Abstract
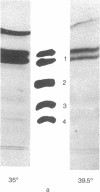
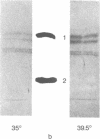
Mouse cells transformed by a temperature-sensitive mutant of simian virus 40 belonging to complementation group A lost their ability to regulate cell growth when grown at the permissive temperature (35 degrees) but showed the low saturation density of cell growth at the restrictive temperature (39.5 degrees) that is characteristic of normal cells in vitro. Biochemical analysis of the membranes of cells grown under the restrictive and the permissive conditions demonstrated no qualitative temperature-dependent differences either in neutral glycolipids or in acidic glycolipids of the cells. Plasma membrane glycoproteins labeled with radioactive glucosamine showed significantly different patterns on both polyacrylamide gel electrophoresis and electrofocusing. When the levels of glycoprotein glycosyltransferases of the cells were examined, the level of sialyltransferase (CMP-N-acetylneuraminytransferase,EC 2.4.99.1) of the cells grown at the restrictive temperature was low compared with that of cells grown at the permissive temperature. Our results indicate that the level of sialyltransferase is under the control of the gene A function of simian virus 40 and consequently is related to alterations in the cell surface glycoproteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekesi J. G., St-Arneault G., Holland J. F. Increase of leukemia L1210 immunogenicity by Vibrio cholerae neuraminidase treatment. Cancer Res. 1971 Dec;31(12):2130–2132. [PubMed] [Google Scholar]
- Bernacki R. J., Bosmann H. B. Activity of a rat-liver glycoprotein: sialyltransferase utilizing desialyzed prothrombin as an acceptor in normal and hypothrombinemic animals. Eur J Biochem. 1973 Feb 15;33(1):49–58. doi: 10.1111/j.1432-1033.1973.tb02653.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Case K. R., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. I. Electrokinetic properties of cells and cell surface glycoprotein:glycosyl transferase activities. Exp Cell Res. 1974 Jan;83(1):15–24. doi: 10.1016/0014-4827(74)90682-x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Cell surface glycosyl transferases and acceptors in normal and RNA- and DNA-virus transformed fibroblasts. Biochem Biophys Res Commun. 1972 Aug 7;48(3):523–529. doi: 10.1016/0006-291x(72)90379-8. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Platelet adhesiveness and aggregation: the collagen:glycosyl, polypeptide:N-acetylgalactosaminyl and glycoprotein:galactosyl transferases of human platelets. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1118–1124. doi: 10.1016/0006-291x(71)90578-x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Brugge J. S., Noonan C. A. Transformation of primate and rodent cells by temperature-sensitive mutants of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):25–36. doi: 10.1101/sqb.1974.039.01.006. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Bagshawe K. D. The effect of neuraminidase on the immunogenicity of the Landschütz ascites tumour: site and mode of action. Br J Cancer. 1968 Sep;22(3):588–594. doi: 10.1038/bjc.1968.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Grimes W. J. Sialic acid transferases and sialic acid levels in normal and transformed cells. Biochemistry. 1970 Dec 22;9(26):5083–5092. doi: 10.1021/bi00828a007. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Murakami W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Jan;59(1):254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Breeding J., Nedrud J. Sialic acid masked membrane antigens of clonal functional glial cells. J Cell Physiol. 1972 Apr;79(2):249–258. doi: 10.1002/jcp.1040790209. [DOI] [PubMed] [Google Scholar]
- Johnston I. R., McGuire E. J., Jourdian G. W., Roseman S. Incorporation of N-acetyl-D-glucosamine into glycoproteins. J Biol Chem. 1966 Dec 10;241(23):5735–5737. [PubMed] [Google Scholar]
- Kemp R. B. Effect of the removal of cell surface sialic acids on cell aggregation in vitro. Nature. 1968 Jun 29;218(5148):1255–1256. doi: 10.1038/2181255a0. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K., Eng L. F. Gangliosides of human myelin: sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973 Oct;21(4):829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y., Avila J., Saral R. The semiautonomous replicon: a molecular model for the oncogenicity of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):17–24. doi: 10.1101/sqb.1974.039.01.005. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Transformation of neuraminidase-treated lymphocytes by soybean agglutinin. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2515–2518. doi: 10.1073/pnas.70.9.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N., Pardee A. B., McAuslan B. R., Burger M. M. Sialic acid contents and controls of normal and malignant cells. Biochim Biophys Acta. 1968 Apr 16;158(1):98–102. doi: 10.1016/0304-4165(68)90076-7. [DOI] [PubMed] [Google Scholar]
- Onodera K., Sheinin R. Macromolecular glucosamine-containing component of the surface of cultivated mouse cells. J Cell Sci. 1970 Sep;7(2):337–355. doi: 10.1242/jcs.7.2.337. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Wickus G. G., Branton P. E., Gaffney B. J., Hirschberg C. B., Fuchs P., Blumberg P. The chick fibroblast cell surface after transformation by Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1173–1180. doi: 10.1101/sqb.1974.039.01.135. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Sanford B. H. An alteration in tumor histocompatibility induced by neuraminidase. Transplantation. 1967 Sep 5;5(5):1273–1279. doi: 10.1097/00007890-196709000-00005. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Springer G. F., Desai P. R. Relation of human blood-groups MN to cancer cell surface antigens and to receptors for oncogenic viruses. Ann Clin Lab Sci. 1974 Jul-Aug;4(4):294–298. [PubMed] [Google Scholar]
- Sudo T., Onodera K. Response of cell surface glycosyl transferases to dibutyryl adenosine-3',5' cyclic monophosphate in virus-transformed and normal cells. Exp Cell Res. 1975 Mar 1;91(1):191–199. doi: 10.1016/0014-4827(75)90157-3. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Rucoslahti E., Nordling S. Neuraminidase stimulates division and sugar uptake in density-inhibited cell cultures. Nat New Biol. 1972 Aug 16;238(85):211–212. doi: 10.1038/newbio238211a0. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. Cellular membrane alterations in neoplasia: a review and a unifying hypothesis. Curr Top Microbiol Immunol. 1969;47:152–176. doi: 10.1007/978-3-642-46160-6_7. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Kuchino T. Temperature-sensitive mutants of simian virus 40 selected by transforming ability. J Virol. 1975 Jun;15(6):1297–1301. doi: 10.1128/jvi.15.6.1297-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y., Yamada M. Synchronized cell division induced by medium change. Exp Cell Res. 1967 Oct;48(1):226–228. doi: 10.1016/0014-4827(67)90309-6. [DOI] [PubMed] [Google Scholar]