8. 10.1016/j.ijid.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Castanheira M, Woosley LN, Diekema DJ, Jones RN, Pfaller MA. 2013. Candida guilliermondii and other species of Candida misidentified as Candida famata: assessment by Vitek 2, DNA sequencing analysis, and matrix-assisted laser desorption ionization–time of flight mass spectrometry in two global antifungal surveillance programs. J. Clin. Microbiol. 51:117–124. 10.1128/JCM.01686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. 2009. Identification and susceptibility profile of Candida fermentati from a worldwide collection of Candida guilliermondii clinical isolates. J. Clin. Microbiol. 47:242–244. 10.1128/JCM.01889-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen RH, Arendrup MC. 2011. Candida palmioleophila: characterization of a previously overlooked pathogen and its unique susceptibility profile in comparison with five related species. J. Clin. Microbiol. 49:549–556. 10.1128/JCM.02071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desnos-Ollivier M, Ragon M, Robert V, Raoux D, Gantier JC, Dromer F. 2008. Debaryomyces hansenii (Candida famata), a rare human fungal pathogen often misidentified as Pichia guilliermondii (Candida guilliermondii). J. Clin. Microbiol. 46:3237–3242. 10.1128/JCM.01451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umamaheswari K, Menon T. 2008. Candida fermentati from HIV patients in Chennai, South India. Int. J. Infect. Dis. 12:e153–e154. 10.1016/j.ijid.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Eddouzi J, Lohberger A, Vogne C, Manai M, Sanglard D. 2013. Identification and antifungal susceptibility of a large collection of yeast strains isolated in Tunisian hospitals. Med. Mycol. 51:737–746. 10.3109/13693786.2013.800239. [DOI] [PubMed] [Google Scholar]
- 28.Moretti A, Fukushima K, Takizawa K, Suzuki M, Vidotto V, Cannizzo FT, Boncio L, Bollo E. 2007. First report of oral colonization by Debaryomyces nepalensis in a dog. Mycopathologia 164:189–192. 10.1007/s11046-007-9044-5. [DOI] [PubMed] [Google Scholar]
- 29.Garner CD, Starr JK, McDonough PL, Altier C. 2010. Molecular identification of veterinary yeast isolates by use of sequence-based analysis of the D1/D2 region of the large ribosomal subunit. J. Clin. Microbiol. 48:2140–2146. 10.1128/JCM.02306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romi W, Keisam S, Ahmed G, Jeyaram K. 2014. Reliable differentiation of Meyerozyma guilliermondii from Meyerozyma caribbica by internal transcribed spacer restriction fingerprinting. BMC Microbiol. 14:52. 10.1186/1471-2180-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mota AJ, Back-Brito GN, Nobrega FG. 2012. Molecular identification of Pichia guilliermondii, Debaryomyces hansenii and Candida palmioleophila. Genet. Mol. Biol. 35:122–125. 10.1590/S1415-47572011005000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen HV, Gaillardin C, Neuveglise C. 2009. Differentiation of Debaryomyces hansenii and Candida famata by rRNA gene intergenic spacer fingerprinting and reassessment of phylogenetic relationships among D. hansenii, C. famata, D. fabryi, C. flareri (=D. subglobosus) and D. prosopidis: description of D. vietnamensis sp. nov. closely related to D. nepalensis. FEMS Yeast Res. 9:641–662. 10.1111/j.1567-1364.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa A, Sugita T, Shinoda T. 1999. Rapid identification of Debaryomyces hansenii/Candida famata by polymerase chain reaction. Med. Mycol. 37:101–104. 10.1080/02681219980000161. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix C, Gicquel A, Sendid B, Meyer J, Accoceberry I, Francois N, Morio F, Desoubeaux G, Chandenier J, Kauffmann-Lacroix C, Hennequin C, Guitard J, Nassif X, Bougnoux ME. 2014. Evaluation of two matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) systems for the identification of Candida species. Clin. Microbiol. Infect. 20:153–158. 10.1111/1469-0691.12210. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Xiao M, Wang H, Gao R, Fan X, Brown M, Gray TJ, Kong F, Xu YC. 2014. Yeast identification algorithm based on use of the Vitek MS system selectively supplemented with ribosomal DNA sequencing: proposal of a reference assay for invasive fungal surveillance programs in China. J. Clin. Microbiol. 52:572–577. 10.1128/JCM.02543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohmann C, Sabou M, Moussaoui W, Prevost G, Delarbre JM, Candolfi E, Gravet A, Letscher-Bru V. 2013. Comparison between the Biflex III-Biotyper and the Axima-SARAMIS systems for yeast identification by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 51:1231–1236. 10.1128/JCM.03268-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linton CJ, Borman AM, Cheung G, Holmes AD, Szekely A, Palmer MD, Bridge PD, Campbell CK, Johnson EM. 2007. Molecular identification of unusual pathogenic yeast isolates by large ribosomal subunit gene sequencing: 2 years of experience at the United Kingdom Mycology Reference Laboratory. J. Clin. Microbiol. 45:1152–1158. 10.1128/JCM.02061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leaw SN, Chang HC, Sun HF, Barton R, Bouchara JP, Chang TC. 2006. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 44:693–699. 10.1128/JCM.44.3.693-699.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borman AM, Linton CJ, Oliver D, Palmer MD, Szekely A, Johnson EM. 2010. Rapid molecular identification of pathogenic yeasts by pyrosequencing analysis of 35 nucleotides of internal transcribed spacer 2. J. Clin. Microbiol. 48:3648–3653. 10.1128/JCM.01071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng X, Fu X, Ling B, Wang L, Liao W, Yao Z. 2013. Development of a singleplex PCR assay for rapid identification and differentiation of Cryptococcus neoformans var. grubii, Cryptococcus neoformans var. neoformans, Cryptococcus gattii, and hybrids. J. Clin. Microbiol. 51:1920–1923. 10.1128/JCM.00064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng X, Fu X, Ling B, Wang L, Liao W, Pan W, Yao Z. 2013. Rapid differentiation of cryptic species within Cryptococcus gattii by a duplex PCR assay. J. Clin. Microbiol. 51:3110–3112. 10.1128/JCM.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacques N, Mallet S, Casaregola S. 2009. Delimitation of the species of the Debaryomyces hansenii complex by intron sequence analysis. Int. J. Syst. Evol. Microbiol. 59:1242–1251. 10.1099/ijs.0.004325-0. [DOI] [PubMed] [Google Scholar]