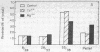Abstract
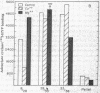
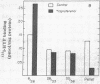
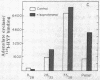
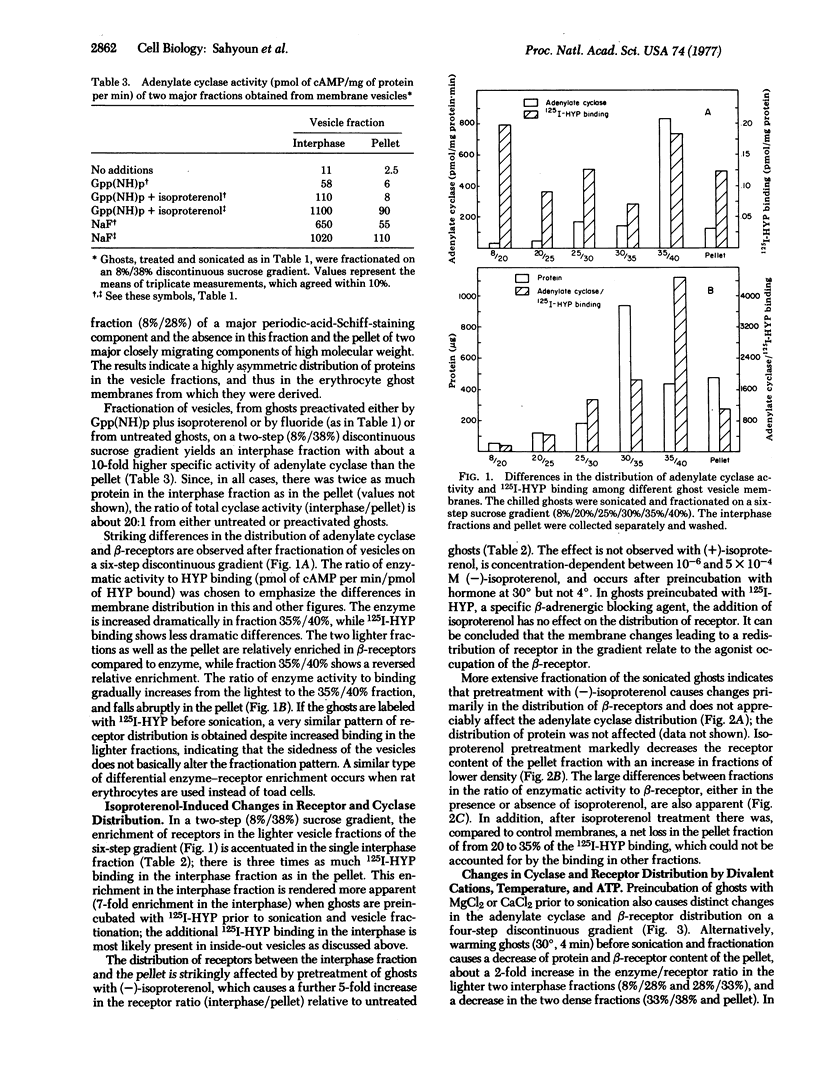
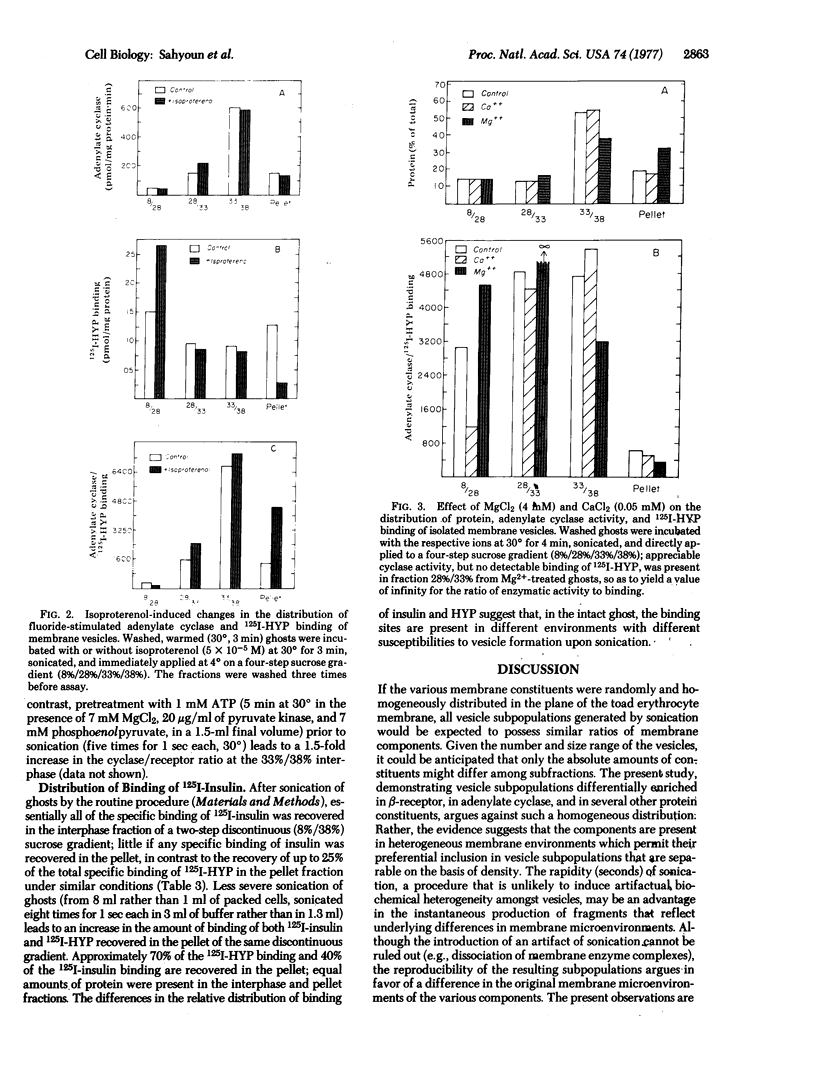
Brief sonication of whole erythrocyte plasma membranes (ghosts) from toads at 4° does not inactivate adenylate cyclase [ATP pyrophosphate-lyase (cyclizing); EC 4.6.1.1] or destroy the receptor binding properties of hydroxybenzylpindolol or insulin. The hormonal (but not the fluoride-induced) stimulation of this enzyme is, however, lost. Fractionation of the small, resealed membrane fragments (vesicles) on discontinuous sucrose gradients results in the separation of vesicle populations differing grossly in size and protein composition. In addition, the distribution of the β-adrenergic receptor, an insulin binding site, and adenylate cyclase among these vesicles fractions differs. The pattern of distribution of these functional structures can be altered differentially by manipulations of the ghosts before sonication. For example, brief preincubation with isoproterenol leads to a change in the relative distribution of β-receptor (but not adenylate cyclase) among the various vesicle fractions; this effect is not obtained with β-receptor antagonists, which block the isoproterenol effect. Exposure of the ghosts to different temperatures, changes in the divalent cation composition of the medium, or the addition of ATP also leads to changes in the distribution of surface markers of the subsequently formed vesicles. The results indicate gross asymmetries in the distribution of protein components within the plane of the membrane and raise important questions regarding the manner whereby functionally related and coupled components, such as hormone receptors and adenylate cyclase, interact.
Keywords: membrane vesicles, β-receptor-enzyme coupling, mobile receptor hypothesis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Cuatrecasas P. Irreversible activation of adenylate cyclase of toad erythrocyte plasma membrane by 5'-guanylylimidodiphosphate. J Membr Biol. 1976;27(3):207–232. doi: 10.1007/BF01869137. [DOI] [PubMed] [Google Scholar]
- Bennett V., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. J Membr Biol. 1975 Jun 3;22(1):29–52. doi: 10.1007/BF01868162. [DOI] [PubMed] [Google Scholar]
- Bennett V., Cuatrecasas P. Preparation of inverted plasma membrane vesicles from isolated adipocytes. Biochim Biophys Acta. 1973 Jul 6;311(3):362–380. doi: 10.1016/0005-2736(73)90316-7. [DOI] [PubMed] [Google Scholar]
- Bennett V., O'Keefe E., Cuatrecasaş P. Mechanism of action of cholera toxin and the mobile receptor theory of hormone receptor-adenylate cyclase interactions. Proc Natl Acad Sci U S A. 1975 Jan;72(1):33–37. doi: 10.1073/pnas.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., O'Keefe E., Cuatrecasaş P. Mechanism of action of cholera toxin and the mobile receptor theory of hormone receptor-adenylate cyclase interactions. Proc Natl Acad Sci U S A. 1975 Jan;72(1):33–37. doi: 10.1073/pnas.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975 Feb 28;187(4178):750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Bennett V., Cuatrecasas P. Membrane receptors as general markers for plasma membrane isolation procedures. The use of 125-I-labeled wheat germ agglutinin, insulin, and cholera toxin. J Biol Chem. 1975 Jan 25;250(2):488–500. [PubMed] [Google Scholar]
- Charness M. E., Bylund D. B., Beckman B. S., Hollenberg M. D., Snyder S. H. Independent variation of beta-adrenergic receptor binding and catecholamine-stimulated adenylate cyclase activity in rat erythrocytes. Life Sci. 1976 Jul 15;19(2):243–249. doi: 10.1016/0024-3205(76)90396-9. [DOI] [PubMed] [Google Scholar]
- Chevalier J., Bourguet J., Hugon J. S. Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res. 1974;152(2):129–140. doi: 10.1007/BF00224690. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Jacobs S., Bennett V. Activation of adenylate cyclase by phosphoramidate and phosphonate analogs of GTP: possible role of covalent enzyme-substrate intermediates in the mechanism of hormonal activation. Proc Natl Acad Sci U S A. 1975 May;72(5):1739–1743. doi: 10.1073/pnas.72.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- De Petris S., Raff M. C., Mallucci L. Ligand-induced redistribution of concanavalin A receptors on normal, trypsinized and transformed fibroblasts. Nat New Biol. 1973 Aug 29;244(139):275–278. doi: 10.1038/newbio244275a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Scott R. E. Modulation of the distribution of plasma membrane intramembranous particles in contact-inhibited and transformed cells. Biochim Biophys Acta. 1975 Aug 20;401(2):213–220. doi: 10.1016/0005-2736(75)90305-3. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Maguire M. E., Gilman A. G., Bourne H. R., Coffino P., Melmon K. L. Beta adrenergic receptors and adenylate cyclase: products of separate genes? Mol Pharmacol. 1976 Nov;12(6):1062–1069. [PubMed] [Google Scholar]
- Jacobs S., Bennett V., Cuatrecasas P. Kinetics of irreversible activation of adenylate cyclase of fat cell membranes by phosphonium and phosphoramidate analogs of gtp1. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):205–223. [PubMed] [Google Scholar]
- Knutton S., Sumner M. C., Pasternak C. A. Role of microvilli in surface changes of synchronized P815Y mastocytoma cells. J Cell Biol. 1975 Sep;66(3):568–576. doi: 10.1083/jcb.66.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H. U., Barber R., McGuire R. F. Glycoprotein-enriched vesicles from sheep erythrocyte ghosts obtained by spontaneous vesiculation. J Biol Chem. 1976 Jun 10;251(11):3500–3510. [PubMed] [Google Scholar]
- Maguire M. E., Wiklund R. A., Anderson H. J., Gilman A. G. Binding of (125I)iodohydroxybenzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976 Mar 10;251(5):1221–1231. [PubMed] [Google Scholar]
- Mukherjee C., Lefkowitz R. J. Desensitization of beta-adrenergic receptors by beta-adrenergic agonists in a cell-free system: resensitization by guanosine 5'-(beta, gamma-imino)triphosphate and other purine nucleotides. Proc Natl Acad Sci U S A. 1976 May;73(5):1494–1498. doi: 10.1073/pnas.73.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Richards J. G., Lorez H. P., Tranzer J. P. Indolealkylamine nerve terminals in cerebral ventricles: identification by electron microscopy and fluorescence histochemistry. Brain Res. 1973 Jul 27;57(2):277–288. doi: 10.1016/0006-8993(73)90136-4. [DOI] [PubMed] [Google Scholar]
- Révész T., Greaves M. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1. Nature. 1975 Sep 11;257(5522):103–106. doi: 10.1038/257103a0. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Furcht L. T., Kersey J. H. Changes in membrane structure associated with cell contact. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3631–3635. doi: 10.1073/pnas.70.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swislocki N. I., Tierney J., Essner E. S. Isolation and characterization of a liver plasma membrane fraction enriched in glucagon-sensitive adenylate cyclase. Arch Biochem Biophys. 1976 May;174(1):291–297. doi: 10.1016/0003-9861(76)90347-7. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G. Organisation of proteins in membranes with special reference to the cytochrome oxidase system. Biochim Biophys Acta. 1974 Dec 16;344(3-4):307–344. doi: 10.1016/0304-4157(74)90011-2. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Saper M. A., Lerner A. B., Fritsch P. Nonrandom distribution of receptors for melanocyte-stimulating hormone on the surface of mouse melanoma cells. J Supramol Struct. 1976;4(1):45–49. doi: 10.1002/jss.400040105. [DOI] [PubMed] [Google Scholar]
- Vial J., Porter K. R. Scanning microscopy of dissociated tissue cells. J Cell Biol. 1975 Nov;67(2PT1):345–360. doi: 10.1083/jcb.67.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haën C. The non-stoichiometric floating receptor model for hormone sensitive adenylyl cyclase. J Theor Biol. 1976 May 21;58(2):383–400. doi: 10.1016/s0022-5193(76)80126-9. [DOI] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Distribution of immunoglobulin on the surface of mouse lymphoid cells as determined by immunoferritin electron microscopy. Antibody-induced, temperature-dependent redistribution and its implications for membrane structure. Eur J Immunol. 1972 Dec;2(6):523–535. doi: 10.1002/eji.1830020611. [DOI] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Normal distribution, patching and capping of lymphocyte surface immunoglobulin studied by electron microscopy. Nat New Biol. 1973 Feb 28;241(113):257–259. doi: 10.1038/newbio241257a0. [DOI] [PubMed] [Google Scholar]