Abstract
Acinetobacter baumannii has emerged as one of the leading pathogens causing hospital-acquired infection. The success of A. baumannii as a pathogen has to a large extent been attributed to its capacity to remodel its genome. Several major epidemic clonal complexes of A. baumannii spread across different health care facilities around the world, each of which contains a subset of diversified strains. However, little is known about the population dynamics during colonization of A. baumannii within hosts. Here, whole-genome sequencing was used to analyze population dynamics of A. baumannii strains isolated from a group of patients at different time points as well as from different sites of a particular patient. Seven out of nine of the sampled A. baumannii strains belonged to the international clone II (CC92 clonal complex). While the A. baumannii strains were found to be stable in three patients, there was a change of A. baumannii strains in one patient. Comparative genomic analysis revealed that the accessory genome of these strains contained a large set of virulence-encoding genes and these virulence factors might play a role in determining population dynamics. Microscale genome modification has been revealed by analysis of single nucleotide polymorphisms (SNPs) between A. baumannii strains isolated from the same patient. Parallel evolutionary traits have been observed during genome diversification when A. baumannii colonize in different patients. Our study suggested that both antibiotic usage and host environment might impose selective forces that drive the rapid adaptive evolution in colonizing A. baumannii.
INTRODUCTION
Hospital-acquired infection (HAI) is one of the major threats to public health nowadays and is a significant cause of morbidity and mortality (1). The majority of HAIs are caused by a few groups of bacterial species, including Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii, which are commonly found in hospital environments (2). Significant increases in antimicrobial resistance have occurred for all of the dominant species causing HAIs, which further complicates therapeutic strategies (2, 3). Surveillance and control strategies in prevention and treatment of HAIs rely profoundly on current understanding of the epidemiology of the main cause of infection (4).
Major epidemic clonal complexes of the bacterial species were observed in HAIs and played important roles in causing outbreaks and spreading resistance (5, 6). These clonal complexes seem to successfully evolve from specific hospital and host environments, and understanding their genomes and physiologies can greatly facilitate development of efficient treatment strategies for HAIs. For example, the emerging strain Clostridium difficile NAP1/027 clonal complex produces higher levels of peak median (interquartile range [IQR]) toxin A and toxin B than other clonal complexes (7). Whole-genome sequencing has greatly facilitated the current understanding of macroscale evolution of HAI-associated pathogens, where a representative subset of strains belonging to the same clonal complex of a pathogen was isolated and compared (8–10). These studies revealed that a large number of recombination and horizontal gene transfer events are actively shaping the genomes of the pathogens. More importantly, parallel evolution has been observed during the colonization of pathogens within different hosts and can be used as a marker to study host-pathogen interactions (8–10).
A. baumannii has emerged as the third most common pathogen of HAIs and has evolved resistance toward most of the available antibiotics (11, 12). Multiple-drug-resistant (MDR) A. baumannii isolates were reported to reside in a large proportion of their human hosts for extended periods of time (up to 42 months posttreatment) (13). The long-term carriage of MDR A. baumannii by these individuals might lead to dissemination to other hospitals. Internationally, there are several epidemic clonal complexes of A. baumannii (e.g., the international clones I, II, and III) spreading across different health care facilities, and each clonal complex contains a subset of diversified strains (14–16). Comparative genomic analysis revealed that A. baumannii outbreaks at hospitals might be polyclonal and provided evidence for gene transfer and genome reorganization among different frequent genotypes (17–21). Understanding the genome diversification within a subset of closely related strains from the same clonal complex and their population dynamics during colonization provides insight for evolution and adaptation of bacterial pathogens (22, 23). We used comparative genomics to investigate a group of closely associated A. baumannii strains isolated from several patients in a hospital of Guangxi Province, China. Pairs of A. baumannii strains were sampled from multiple patients, and comparative genomic analysis revealed both macroscale and microscale population dynamics.
MATERIALS AND METHODS
Isolate collection and preliminary analysis.
Ethical approval for the carriage study was obtained from the First Affiliated Hospital of Guangxi Medical University (reference no. 2012 [KY-E-010]). A. baumannii isolates were isolated from clinical specimens. Bacteria identification and susceptibility tests were initially performed using automatic bioanalysis with Vitek-2 and Kirby-Bauer (disk diffusion assay) methods. Two individual A. baumannii colonies of each sample were picked and stored in a freezer at −80°C. Bacterial genome DNA was isolated, and randomly amplified polymorphic DNA (RAPD)-PCR typing was carried out using the primers M13 (5′-GAGGGTGGCGGTTCT-3′) and DAF4 (5′-CGGCAGCGCC-3′) as previously described (24).
Antimicrobial susceptibility assay.
Susceptibilities of the A. baumannii strains to aminoglycosides (amikacin, gentamicin, and tobramycin), penicillins (ampicillin and piperacillin), carbapenems (imipenem), cephalosporins (cefazolin, cefotiam, cefepime, ceftazidime, ceftriaxone, and sulperazon [cefoperazone plus sulbactam]), monobactams (aztreonam),quinolones (ciprofloxacin and levofloxacin), nitrofurantoin, penicillins plus β-lactamase inhibitors (ampicillin-sulbactam), and folate pathway inhibitors (sulfamethoxazole-trimethoprim [SMZ-TMP]) were evaluated by precisely determining the MIC according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (25).
Sequencing, assembly, annotation, and typing.
Whole-genome DNA of the A. baumannii strains was purified using a Wizard genomic DNA purification kit (Promega) and sequenced on an Illumina HiSeq 2500 platform generating 101-bp-long paired-end reads. The Cutadapt tool (http://code.google.com/p/cutadapt/) was used to remove adapters and low-quality bases. Velvet (26) was used to assemble reads into contigs de novo for each genome. Average insert sizes were 340 (LY1 to LY6) or 375 (LY7 to LY9) nucleotides, and the average genomic coverage depths were 261- to 336-fold. The genome sequence files were submitted to the Rapid Annotations using Subsystem Technology (RAST) server (27) for bacterial genome annotation. The A. baumannii genome sequences were submitted to the ResFinder database (28), with a 98% threshold for identification of genes involved in antibiotic resistance. Multilocus sequence typing (MLST) of different A. baumannii strains was performed using the MLST server (http://cge.cbs.dtu.dk/services/MLST/) (29). Both PubMLST typing and Pasteur's MLST typing were performed by using the MLST server. e-BURST analyses were performed based on sequence types (STs) identified from both the PubMLST typing and the Pasteur's MLST typing methods by using the goeBURST 1.2.1 program (30). eBURST was performed using 6 as the minimum identical loci for the definition of a clonal complex (CC) and 3 as the minimum single-locus variants. CCs were named according to the number of the predicted founder ST except for CC92, which has been well defined in the literature. If no founder ST was predicted by eBURST, the CC was named for the first ST assigned.
Detection and filtering of nucleotide differences.
Nucleotide differences were generated from the CLC Genomics Workbench 6.5 (CLC bio). Paired-end reads in FASTQ format of different genomes were first mapped against the A. baumannii ACICU genome (GenBank accession number NC_010611) and then further compared. This list of SNPs was then filtered to remove those SNPs that were likely to have been caused by alignment or sequencing errors by setting a probability score of ≥90%.
Whole-genome alignments and phylogenetic tree construction.
The genomes of the nine sequenced A. baumannii clinical isolates were first compared to the genomes of 16 A. baumannii strains available from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database by using Progressive Mauve (31). Multiple genome alignment was used to compare the genomes of the seven sequenced A. baumannii clinical isolates (LY3 to LY9) that belong to the international clone II by using Progressive Mauve. Phylogenetic tree diagrams were prepared using the software FigTree version 1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/).
Pan/core genomic analysis.
Core and pan-genomes were calculated using BLAST with cutoffs of 50% identity and 50% coverage of the longest gene. Curves for the pan-genome and core genome were generated by the CMG-biotools package (32). The identified core and accessory proteins were assigned to clusters of orthologous groups (COGs) and their abundances were compared statistically as described in Rodriguez-Brito et al. (33) and Allen et al. (34) using a subsample size of 500 and 1,000 bootstrap replicates at a statistical confidence of 99%. The identified core and accessory proteins were BLASTP searched against the Virulence Factors Database (VFDB) of pathogenic bacteria (35) to identify virulence genes by using Bio-Edit (Ibis Biosciences, Carlsbad, CA, USA) (http://www.mbio.ncsu.edu/bioedit/bioedit.html) (minimum 50% identity with an E value of <1e−5).
Nucleotide sequence accession numbers.
The whole-genome shotgun bioproject for A. baumannii LY1 to LY9 has been deposited at DDBJ/EMBL/GenBank under the accession numbers JDSV00000000, JDSW00000000, JDSX00000000, JDSY00000000, JDSZ00000000, JDTA00000000, JDTB00000000, JDTC00000000, and JDTD00000000, respectively.
RESULTS AND DISCUSSION
Population dynamics of A. baumannii during patient colonization.
Nine representative A. baumannii isolates were collected from a group of patients who had been admitted into the intensive care units (ICUs) of the First Affiliated Hospital of Guangxi Medical University from March to April 2013 (Fig. 1A). LY1 and LY2 were isolated sequentially with a 10-day interval from sputum samples of a pneumonia patient (patient 1). LY3 and LY4 were isolated sequentially with a 4-day interval from sputum samples of an acute pulmonary thromboembolism patient (patient 2). LY5 and LY6 were isolated sequentially with a 16-day interval from sputum samples of a chronic obstructive pulmonary disease (COPD) patient (patient 3). LY7, LY8, and LY9 were isolated sequentially with a 14-day interval from a wound surface and a central venous catheter of a burn patient (patient 4). RAPD-PCR analysis showed that several of these A. baumannii isolates might have come from a clonal complex (see Fig. S1 in the supplemental material), and this might be a good case for studying the population dynamics during host colonization.
FIG 1.
PubMLST typing of A. baumannii clinical isolates (A) and eBURST analysis of the STs from the A. baumannii MLST databases (http://pubmlst.org/abaumannii/) (B). (C and D) Partial snapshots (red circles in B) of two A. baumannii clonal complexes. Groups are formed by linking all STs that are single-locus variants (SLVs) and are commonly denoted as clonal complexes. Light green color indicates group founder; dark green, subgroup founder; light blue, common node. ST types of A. baumannii LY1 to LY9 were highlighted in panels C and D by red underlining. LY4 (ST208), LY5 (ST208), and LY9 (ST208) belong to the founder of the largest A. baumannii clonal complex (CC92). LY3 (ST195), LY6 (ST457), LY7 (ST457), and LY8 (ST457) also belong to CC92. LY1 (ST373) and LY2 (ST373) belong to another A. baumannii clonal complex where ST17 is the founder.
We then used an Illumina HiSeq 2500 platform to sequence the genomes of nine A. baumannii isolates. The general characteristics of the genomes of A. baumannii clinical isolates LY1 to LY9 can be found in Table S1 in the supplemental material. To track the origin of the nine A. baumannii isolates, multilocus sequence typing (MLST) types of these isolates were analyzed by using both PubMLST (Bartual's) scheme and Pasteur's MLST scheme followed by eBURST analysis (see the Materials and Methods). The PubMLST scheme gave good discrimination of the nine A. baumannii isolates by showing that these isolates belonged to four different ST types (ST373, ST195, ST208, and ST457) (Fig. 1A). The Pasteur's MLST scheme gave less discrimination of the nine A. baumannii isolates and showed that these isolates belonged to two different ST types (ST2 and ST40) (see Table S2 and Fig. S2 in the supplemental material). eBURST analysis based on the PubMLST scheme showed that the nine A. baumannii isolates belonged to two clonal complexes (CC92 and CC17 in the red circles in Fig. 1B). LY4, LY5, and LY9 belonged to the founder (ST208) of A. baumannii clonal complex CC92, which corresponds to the well-known international clone II (36) (Fig. 1C). LY3 (ST195), LY6 (ST457), LY7 (ST457), and LY8 (ST457) also belong to the A. baumannii clonal complex CC92 (Fig. 1C). Together with findings from a recent study, our data suggest that ST208 represents an emerging lineage in China (37). LY1 (ST373) and LY2 (ST373) belong to another A. baumannii clonal complex, CC17, for which ST17 is the founder (Fig. 1D).
The nine sequenced A. baumannii genomes were compared with the genomes of 16 other A. baumannii strains (their genome sequences were downloaded from the NCBI FTP site) using Progressive Mauve. The phylogenetic tree based on the neighbor-joining algorithm showed that the genomes of the LY3 to LY9 strains were closely associated with each other and belonged to international clone II (Fig. 2). Multiple genome alignment was performed to compare the genomes of the LY3 to LY7 strains that belong to international clone II (Fig. 3). Together with the eBURST analysis, the phylogenetic tree and multiple genome alignment clearly showed that while there were several A. baumannii strains belonging to international clone II (CC92) in the hospital environment (perhaps the ICU where all the patients had stayed), the A. baumannii strains causing infections were relatively stable during hospitalization of patients. The representative A. baumannii strains isolated from different time points from three of the patients did not change (patients 1, 3, and 4). The LY3 (ST195) strain was replaced by LY4 (ST208) in patient 2 (Fig. 1).
FIG 2.
A phylogenetic tree showing the 9 A. baumannii clinical isolates in relation to 16 other A. baumannii strains. The international clonal groups are marked beside the phylogenetic tree. This phylogenetic tree was produced by pairwise genome comparisons by Progressive Mauve.
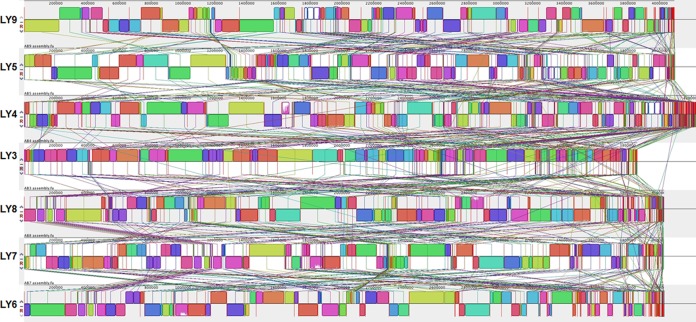
FIG 3.
Multiple genome alignment performed using Mauve software and the genomes of A. baumannii LY3 to LY9. Each genome is laid out horizontally with homologous segments (locally collinear blocks [LCBs]) outlined as colored rectangles. Regions inverted relative to LY9 are set below those that match in the forward orientation. Lines collate aligned segments between genomes. Average sequence similarities within an LCB, measured in sliding windows, are proportional to the heights of interior colored bars.
Role of antibiotic resistance on population dynamics during colonization.
To investigate the role of antibiotic resistance in population dynamics during colonization, we compared the antibiotic resistance profiles (see Table S3 in the supplemental material) of our A. baumannii strains and the antibiotic treatments the patients had received (see Table S4). The predicted antibiotic-resistant genes from LY3 to LY9 as well as several other international clone II A. baumannii strains are listed in Fig. 4. Genomic analysis and MIC assay showed that all of the LY3 to LY9 strains are multiple-drug-resistant (MDR) strains, with the ST208 strains (LY4, LY5, and LY9) being the most resistant ones (Fig. 4). The MDR phenotype to a large extent explained the success of the A. baumannii international II clone strains throughout the world. There is a good correlation between the presences of certain resistance genes and MIC measurement. LY5 and LY9 lacked the β-lactam-resistant blaTEM-1 gene (Fig. 4) compared to other LY strains and thus were more sensitive to ampicillin-sulbactam than were other LY strains (see Table S3 in the supplemental material). LY6, LY7, and LY8 lacked the ciprofloxacin-modifying enzyme aac(6′)-Ib-cr (Fig. 4) compared to other LY strains (except LY3) and thus were more sensitive to levofloxacin than were other LY strains (see Table S3). The later isolate LY4 from patient 2 was more resistant to amikacin and SMZ-TMP than the early isolate LY3 (see Table S3). However, patient 2 did not receive treatment using these two classes of antibiotics (see Table S4 in the supplemental material). Thus, there was no evidence indicating that antibiotic usage played a role in the strain replacement.
FIG 4.
Distribution of the antibiotic resistance genes present in the selected international clone II A. baumannii strains in this study.
Virulence factors might play a role in macroscale evolution of A. baumannii.
To further identify other factors that might contribute to the population dynamics during colonization, we have evaluated the pan-genome and core genome of the LY3 to LY9 strains together with eight fully sequenced A. baumannii strains from international clone II (Fig. 2). The results of all permutations of the order of addition for each of the seven genomes are presented in Fig. 5. As expected, the number of genes in the core genomes initially decreases and the number in the pan-genomes initially increases with the addition of each new sequence. Extrapolation of the curve indicates that the number of genes in the core genomes reaches a minimum of 2,895 and will remain relatively constant (Fig. 5). In accordance with the core-genome size, the pan-genome size reaches a maximum of 4,765 genes (Fig. 5). Interestingly, LY4 was found to contain many more accessory genes than other LY strains from our study (Fig. 5), which correlates well with its larger genome size compared to other LY strains (Fig. 3).
FIG 5.
Curves for the core genomes, pan-genomes, and numbers of new genes of A. baumannii LY3 to LY9 together with eight fully sequenced A. baumannii strains from international clone II.
Genes belonging to core and accessory genomes have been classified according to their predicted functional roles (Fig. 6). As expected, the vast majority of genes making up the core genome belong to the groups for amino acid metabolism and transport, coenzyme metabolism, lipid metabolism, energy production and conversion, and inorganic ion transport and metabolism. Compared to the accessory genome, the core is enriched in predicted proteins belonging to COG category C (energy production and conversion). The accessory genome is enriched in cell wall/membrane/envelop biogenesis (M), DNA replication and repair (L), and translation (J) categories (Fig. 6). Genes that encode antimicrobial resistance-associated enzymes and efflux pumps, transposon systems (23 out of 605 annotated), and phage components (46 out 605 annotated) are highly abundant in the accessory genome. This finding suggests that the majority of specific traits might be acquired by lateral gene transfer events.
FIG 6.
The identified core and accessory proteins were assigned to clusters of orthologous groups (COGs). The y axis indicates the percentage of genes in a specific function cluster. *, Statistical confidence of 99%.
To investigate whether virulence factors play a role in population dynamics during colonization, we predicted the virulence factors encoded by the core and accessory genomes by BLASTP, searching for them against the Virulence Factors Database (VFDB) of pathogenic bacteria (35). With a cutoff of a minimum 50% identity and with an E value of <1e−5, 33 and 32 virulence genes were identified from the core and accessory genomes of selected A. baumannii strains, respectively. The accessory genome of selected A. baumannii strains includes genes involved in fimbrial protein biosynthesis, polysaccharide biosynthesis, hemolysin synthesis, lipopolysaccharide biosynthesis, and clumping factor B biosynthesis (see Table S5 in the supplemental material). These virulence factors might determine the fitness and population dynamics of A. baumannii during colonization.
Microscale evolution of A. baumannii within host.
We analyzed the SNPs between the almost identical genomes of LY5 (patient 3) and LY9 (patient 3), the genomes of LY6 (patient 4, wound secretion) and LY7 (patient 4, wound secretion), and between the genomes of the LY6 (patient 4, wound secretion) and LY8 strains (patient 4, central venous catheter). We discovered 216 single nucleotide polymorphisms (SNPs) and short indels (see Table S6 in the supplemental material), compromising 141 synonymous, 56 nonsynonymous, and 19 intergenic SNPs between LY5 and LY9 (see Table S6). We discovered 157 SNPs and short indels (see Table S7) comprising 91 synonymous, 42 nonsynonymous, and 24 intergenic SNPs between LY6 and LY7 (see Table S7). A very interesting finding was that 122 SNPs and short indels (see Table S8) compromising 68 synonymous, 28 nonsynonymous, and 26 intergenic SNPs were discovered between LY6 and LY8 over a period of 13 days (see Table S8). Unlike LY7, which was isolated from the same location of a wound surface as LY6, LY8 was isolated from the central venous catheter of patient 4. There are fewer SNPs between LY6 and LY8 than SNPs between LY6 and LY7. Also, most of the SNPs between LY6 and LY8 are included in the SNPs between LY6 and LY7 (Table 1). This result showed that A. baumannii has a higher mutation rate in the infection site than on the catheter surface, where the biofilm mode of growth is normally employed by A. baumannii.
TABLE 1.
Comparison of mutations between LY6 and LY7 and LY6 and LY8
| CP000863 (CDS) (LY6 and LY7a) | Coding region change(s) | CP000863 (CDS) (LY6 and LY8) | Coding region change(s) |
|---|---|---|---|
| ACICU_00073 | ACC55385.1:c.675A>T | ACICU_00073 | ACC55385.1:c.675A>T |
| ACICU_00087 | ACC55399.1:c.87A>T | ACICU_00087 | ACC55399.1:c.87A>T |
| ACICU_00087 | ACC55399.1:c.104G>C | ACICU_00087 | ACC55399.1:c.65_66delCTinsAG |
| ACICU_00088 | ACC55400.1:c.202A>C | ACICU_00866 | ACC56178.1:c.119_120delGCinsCT |
| ACICU_00219 | ACC55531.1:c.236_237delGCinsCT | ACICU_00219 | ACC55531.1:c.236_237delGCinsCT |
| ACICU_00219 | ACC55531.1:c.231_232delCAinsTC | ACICU_00219 | ACC55531.1:c.231_232delCAinsTC |
| ACICU_01053 | ACC56365.1:c.288_289delTGinsGA | ACICU_01053 | ACC56365.1:c.288_289delTGinsGA |
| ACICU_01053 | ACC56365.1:c.291G>A | ACICU_01053 | ACC56365.1:c.291G>A |
| ACICU_01053 | ACC56365.1:c.298G>T | ACICU_01053 | ACC56365.1:c.298G>T |
| ACICU_01053 | ACC56365.1:c.303_304delAAinsGG | ACICU_01053 | ACC56365.1:c.303_304delAAinsGG |
| ACICU_01053 | ACC56365.1:c.315_316delTAinsCC | ACICU_01053 | ACC56365.1:c.315_316delTAinsCC |
| ACICU_01053 | ACC56365.1:c.318_319delAAinsGC | ACICU_01053 | ACC56365.1:c.318_319delAAinsGC |
| ACICU_01060 | ACC56372.1:c.3414G>C | ACICU_01060 | ACC56372.1:c.3429_3430delTGinsCA |
| ACICU_01060 | ACC56372.1:c.107T>A | ACICU_01060 | ACC56372.1:c.107T>A |
| ACICU_01061 | ACC56373.1:c.367T>G | ||
| ACICU_02165 | ACC57477.1:c.5938A>C | ||
| ACICU_02178 | ACC57490.1:c.1333T>A | ||
| ACICU_02178 | ACC57490.1:c.93A>T | ACICU_02178 | ACC57490.1:c.93A>T |
| ACICU_02180 | ACC57492.1:c.181T>A | ||
| ACICU_02180 | ACC57492.1:c.172C>T | ||
| ACICU_02215 | ACC57527.1:c.145C>A | ACICU_02215 | ACC57527.1:c.145C>A |
| ACICU_02223 | ACC57535.1:c.19_20delAAinsGG | ||
| ACICU_02244 | ACC57556.1:c.503C>G | ||
| ACICU_02711 | ACC58023.1:c.285_288delACAAinsCTTG | ACICU_02711 | ACC58023.1:c.285_288delACAAinsCTTG |
| ACICU_02711 | ACC58023.1:c.127C>T | ACICU_02711 | ACC58023.1:c.127C>T |
| ACICU_02711 | ACC58023.1:c.165A>T | ACICU_02711 | ACC58023.1:c.145_147delGGTinsAAC |
| ACICU_02711 | ACC58023.1:c.162_163delTGinsAA | ACICU_02711 | ACC58023.1:c.140_141delCTinsTC |
| ACICU_02711 | ACC58023.1:c.160G>A | ACICU_02711 | ACC58023.1:c.206A>G |
| ACICU_02712 | ACC58024.1:c.525_526delTTinsCG | ||
| ACICU_02712 | ACC58024.1:c.420C>A | ||
| ACICU_02712 | ACC58024.1:c.526G>T | ||
| ACICU_02938 | ACC58250.1:c.8delA | ACICU_02938 | ACC58250.1:c.8delA |
| ACICU_02945 | ACC58257.1:c.201_211insC | ACICU_02945 | ACC58257.1:c.201_211insC |
| ACICU_03412 | ACC58722.1:c.897_898delTGinsAC | ||
| ACICU_03412 | ACC58722.1:c.881C>G | ||
| ACICU_03412 | ACC58722.1:c.873A>T | ||
| ACICU_03412 | ACC58722.1:c.213_215delACCinsTTT | ||
| ACICU_03412 | ACC58722.1:c.206T>A | ACICU_03437 | ACC58746.1:c.388T>C |
| ACICU_03412 | ACC58722.1:c.191G>C | ACICU_03563 | ACC58872.1:c.2853T>A |
| ACICU_03453 | ACC58762.1:c.450T>G | ACICU_03453 | ACC58762.1:c.450T>G |
| ACICU_03575 | ACC58884.1:c.132T>G | ACICU_03575 | ACC58884.1:c.132T>G |
| ACICU_03575 | ACC58884.1:c.125T>C | ACICU_03575 | ACC58884.1:c.125T>C |
The same mutations in two pairs are highlighted in bold type. CDS, coding sequence.
To investigate the selective forces acting on A. baumannii genomes, the rate of nonsynonymous substitutions (dN) relative to the rate of synonymous substitutions (dS) of the two subcolonies were calculated: dN/dS = 0.40 for LY5/LY9 and dN/dS = 0.46 for LY6/LY7, respectively. These results indicated that negative selection is prevalent in A. baumannii during short-term host colonization. The dN/dS = 0.41 for LY6/LY8, which is lower than the 0.46 of LY6/LY7, which showed that different infection sites might pose different selective pressure on microscale population dynamics.
Parallel evolution of A. baumannii occurs in vivo.
Independent evolution of similar traits by different lineages originating from similar ancestral conditions (parallel evolution) was previously reported to take place in other bacterial species during long-term colonization or laboratory evolution experiments (8, 38). Parallel evolution can be used to identify important biomarkers of chronic infections. We observed that parallel evolution has taken place during short-term in vivo colonization of the two subcolonies (Table 2).
TABLE 2.
Parallel evolutionary traits of A. baumannii clinical isolates revealed by mutation
| Mutation and isolates | Type | Product (CP000863 [CDS]) | Coding region change |
|---|---|---|---|
| ACICU_03412 | |||
| LY5 and LY9 | SNV | Putative silver efflux pump | ACC58722.1:c.979A>C |
| LY5 and LY9 | SNV | Putative silver efflux pump | ACC58722.1:c.976A>G |
| LY5 and LY9 | MNV | Putative silver efflux pump | ACC58722.1:c.971_972delCCinsAT |
| LY5 and LY9 | SNV | Putative silver efflux pump | ACC58722.1:c.964G>A |
| LY5 and LY9 | SNV | Putative silver efflux pump | ACC58722.1:c.958C>A |
| LY5 and LY9 | MNV | Putative silver efflux pump | ACC58722.1:c.951_952delAGinsTA |
| LY5 and LY9 | MNV | Putative silver efflux pump | ACC58722.1:c.945_948delCCGTinsTAAA |
| LY5 and LY9 | SNV | Putative silver efflux pump | ACC58722.1:c.910C>T |
| LY5 and LY9 | SNV | Putative silver efflux pump | ACC58722.1:c.191G>C |
| LY6 and LY7 | MNV | Putative silver efflux pump | ACC58722.1:c.897_898delTGinsAC |
| LY6 and LY7 | SNV | Putative silver efflux pump | ACC58722.1:c.881C>G |
| LY6 and LY7 | SNV | Putative silver efflux pump | ACC58722.1:c.873A>T |
| LY6 and LY7 | MNV | Putative silver efflux pump | ACC58722.1:c.213_215delACCinsTTT |
| LY6 and LY7 | SNV | Putative silver efflux pump | ACC58722.1:c.206T>A |
| LY6 and LY7 | SNV | Putative silver efflux pump | ACC58722.1:c.191G>C |
| ACICU_01060 | |||
| LY5 and LY9 | MNV | Phage-related minor tail protein | ACC56372.1:c.126_127delCAinsTG |
| LY5 and LY9 | SNV | Phage-related minor tail protein | ACC56372.1:c.2431A>T |
| LY6 and LY7 | SNV | Phage-related minor tail protein | ACC56372.1:c.3414G>C |
| LY6 and LY7 | SNV | Phage-related minor tail protein | ACC56372.1:c.107T>A |
| ACICU_02165 | |||
| LY5 and LY9 | SNV | Phage-related protein | ACC57477.1:c.5938A>C |
| LY5 and LY9 | SNV | Phage-related protein | ACC57477.1:c.10025C>G |
| LY5 and LY9 | MNV | Phage-related protein | ACC57477.1:c.10022_10023delGAinsTC |
| LY6 and LY7 | SNV | Phage-related protein | ACC57477.1:c.5938A>C |
SNV, single-nucleotide variant; MNV, multinucleotide variant.
The most obvious parallel evolution event is a mutation in the ACICU_03412 gene, which encodes a putative silver efflux pump, CzcA, in the two A. baumannii subcolonies (Table 2). Two genetic loci with high mutation rates were found in the ACICU_03412 gene (Table 2). Homologs of the ACICU_03412 gene exist widely in different bacterial species. It was reported that CzcA as well as other putative efflux transporters were induced by physiological concentrations of NaCl and might contribute to increased tolerance of A. baumannii to various antibiotics such as aminoglycosides, carbapenems, and colistin (39).
Another parallel evolution event is the mutation in genes encoding phage-related proteins. Mutations in the ACICU_01060 gene that encodes a phage-related minor tail protein were found in three A. baumannii lineages during short-term colonization (Table 2). Mutations were found in the ACICU_02165 gene that encodes phage-related proteins in subcolony I (LY5 and LY9) and subcolony IV (LY6 and LY7) (Table 2). Further investigations will be carried out to elucidate the impact of these mutations on the phage activities of A. baumannii.
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniela Drautz, Yap Zhei Hwee, and Rikky Wenang Purbojati for helping with the DNA sequencing experiments.
The genomic analysis part of the research is supported by the National Research Foundation and Ministry of Education Singapore under its Research Centre of Excellence Programme and a start-up grant (M4330002.C70) from Nanyang Technological University, Singapore. This work was supported by grants from the Danish Council for Strategic Research (M.G.). The clinical part of the research was supported by the National Natural Sciences Foundation of China (grant numbers 81260002 and 81260663) and the General Project of the Guangxi Education Department (grant numbers 200710MS156 and 201010LX039).
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 20 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00921-14.
REFERENCES
- 1.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. 2007. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaynes R, Edwards JR; National Nosocomial Infections Surveillance System. 2005. Overview of nosocomial infections caused by Gram-negative bacilli. Clin. Infect. Dis. 41:848–854. 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 3.Weber DJ, Raasch R, Rutala WA. 1999. Nosocomial infections in the ICU: the growing importance of antibiotic-resistant pathogens. Chest 115(3 Suppl):34S–41S. 10.1378/chest.115.suppl_1.34S. [DOI] [PubMed] [Google Scholar]
- 4.Struelens MJ. 1998. The epidemiology of antimicrobial resistance in hospital acquired infections: problems and possible solutions. BMJ 317:652–654. 10.1136/bmj.317.7159.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witte W, Cuny C, Klare I, Nubel U, Strommenger B, Werner G. 2008. Emergence and spread of antibiotic-resistant Gram-positive bacterial pathogens. Int. J. Med. Microbiol. 298:365–377. 10.1016/j.ijmm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Livermore DM. 2009. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 64(Suppl 1):i29–i36. 10.1093/jac/dkp255. [DOI] [PubMed] [Google Scholar]
- 7.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084. 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Jelsbak L, Marvig RL, Damkiaer S, Workman CT, Rau MH, Hansen SK, Folkesson A, Johansen HK, Ciofu O, Hoiby N, Sommer MO, Molin S. 2011. Evolutionary dynamics of bacteria in a human host environment. Proc. Natl. Acad. Sci. U. S. A. 108:7481–7486. 10.1073/pnas.1018249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He M, Sebaihia M, Lawley TD, Stabler RA, Dawson LF, Martin MJ, Holt KE, Seth-Smith HM, Quail MA, Rance R, Brooks K, Churcher C, Harris D, Bentley SD, Burrows C, Clark L, Corton C, Murray V, Rose G, Thurston S, van Tonder A, Walker D, Wren BW, Dougan G, Parkhill J. 2010. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc. Natl. Acad. Sci. U. S. A. 107:7527–7532. 10.1073/pnas.0914322107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snitkin ES, Zelazny AM, Montero CI, Stock F, Mijares L, Murray PR, Segre JA. 2011. Genome-wide recombination drives diversification of epidemic strains of Acinetobacter baumannii. Proc. Natl. Acad. Sci. U. S. A. 108:13758–13763. 10.1073/pnas.1104404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 46:1254–1263. 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 12.Tan SY, Chua SL, Liu Y, Hoiby N, Andersen LP, Givskov M, Song Z, Yang L. 2013. Comparative genomic analysis of rapid evolution of an extreme-drug-resistant Acinetobacter baumannii clone. Genome Biol. Evol. 5:807–818. 10.1093/gbe/evt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchaim D, Navon-Venezia S, Schwartz D, Tarabeia J, Fefer I, Schwaber MJ, Carmeli Y. 2007. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J. Clin. Microbiol. 45:1551–1555. 10.1128/JCM.02424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dessel H, Dijkshoorn L, van der Reijden T, Bakker N, Paauw A, van den Broek P, Verhoef J, Brisse S. 2004. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 155:105–112. 10.1016/j.resmic.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. 2013. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 41:11–19. 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Snitkin ES, Zelazny AM, Montero CI, Stock F, Mijares L, Program NCS, Murray PR, Segre JA. 2011. Genome-wide recombination drives diversification of epidemic strains of Acinetobacter baumannii. Proc. Natl. Acad. Sci. U. S. A. 108:13758–13763. 10.1073/pnas.1104404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio. 5:e00963–13. 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, MacDonald IJ, Martin KM, Russo T, Campagnari AA, Hujer AM, Bonomo RA, Gill SR. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053–8064. 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Nocera PP, Rocco F, Giannouli M, Triassi M, Zarrilli R. 2011. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol. 11:224. 10.1186/1471-2180-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahl JW, Gillece JD, Schupp JM, Waddell VG, Driebe EM, Engelthaler DM, Keim P. 2013. Evolution of a pathogen: a comparative genomics analysis identifies a genetic pathway to pathogenesis in Acinetobacter. PLoS One 8:e54287. 10.1371/journal.pone.0054287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molloy S. 2012. Bacterial evolution: parallel lives. Nat. Rev. Genet. 13:4. 10.1038/nrg3135. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Rau MH, Yang L, Hoiby N, Molin S, Jelsbak L. 2011. Bacterial adaptation during chronic infection revealed by independent component analysis of transcriptomic data. BMC Microbiol. 11:184. 10.1186/1471-2180-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanthi M, Sekar U, Sowmiya M, Malathi J, Kamalanathan A, Sekar B, Madhavan HN. 2013. Clonal diversity of New Delhi metallobetalactamase-1 producing Enterobacteriaceae in a tertiary care centre. Indian J. Med. Microbiol. 31:237–241. 10.4103/0255-0857.115627. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-S19 Clinical and Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 26.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67:2640–2644. 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 50:1355–1361. 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francisco AP, Bugalho M, Ramirez M, Carrico JA. 2009. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics 10:152. 10.1186/1471-2105-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vesth T, Lagesen K, Acar O, Ussery D. 2013. CMG-biotools, a free workbench for basic comparative microbial genomics. PLoS One 8:e60120. 10.1371/journal.pone.0060120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Brito B, Rohwer F, Edwards RA. 2006. An application of statistics to comparative metagenomics. BMC Bioinformatics 7:162. 10.1186/1471-2105-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen MA, Lauro FM, Williams TJ, Burg D, Siddiqui KS, De Francisci D, Chong KW, Pilak O, Chew HH, De Maere MZ, Ting L, Katrib M, Ng C, Sowers KR, Galperin MY, Anderson IJ, Ivanova N, Dalin E, Martinez M, Lapidus A, Hauser L, Land M, Thomas T, Cavicchioli R. 2009. The genome sequence of the psychrophilic archaeon, Methanococcoides burtonii: the role of genome evolution in cold adaptation. ISME J. 3:1012–1035. 10.1038/ismej.2009.45. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Xiong Z, Sun L, Yang J, Jin Q. 2012. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res. 40:D641–D645. 10.1093/nar/gkr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mugnier PD, Poirel L, Naas T, Nordmann P. 2010. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii. Emerg. Infect. Dis. 16:35–40. 10.3201/eid1601.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Qiao F, Yu R, Gao Y, Zong Z. 2013. Clonal diversity of Acinetobacter baumannii clinical isolates revealed by a snapshot study. BMC Microbiol. 13:234. 10.1186/1471-2180-13-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crozat E, Philippe N, Lenski RE, Geiselmann J, Schneider D. 2005. Long-term experimental evolution in Escherichia coli. XII. DNA topology as a key target of selection. Genetics 169:523–532. 10.1534/genetics.104.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hood MI, Jacobs AC, Sayood K, Dunman PM, Skaar EP. 2010. Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations. Antimicrob. Agents Chemother. 54:1029–1041. 10.1128/AAC.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.