Abstract
Quorum sensing is a cell-cell communication process in which bacteria use the production and detection of extracellular chemicals called autoinducers to monitor cell population density. Quorum sensing allows bacteria to synchronize the gene expression of the group, and thus, act in unison. Here, we review the mechanisms involved in quorum sensing with a focus on the Vibrio harveyi and Vibrio cholerae quorum-sensing systems. We discuss the differences between these two quorum-sensing systems and the differences between them and other paradigmatic bacterial signal transduction systems. We argue that the Vibrio quorum-sensing systems are optimally architected to precisely translate extracellular autoinducer information into internal changes in gene expression. We describe how studies of the V. harveyi and V. cholerae quorum-sensing systems have revealed some of the fundamental mechanisms underpinning the evolution of collective behaviors.
Keywords: Quorum sensing, Autoinducers, Signal transduction, Feedback regulation, Small RNAs
Quorum Sensing
Bacterial processes such as biofilm formation, virulence factor secretion, bioluminescence, antibiotic production, sporulation, and competence for DNA uptake are often critical for survival. However, these behaviors are seemingly futile if performed by a single bacterium acting alone. Yet, we know that bacteria perform these tasks effectively. How do bacteria manage? We now understand that, through a process called quorum sensing, bacteria synchronously control gene expression in response to changes in cell density and species complexity. Quorum sensing allows bacteria to switch between two distinct gene expression programs: one that is favored at low-cell-density (LCD) for individual, asocial behaviors, and another, that is favored at high-cell-density (HCD) for social, group behaviors (reviewed in 87, 88, 134, 139).
The fundamental steps involved in detecting and responding to fluctuations in cell number are analogous in all known quorum-sensing systems. First, low molecular weight molecules called autoinducers are synthesized intracellularly. Second, these molecules are either passively released or actively secreted outside of the cells. As the number of cells in a population increases, the extracellular concentration of autoinducer likewise increases. Third, when autoinducers accumulate above the minimal threshold level required for detection, cognate receptors bind the autoinducers and trigger signal transduction cascades that result in population-wide changes in gene expression. Thus, quorum sensing enables cells in a population to function in unison and, in so doing, they carry out behaviors as a collective.
In this review, following a short synopsis of canonical quorum-sensing systems, we focus on the quorum-sensing systems of Vibrio harveyi and Vibrio cholerae. Studies of these two paradigmatic systems have revealed fundamental molecular mechanisms underpinning small-molecule biosynthesis, signal detection and transduction, information processing, small RNA-mediated post-transcriptional control of mRNA levels, and transcriptional gene regulation (37, 39, 57, 75, 97, 124, 135). Furthermore, analysis of the similarities and differences in these two related quorum-sensing systems, as well as assessing differences between these two systems and other bacterial signal transduction systems provides insight into how evolution has incorporated distinct features into bacterial sensory networks that solve unique biological information processing challenges.
LuxIR-Type Quorum-Sensing Systems in Gram-Negative Bacteria
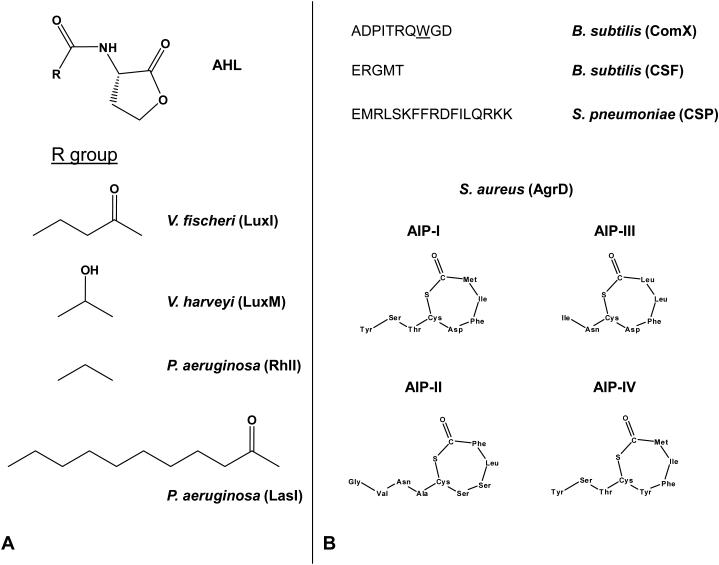
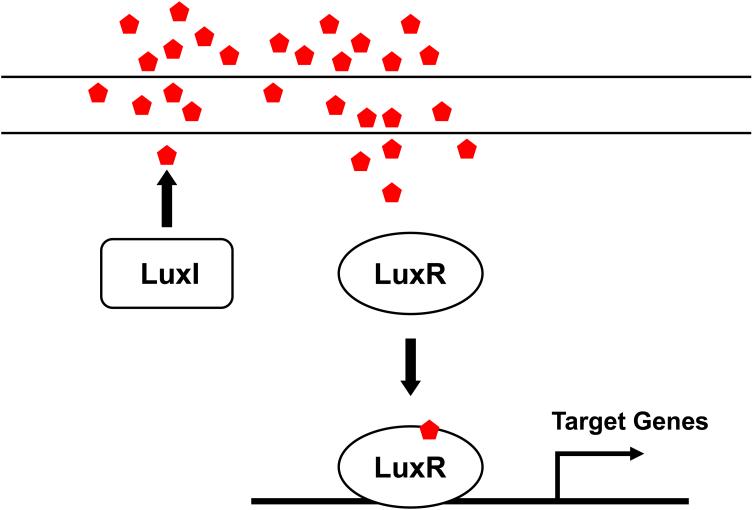
Acyl homoserine lactones (AHLs) are a major class of autoinducer signal used by Gram-negative proteobacteria for intraspecies quorum sensing. AHLs are composed of homoserine lactone (HSL) rings carrying acyl chains of C4 to C18 in length (25). These side chains harbor occasional modification, notably at the C3 position, or unsaturated double bonds (Figure 1A). The first AHL autoinducer and its cognate regulatory circuit was discovered in the bioluminescent marine bacterium Vibrio fischeri, which colonizes the light organ of the Hawaiian Bobtail Squid Euprymna scolopes (100). The nutritious environment inside the light organ of the squid allows the bacteria to grow to high cell density, and, using quorum sensing, to activate expression of the luciferase operon. The squid host uses the bacterial-produced light to counter-illuminate itself in an anti-predation strategy (100). Two proteins, LuxI and LuxR, are essential for quorum-sensing control of bioluminescence in V. fischeri (Figure 2). LuxI is the synthase of the quorum-sensing autoinducer N-3-(oxo-hexanoyl)-homoserine lactone (3OC6HSL, Figure 1) (19, 104). LuxI catalyzes acylation and lactonization reactions between the substrates S-adenosylmethionine (SAM) and hexanoyl-ACP (81, 104). Following synthesis, 3OC6HSL diffuses freely in and out of the cell, and its concentration increases as the cell density of the population increases (49). LuxR is the cytoplasmic receptor for 3OC6HSL as well as the transcriptional activator of the luciferase luxICDABE operon (18, 19). Without the 3OC6HSL ligand, the LuxR protein is unstable and is rapidly degraded. When 3OC6HSL accumulates, it is bound by LuxR, and the LuxR-AHL complex recognizes a consensus binding sequence (lux box) upstream of the luxICDABE operon and activates its expression (114). The luxAB genes encode the subunits of luciferase, and luxCDE encode the fatty acid reductase complex which produces and recycles the luciferase aldehyde substrate (74). Because expression of luxI is also activated by 3OC6HSL-bound LuxR, when the quorum-sensing circuit engages, autoinducer production is induced, and the surrounding environment is flooded with the signal molecule. This “autoinduction” positive feedback loop is presumed to enforce synchrony as the population of cells switches from LCD mode to HCD quorum-sensing mode.
Figure 1.
Structures of bacterial autoinducers. (A) Homoserine lactone autoinducers produced by different Gram-negative bacteria. (B) Amino acid sequences of three peptide autoinducers, ComX, CSF, and CSP, produced by Gram-positive bacteria. The underlined tryptophan in B. subtilis ComX is isoprenylated. The four different AIPs produced by S. aureus. (C) DPD, the precursor to AI-2. In the presence of boron, AI-2 exists as S-THMF-borate. In the absence of boron, AI-2 exists as R-THMF. (D) Structure of V. cholerae CAI-1 and Amino-CAI-1. (E) Structure of the PQS autoinducer of P. aeruginosa.
Figure 2.
A canonical Gram-negative LuxIR-type quorum-sensing system. Red pentagons denote AHL autoinducers. Refer to text for details.
The LuxI/LuxR regulatory system of V. fischeri is considered the paradigm for the control of gene expression by quorum sensing in Gram-negative bacteria (Figure 2) (19). Homologues of luxI and luxR have been identified in a large number of bacterial genomes and these other LuxIR-type quorum-sensing systems control global cell-density dependent gene expression (11). Positive feedback loops consisting of LuxR-type proteins activating luxI-type gene expression are commonly wired into these AHL quorum-sensing systems (18, 26, 108). Some well studied AHL quorum-sensing systems include the LasI/LasR-RhlI/RhlR system of Pseudomonas aeruginosa that controls virulence factor gene expression and biofilm formation (89-91, 140), the TraI/TraR system of Agrobacterium tumefaciens that regulates transfer of the oncogenic Ti plasmid to the plant host (26, 41, 96), and the EsaI/EsaR system in Pantoea stewartii that controls exopolysaccharide production, adhesion, and plant colonization (131, 132). In an interesting twist on these circuits, the EsaI/EsaR system functions reciprocally to other LuxIR-type systems. Unliganded EsaR binds DNA and represses transcription. Autoinducer binding to EsaR promotes DNA release, and target gene expression (79, 130).
AHL autoinducer molecules are typically unique in that, a particular AHL molecule is detected only by the species that produces it. Therefore, it is suggested that AHL-type quorum-sensing systems predominately foster intra-species cell-cell communication. Signal specificity is attributed to the fact that LuxR-type protein folding requires the presence of the AHL ligand (107, 127, 149, 150). For example, TraR in the absence of its cognate ligand (3OC8HSL) is unstructured and rapidly degraded (149, 150). Supporting this notion is the finding that the crystal structure of the 3OC8HSL-TraR complex reveals that the ligand is completely buried within the protein (128, 146). Solution structure of the ligand binding domain complexed with C8HSL of SdiA, a TraR homolog in E. coli, reveals similar organization (145). Although P. aeruginosa LasR shares low overall homology with TraR and SdiA, the LasR ligand binding domain nonetheless shows significant structural similarity to these two proteins (8). Importantly, the putative signal binding pocket of LasR is more spacious than that of TraR and SdiA. The presumption is that this arrangement accommodates the larger cognate ligand, C12HSL (8).
Biochemical analyses of LuxR-type proteins suggest that there exist three classes of these receptors (reviewed in 106). Class 1 receptors (e.g. LasR) require AHL for folding and have exquisitely tight affinity for their ligands. Class 2 receptors (e.g. LuxR of V. fischeri) also require AHLs for folding but the mature proteins bind to their ligands reversibly. Class 3 receptors (e.g. EsaR) do not require AHLs for folding and have reversible ligand binding. The absolute requirement for AHLs for protein folding in Class 1 and 2 LuxR-type receptors suggests that these receptors are refractory to sudden increases in AHL concentrations in the environment since protein translation is a relatively slow process (106). It is further suggested that the difference in ligand binding affinity between these receptors is the main criterion controlling their robustness to perturbation (106). For example, the folded Class 1 receptor TraR remains active for a prolonged period of time even when its cognate AHL is removed (63), whereas the folded Class 2 receptor LuxR is inactivated within minutes after its cognate AHL is removed (49).
Two different structures of AHL synthases, LasI from P. aeruginosa that synthesizes 3OC12HSL and EsaI from P. stewartii that synthesizes 3OC6HSL, have been reported (31, 137). By comparing the two structures, it appears that although LasI and EsaI synthesize different molecules, the core domains which contain 74 conserved amino acid residues are highly similar (31, 137). However, the acyl chain binding pockets differ dramatically. The binding pocket of EsaI sits in an enclosed cavity surrounded by numerous other residues (137). By contrast, the substrate binding pocket in LasI is an elongated tunnel (31). These structural features suggest that EsaI can only accommodate substrates with relatively short acyl-chain lengths while LasI has no steric restriction on the substrate acyl-chain length (31, 137). Thus, it is not understood how LasI selects only the appropriate substrate (i.e. C12-ACP) for reaction. One hypothesis is that substrates with acyl chains longer than C12 are used by the cell for other essential processes such as LPS biosynthesis and this limits their availability to LasI (31). Like LasI and EsaI, many other LuxI-type AHL quorum-sensing synthases use the intracellular fatty acid pool as the source of substrate for AHL synthesis. One notable exception is the newly discovered RpaI/RpaR system in the photosynthetic bacterium Rhodopseudomonas palustris. In this case, the signal, p-coumaroyl-HSL, is generated from p-coumaric acid obtained from the extracellular environment (103). Since p-coumarate is a major by-product of lignin degradation in plants, it is proposed that the signal p-coumaroyl-HSL is used for both intra-species signaling among bacterial cells and inter-kingdom signaling between bacterial and plant cells (103).
Two-Component Quorum-Sensing Systems in Gram-Positive Bacteria
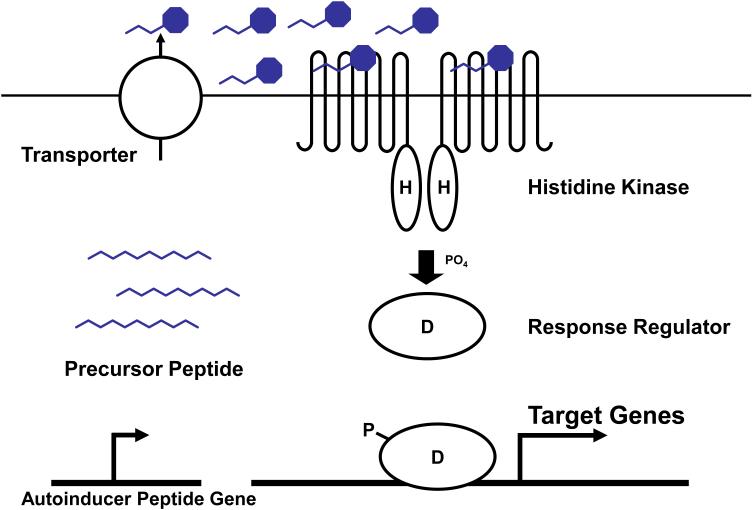
Gram-positive bacteria primarily use modified oligopeptides as autoinducers in quorum-sensing controlled gene expression systems (Figure 1B and Figure 3) (35, 46, 110). Because peptides are impermeable to biological membranes, secretion of quorum sensing peptides is usually mediated by specialized transporters. In addition, modifications to the initially synthesized peptides, such as processing and cyclization, are often associated with secretion (Figure 1, (35, 45, 69, 70, 110)). One major difference between LuxIR-based and oligopeptide-based quorum-sensing systems is the location of the cognate receptors; while the LuxR-type receptors are cytoplasmic, the sensors for oligopeptide autoinducers in Gram-positive bacteria are membrane-bound. The membrane-bound receptors, so-called two-component signaling proteins, transduce information via a series of phosphorylation events (40, 42, 109).
Figure 3.
A canonical Gram-positive two-component-type quorum-sensing system. Purple octagons denote processed/modified peptide autoinducers. Refer to text for details.
A typical two-component system consists of a membrane-bound histidine kinase receptor and a cognate cytoplasmic response regulator, which functions as a transcriptional regulator (40, 42, 109). A general model for oligopeptide-mediated quorum sensing is depicted in Figure 3. As in AHL quorum-sensing systems, the concentration of secreted oligopeptide autoinducer increases as the cell density increases. Peptide binding to the membrane-bound histidine kinase receptor stimulates its intrinsic autophosphorylation activity, resulting in ATP-driven phosphorylation of a conserved histidine residue (H) in the cytoplasm. The phosphate group is subsequently transferred to the conserved aspartate residue (D) of a cognate response regulator. Phosphorylated response regulators are active and they function as DNA-binding transcription factors to modulate expression of target genes. In many cases, the genes encoding the oligopeptide autoinducer precursor, the histidine kinase receptor, and the response regulator form an operon and its expression is auto-induced by quorum sensing (46, 95). In such cases, similar to what was described above for the LuxR/LuxI systems, this configuration produces positive feedback and accelerates the transition from the LCD to the HCD quorum-sensing mode of gene expression. Examples of peptide-based quorum-sensing systems include the ComD/ComE system of Streptococcus pneumoniae that controls competence development (94), the AgrC/AgrA system of Staphylococcus aureus that controls pathogenesis (46), and the ComP/ComA system of Bacillus subtilis that controls competence and sporulation (69).
Unlike Gram-negative bacterial AHLs, Gram-positive peptide autoinducers are not variations on a single core molecule. Rather, peptide autoinducers are genetically encoded, and thus each species of bacteria is capable of producing a peptide signal with a unique sequence (Figure 1B). Consistent with this, although Gram-positive quorum-sensing receptors are members of the histidine kinase protein family and thus share overall homology, little homology exists in their transmembrane ligand sensing domains and this likely determines their specificity (69, 87, 94). While no Gram-positive quorum-sensing receptor has yet been crystallized, elegant genetic and biochemical studies have defined the S. aureus Agr quorum-sensing receptor-ligand interaction and it stands as the paradigm for understanding signal transduction in peptide quorum-sensing systems (Reviewed in 87).
The S. aureus Agr autoinducer is denoted AIP (for Auto-inducing peptide) and is encoded as a longer precursor peptide by the agrD gene. Processing (truncation and cyclization) and secretion occurs via the AgrB transporter. Extracellular AIP is detected by the AgrC histidine kinase receptor and signal transduction occurs by phosphorelay to the AgrA response regulator (87). There are four S. aureus AIP specificity groups (I-IV) which are defined by the particular AIP peptide sequence (Figure 1B). The mature AIPs are seven to nine residues in length with a five-membered ring formed between the sulfur atom from a central cysteine and the C-terminus via a thiolactone bond. The two bulky hydrophobic residues of each AIP are involved in AgrC binding, and the ring structure is critical for activity (67, 70, 142). The AIPs and their cognate receptors are co-evolving such that productive signaling interactions only occur between a particular AIP and the hypervariable region of its cognate AgrC transmembrane domain (43, 45). And, remarkably, interaction between an AIP and a non-cognate AgrC receptor inhibits quorum sensing (43, 45, 66). Mutagenesis studies pinpoint the residues in the extracellular loop connecting transmembrane helices 3 and 4 as critical for AIP discrimination, while mutations in Ile171 broaden AIP specificity (27, 28, 44).
Additional Features of Gram-Negative and Gram-Positive Quorum-Sensing Systems
Integration of additional regulatory features is common in both Gram-negative and Gram-positive quorum-sensing systems. We name only a few here. In P. aeruginosa, the CRP homolog Vfr is activated by binding the second messenger molecule cyclic-AMP (141). Activated Vfr induces transcription of lasR when P. aeruginosa enters into stationary phase (1). One of the target genes activated by the S. pneumoniae ComD/ComE system is comX, which encodes an alternative sigma factor that is essential for transcription of a set of late competence genes encoding proteins involved in genetic exchange such as DNA uptake and recombination (55, 61, 62). Interestingly, competence only occurs for a short period of time because ComX disappears soon after competence has developed. The ATP-dependent protease ClpEP has been implicated in specific degradation of ComX and termination of competence (116). These and other accessory control mechanisms allow bacteria to integrate environmental cues in addition to autoinducer information into their quorum-sensing networks presumably to extract maximal information from their surroundings and fine tune their transitions into and out of LCD and HCD gene expression programs.
The Quorum-Sensing Network Architecture in V. harveyi and V. cholerae
Although LuxIR systems similar to that of V. fischeri have been identified in distantly related bacteria, analyses of quorum sensing in other Vibrios, most notably V. harveyi and V. cholerae, show that their systems do not conform to the common LuxIR theme. These two species possess neither luxI nor luxR genes similar to V. fischeri and other Gram-negative quorum-sensing bacteria. However, V. harveyi and V. cholerae do possess sets of quorum-sensing components that are highly similar to one another (37, 57, 75, 124, 148). This close similarity initially suggested functional equivalence among the components, however, detailed analyses of the roles of the individual components and the overall function of the networks reveal that these two quorum-sensing circuits operate by surprisingly different means (37, 57, 75, 126). We hypothesize that, these differences have evolved to allow these two closely related species to adapt to their drastically different lifestyles.
V. harveyi is a free living marine bacterium and is an important pathogen of marine organisms (2). V. cholerae, by contrast, is the etiological agent of the disease cholera and its life cycle consists of alternations between human hosts and the aquatic environment (20). Quorum sensing in V. harveyi activates bioluminescence and metalloprotease production and represses type III secretion (9, 36). In V. cholerae, quorum sensing represses biofilm formation and virulence factor production and activates protease production (32, 47, 147, 148). Quorum sensing also promotes genetic exchange between V. cholerae cells in the presence of chitin, which is believed to be important for serogroup conversion (6, 7, 73).
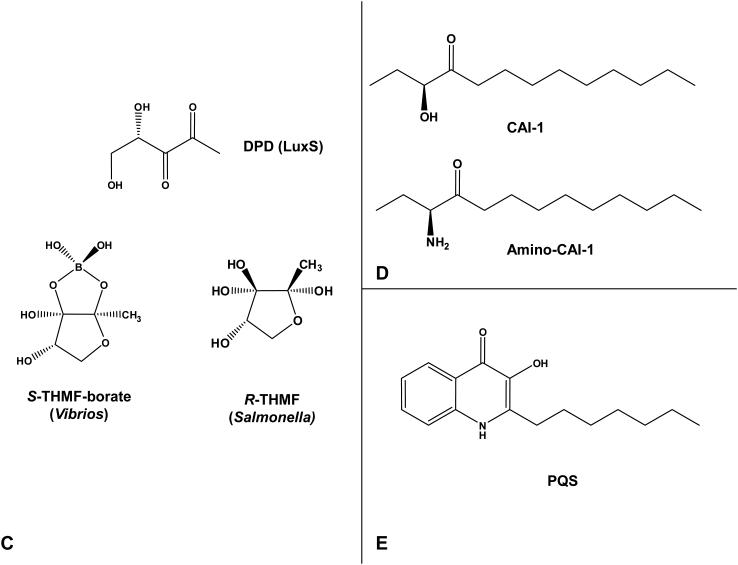
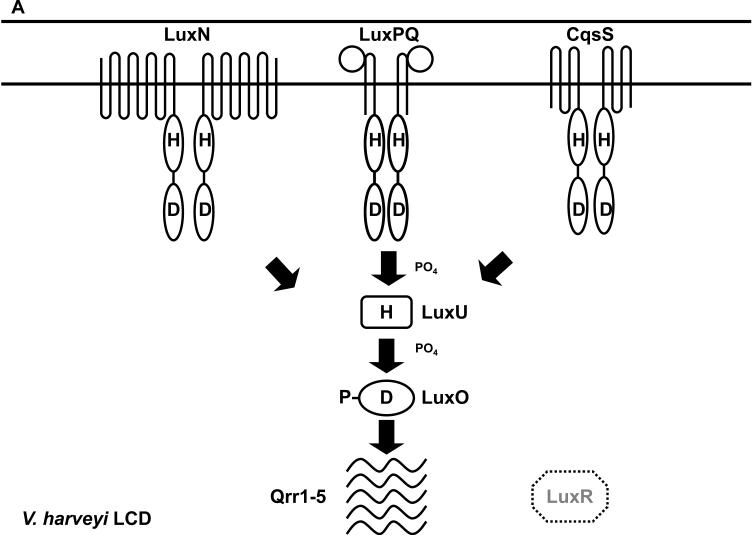
We first outline the quorum-sensing circuit in V. harveyi, a hybrid of the canonical Gram-negative and Gram-positive bacterial quorum-sensing systems (Figure 4). As in other Gram-negative quorum-sensing bacteria, V. harveyi produces, detects, and responds to an AHL autoinducer (3OHC4-HSL) denoted HAI-1 for V. harveyi autoinducer 1 (Figure 1A) (10). Two additional autoinducer molecules called AI-2 and CAI-1 are produced and detected by V. harveyi (Figure 1C and D, respectively). AI-2 is a set of interconverting molecules derived from the shared precursor 4,5-dihydroxy-2,3-pentanedione (DPD) (105). The active form of AI-2 in V. harveyi contains boron (13). CAI-1 has not been purified from V. harveyi, but it is presumed to be related to CAI-1 produced by V. cholerae, which has been identified as (S)-3-hydroxytridecan-4-one (39).
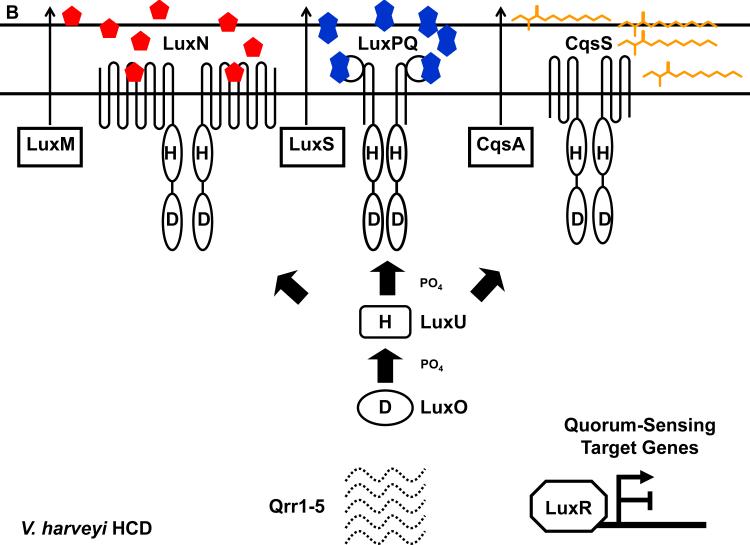
Figure 4.
The V. harveyi quorum-sensing circuit. (A) Signal transduction at LCD. During this stage, autoinducer levels are low and the LuxN, LuxPQ, and CqsS receptors function as kinases. LuxO is phosphorylated, the Qrr1-5 sRNAs are transcribed, and LuxR protein is not produced. (B) Signal transduction at HCD. During this stage, autoinducer levels are high and the LuxN, LuxPQ, and CqsS receptors function as phosphatases. LuxO is unphosphorylated, Qrr1-5 sRNAs are not transcribed, and LuxR protein is produced. Solid and dotted lines denote regulatory factors that are produced and not produced, respectively. Refer to text for details.
HAI-1 is synthesized by the LuxM synthase, which shows no homology to the LuxI-type AHL synthases although it carries out the same biochemistry (3, 4). DPD is synthesized by the LuxS enzyme, and luxS exists in hundreds of bacterial genomes (105, 117). We provide further detail about LuxS and AI-2 below. CAI-1 is synthesized by the CqsA synthase, and cqsA shows sequence homology to aminotransferases. CqsA homologs have been identified in several sequenced Vibrio genomes as well as Legionella pneumophila (37, 39, 75, 113, 122, 123).
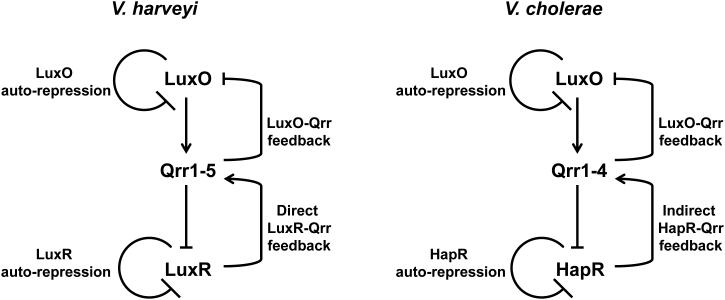
Detection of the V. harveyi autoinducers does not occur through LuxR-type proteins. Rather, membrane-bound histidine kinases act as cognate receptors for all three autoinducers (Figure 4). HAI-1 is detected by the LuxN histidine kinase (24, 37, 120). AI-2 is detected by the periplasmic protein LuxP in complex with the LuxQ histidine kinase (4, 37, 84, 85). CAI-1 is detected by the CqsS histidine kinase (37, 39, 75). LuxN, LuxQ, and CqsS are bi-functional two-component enzymes that possess both kinase and phosphatase activities. At LCD (Figure 4A), the receptors are devoid of their respective ligands, and in this mode, their kinase activities predominate, resulting in phosphorylation of conserved histidine residues by ATP. The phosphate group is subsequently transferred to the conserved aspartate residue located in the receiver domain at the C-terminus of each receptor. Phosphate from all three receptors is subsequently transduced to a single phosphotransfer protein, LuxU. LuxU transfers the phosphate to a response regulator called LuxO. LuxO belongs to the NtrC family of response regulators and requires phosphorylation to act as a transcriptional activator (5, 23, 58).
Together with σ54-loaded RNA polymerase, phosphorylated LuxO (LuxO-P) activates transcription of genes encoding five small regulatory RNAs (sRNAs) called Qrr1-5 (Figure 4A). The main target of the Qrr sRNAs is the mRNA encoding the quorum-sensing master transcriptional regulator LuxR, which is not similar to the LuxR described above in LuxIR-type quorum-sensing systems. At LCD, the Qrr sRNAs are transcribed, and directed by the RNA chaperone Hfq, Qrr1-5 base pair with and destabilize the luxR mRNA transcript (124). Therefore, at LCD, LuxR protein is not made. When autoinducer concentration is above the threshold level required for detection (e.g. at HCD, Figure 4B), binding of autoinducers to the cognate receptors switches the receptors from kinases to phosphatases. Phosphate flow in the signal transduction pathway is reversed, resulting in dephosphorylation and inactivation of LuxO. Therefore, at HCD, qrr1-5 are not transcribed, luxR mRNA is stabilized, and LuxR protein is produced. LuxR acts as both a transcriptional activator and a transcriptional repressor. In addition to the luciferase operon, LuxR regulates at least another 50 genes including those encoding the type III secretion apparatus and metalloproteases (36, 97, 135).
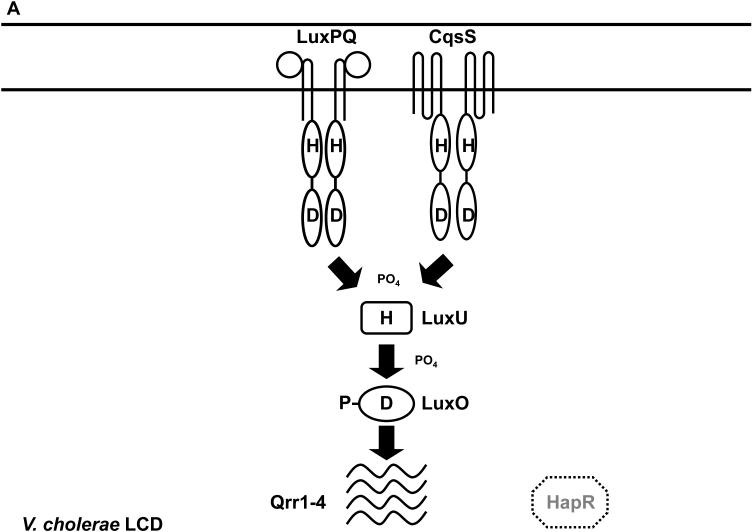
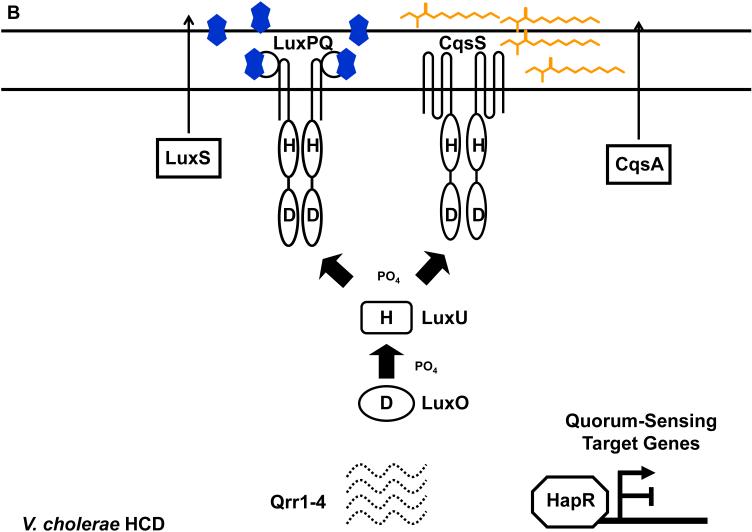
The components and the wiring of the V. cholerae quorum-sensing system (Figure 5) appear extremely similar to their V. harveyi counterparts with only two notable differences. First, V. cholerae does not have the LuxM synthase and does not make HAI-1. Consistent with this, V. cholerae also does not have the LuxN sensor and it does not detect HAI-1. Second, only four sRNAs genes lie downstream of LuxO-P in the V. cholerae cascade (57). The functional homolog of the V. harveyi LuxR master regulator is called HapR in V. cholerae. HapR, like LuxR, acts as both an activator and a repressor of gene expression. At HCD (Figure 5B), HapR activates a gene encoding the Hap protease (47) and represses genes important for biofilm formation and virulence factor production (32, 147).
Figure 5.
The V. cholerae quorum-sensing circuit. (A) Signal transduction at LCD. During this stage, autoinducer levels are low and the CqsS and LuxPQ receptors function as kinases. LuxO is phosphorylated, the Qrr1-4 sRNAs are transcribed, and HapR protein is not produced. (B) Signal transduction at HCD. During this stage, autoinducer levels are high and the CqsS and LuxPQ receptors function as phosphatases. LuxO is unphosphorylated, Qrr1-4 sRNAs are not transcribed, and HapR protein is produced. Solid and dotted lines denote regulatory factors that are produced and not produced, respectively. Refer to text for details.
Based on sequence homology and circuit configuration, at first analysis, it appears that the V. harveyi and V. cholerae systems are nearly identical, and in turn, should function analogously. However, systematic analyses show striking differences in how the two systems operate. These differences could not have been uncovered through genomic sequence comparisons alone. In the following sections, we compare and contrast these two quorum-sensing systems. First, we outline each system’s core components; the multiple autoinducers and their synthases and the molecular mechanisms used by the cognate receptors for signal detection and transduction. Second, we discuss the distinct mechanisms by which the V. harveyi and V. cholerae Qrr sRNAs function. Third, we discuss how feedback regulation is integrated into the two circuits to uniquely optimize their outputs.
It is interesting to note that, many of the quorum-sensing components of the V. harveyi and V. cholerae systems exist in other Vibrio species that harbor LuxIR-type systems. For instance, in addition to the extensively-studied LuxIR AHL quorum-sensing system, V. fischeri possesses homologs of the V. harveyi cascade: LuxMN and LuxS/LuxPQ as well as LuxU, LuxO, one Qrr sRNA, and a V. harveyi-like LuxR called LitR (22). The LuxM and LuxN homologs in V. fischeri are called AinS and AinR, respectively (17, 29, 51, 64, 65). The signal produced by AinS is C8HSL (34). Presumably, like V. harveyi, at low autoinducer concentrations, the LuxQ and AinR kinases transfer phosphate through LuxU to LuxO which activates transcription of the gene encoding the single Qrr sRNA, which prevents production of LitR. Interestingly, LitR activates transcription of ainS generating another positive feedback loop in the V. fischeri network (64). Moreover, LitR activates transcription of luxI linking the LuxS/LuxPQ and AinS/AinR systems to the canonical LuxI/LuxR system (22).
Vibrio anguillarum, the causative agent of terminal hemorrhagic septicemia in marine fish, possesses three parallel quorum-sensing systems homologous to those of V. harveyi (14, 15, 78). VanM, the LuxM homolog, produces two autoinducers C6HSL and 3OC6HSL. Both autoinducers are detected by the VanN receptor (15). Unlike V. harveyi and V. cholerae, the mRNA of vanT (the homolog of luxR/hapR/litR) is stable at LCD and further production is not induced by quorum sensing (15). It is proposed that inhibition of translation of vanT mRNA at LCD occurs through some other unidentified mechanism (77).
Synthesis of V. harveyi and V. cholerae Autoinducers
The autoinducers HAI-1, AI-2, and CAI-1 are synthesized by the cytoplasmic enzymes LuxM, LuxS, and CqsA, respectively (3, 37, 39, 75, 105). As mentioned, LuxM has no significant sequence homology to LuxI of V. fischeri but it is similar to another AHL synthase AinS (29). Mechanistic studies of LuxM synthesis of 3OHC4HSL (Figure 1A) have not been performed. However, parallel studies on AinS show that, analogous to the LuxI class of enzymes, the AinS AHL synthase (and presumably the LuxM synthase) also uses SAM and acyl-CoA or acyl-ACP as substrates to produce its particular AHL (34).
The LuxS synthase produces 4,5-dihydroxy-2,3-pentanedione (DPD) (Figure 1C), which is the precursor to a set of interconverting molecules that are generically called AI-2. Specifically, DPD is produced from SAM in three enzymatic steps (105). First, methyltransferase enzymes catalyze transfer of the methyl group on SAM to particular substrates to produce products such as DNA, RNA, and proteins. S-adenosylhomocysteine (SAH) is formed as a toxic byproduct of these reactions. The Pfs nucleosidase relieves the SAH toxicity by cleaving adenine from SAH to form S-ribosylhomocysteine (SRH) (105). SRH is next hydrolyzed by LuxS to form two products: homocysteine, and DPD. DPD is unstable, and spontaneously converts into different moieties in solution (105). In the marine environment, where the borate concentration can reach 0.4 mM, DPD cyclizes and reacts with borate to form (2S, 4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran borate (S-THMF borate), which is the active form of the AI-2 autoinducer used by V. harveyi and V. cholerae (Figure 1C) (13). In terrestrial environments where boron is limited, (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (R-THMF), an unborated rearranged DPD moiety derived from DPD, is the form of AI-2 used by enteric bacteria such as Escherichia coli and Salmonella typhimurium (Figure 1C) (76). The AI-2 synthase LuxS has gained attention because LuxS homologs exist in hundreds of bacterial genomes, and AI-2 is proposed to be a rather universal signal that fosters inter-species cell-cell communication (21, 143). In V. harveyi and V. cholerae, and in some other quorum-sensing bacteria, AI-2 clearly acts as a bona fide autoinducer signal (98, 121). Nonetheless, some studies suggest that phenotypes observed in luxS mutants of other bacterial species stem from LuxS’s role in metabolism of SAM (129). Both possibilities could be correct.
CAI-1 was recently purified from V. cholerae and identified as (S)-3-hydroxytridecan-4-one (Figure 1D) and, as mentioned, its synthesis depends on CqsA, an enzyme with similarity to aminotransferases (38, 39). Purified protein studies show that 3-aminotridecane-4-one (amino-CAI-1, Figure 1D) is in fact the CqsA product and its substrates are (S)-2-aminobutyrate and decanoyl-CoA (38). Crystallographic analysis combined with mutagenesis and spectral analyses verify these findings and demonstrate that V. cholerae CqsA produces amino-CAI-1 by a pyridoxal phosphate-dependent aminotransferase reaction (38). Both amino-CAI-1 and CAI-1 are detected by the V. cholerae CqsS receptor and they have comparable biological activities, however, CAI-1, not amino-CAI-1, is the major form of the molecule in cell-free culture fluids (38). The current understanding is that, once synthesized, amino-CAI-1 is immediately converted into CAI-1 presumably via another enzyme, and the latter molecule is the predominant form of the signaling molecule encountered by V. cholerae (38). As mentioned, V. harveyi and several other Vibrio species possess homologs of both cqsA and cqsS, therefore, it is proposed that the CqsA/CqsS system is used for inter-Vibrio communication. Consistent with this, cell-free culture fluids from V. harveyi and other Vibrio species that possess CqsA activate gene expression in a V. cholerae CAI-1 reporter strain (37). Whether the CAI-1 autoinducers from V. harveyi and other Vibrio species are identical to V. cholerae CAI-1 or whether they are closely related molecules remains to be addressed. One oddity is that cqsA and cqsS, called lqsA and lqsS, exist in the distantly related bacterium Legionella pneumophila. LqsA produces 3-hydroxypentadecan-4-one; a molecule with a longer hydrocarbon chain than CAI-1 (113). The Lqs system promotes host-bacterial interactions in the L. pneumophila stationary-phase virulence regulatory network (122, 123).
Signal Transduction through the V. harveyi and V. cholerae Quorum-Sensing Receptors
As discussed above, both the V. harveyi and V. cholerae quorum-sensing systems employ multiple two-component proteins and a phosphorylation-dephosphorylation cascade for signal transduction (37, 75). At LCD, when autoinducer concentration is low, LuxN, LuxPQ, and CqsS quorum-sensing receptors function as kinases (Figures 4A and 5A). At HCD, when autoinducer concentration is high, the receptors function as phosphatases (Figures 4B and 5B). Although hundreds of two-component systems are known, it is unclear how the phosphorylation activities of particular histidine sensor kinases are regulated by specific signals because only a few ligands have been identified. The V. harveyi quorum-sensing system has emerged as an important system for understanding two-component receptor signal transduction across the membrane because the structures of the HAI-1 and AI-2 autoinducers have been known for some time and synthetic molecules are available. These two features made analysis of two-component signaling across the membrane feasible. Two particular studies, one of AI-2-LuxPQ signaling and one of HAI-1-LuxN signaling provide insight into how ligand binding elicits the switch in a two-component receptor from kinase to phosphatase (13, 84, 85, 120).
The first informative study concerns AI-2 and LuxPQ. The crystal structures of LuxP in complex with the periplasmic domain of LuxQ (LuxQp) in both the AI-2-free and AI-2-bound forms were solved and compared (84, 85). LuxP, like other periplasmic binding proteins, binds AI-2 in a cleft formed between two similar domains connected by a three stranded hinge. LuxQp is composed of two tandem PAS domains with no sequence homology to one another or to other PAS folds. Unliganded LuxP adopts an open conformation representing the AI-2 receptive state in the apo-LuxP/LuxQp complex. Mutations that destabilize or eliminate the interfaces between LuxP and LuxQp decrease the concentration of AI-2 required to convert LuxQ from kinase to phosphatase, suggesting that interactions between unliganded LuxP and LuxQp inhibit the conversion of LuxQ from kinase to phosphatase. Binding of AI-2 to LuxPQp induces significant conformational and organizational changes in the complex. First, the two domains in the AI-2-bound LuxP close via a dramatic conformational change. This event, however only minimally alters the LuxQp conformation. What is critical is that, AI-2 binding alters LuxPQp-LuxPQp dimerization with the two LuxPQp dimers undergoing an approximately 140° rotation around an axis between them. Mutations constructed to investigate the role of the new interface formed between the LuxPQp-LuxPQp dimers when AI-2 binds showed that they decrease responsiveness to AI-2 suggesting that the mutations inhibit the two LuxPQp dimers from making the interface required to promote phosphatase activity. Therefore, increased AI-2 is required to switch LuxQ to phosphatase mode. The current model proposes that two LuxPQ complexes form a symmetric hetero-tetramer (LuxPQ-LuxPQ) in the absence of AI-2 at LCD. Analogous to what has been shown for the histidine kinases EnvZ and NtrB (86, 144), autophosphorylation is presumed to occur by cytoplasmic cross-phosphorylation between two LuxQ histidine kinase monomers (although this has not been verified for LuxQ). At HCD, AI-2 binding to LuxP causes a large rotation of one of the LuxPQ subunits relative to the other LuxPQ subunit. This rotational change disrupts the symmetry of the LuxPQ-LuxPQ tetramer, and thus prevents cross-phosphorylation between the cytoplasmic regions of the two LuxQ monomers. Moreover, the crystal structures predict that this asymmetric architecture prevents formation of higher order oligomers, suggesting that AI-2-bound LuxPQ tetramers cannot cluster (85). This arrangement potentially reduces premature commitment to quorum sensing due to signal noise (see below).
The second informative signaling study concerns LuxN. The proposed autoinducer binding domain of LuxN contains nine transmembrane-spanning (TM) helices with the N-terminus located on the periplasmic side of the inner membrane (48). Because of this topology, no detailed structural information is available. Rather, an approach that exploited mutagenesis and suppressor analyses, together with HAI-1 antagonist studies and mathematical modeling was used to define the HAI-1 binding site and the in vivo signaling parameters of the LuxN receptor (120). Mutations in TMs 4, 5, 6, and 7, and the intervening periplasmic loops 2 and 3 of LuxN render V. harveyi non-responsive to HAI-1. Analyses of the mutants showed that one class does not bind HAI-1 and thus does not switch from kinase to phosphatase. A second class of mutants has reduced affinity for HAI-1 and thus can switch from kinase to phosphatase albeit only at high concentrations of HAI-1.
A potent competitive HAI-1 antagonist was identified from a high-throughput chemical screen (120). This antagonist was used to further probe the LuxN/HAI-1 interaction. By simultaneously varying the amounts of antagonist and HAI-1, HAI-1 dose-response curves could generated, which first, defined the HAI-1 EC50 value for wild type LuxN to be 20 nM. Second, the data from all the curves could be collapsed into a single curve. The principal underlying the data fitting is that there is a fixed relation between the kinase and phosphatase configurations of LuxN such that the probability for LuxN to be a kinase depends on the free energy difference between the two configurations. Using this analysis, the dissociation constants (KD) for HAI-1 for wild type LuxN and the various LuxN mutants were determined. In the phosphatase state, KHAI-1 is about 1 nM, and in the kinase state, Kantagonist is about 500 nM. The mutants could then be classified as those that affect HAI-1 binding (i.e. with altered KD), and those that do not affect HAI-1 binding (similar KD) but have altered free energy differences between the two configurations (kinase and phosphatase) of LuxN. One additional prediction from these analyses is that the probability for LuxN to be a kinase in the absence of HAI-1 is about 96%, which explains the large ratio between the EC50 value (20 nM) and the underlying KD (1 nM).
This final observation of a large difference in ligand EC50 and KD differs dramatically from what has been found in the classic chemotaxis two-component signaling network where there exists only a small difference (~0.1) between EC50 and KD. Thus, in the chemotaxis system, there is a roughly equal probability for the receptor to be a kinase or a phosphatase when ligand concentration is low (50). The difference between the receptors in the quorum-sensing and chemotaxis circuits apparently allows each system to solve its respective biological problem effectively. In chemotaxis, bacteria need to respond rapidly to small alterations in signal concentrations, therefore, the receptors are poised to immediately change from kinase to phosphatase and vice versa by spending half of the time in each state. Furthermore, chemotaxis receptors are clustered in arrays that promote amplification of the signal (68, 112, 115). By contrast, quorum-sensing receptors, as discussed above, do not cluster and distribute evenly on the bacterial inner membrane (85), so they do not amplify small perturbations in ligand concentration. Furthermore, switching from kinase to phosphatase requires a significant buildup of autoinducer since the receptors spend almost 100% of the time in the kinase state (120). An overall reduced signal sensitivity in the quorum-sensing system presumably prevents accidental commitment to group behavior in response to signal noise.
Although each of the above studies was focused exclusively on one V. harveyi receptor: structural analysis of LuxPQ and antagonism analysis of LuxN, it is assumed that the intrinsic signaling properties (i.e. the non-clustering of receptors observed for LuxPQ and the low signal sensitivity observed for LuxN) will apply generally to the other V. harveyi and V. cholerae quorum-sensing receptors (e.g. V. harveyi and V. cholerae CqsS, V. cholerae LuxPQ). In fact, studies on V. cholerae suggest that the CqsS receptor also has low signal sensitivity (W.-L. Ng, unpublished).
Functions of Qrr sRNAs in V. harveyi and V. cholerae: Additivity Versus Redundancy
At the heart of V. harveyi and V. cholerae quorum-sensing circuits lie multiple Qrr sRNAs, and the precisely controlled levels of these sRNAs dictate whether cells switch into or out of quorum-sensing behavior (Figures 4 and 5). At LCD, the Qrr sRNAs are transcribed and prevent production of LuxR/HapR. Qrr sRNAs function by Hfq-assisted base pairing with the mRNA of luxR/hapR, which blocks the translation and destabilizes the transcript (57, 124). At HCD, the Qrr sRNAs are not transcribed, the luxR/hapR mRNA accumulates and LuxR/HapR protein is produced. As mentioned earlier, V. harveyi possess five Qrr sRNAs (Qrr1-5) and V. cholerae possess four Qrr sRNAs (Qrr1-4). The LCD transcription of the Qrr sRNAs is controlled by LuxO-P in both V. harveyi and V. cholerae. In V. harveyi, the steady state level at LCD is highest for Qrr4, followed by Qrr2, Qrr3, Qrr1, and then Qrr5 (124). In V. cholerae, a similar pattern is observed (i.e. Qrr4 > Qrr2 ≈ Qrr3 > Qrr1) (56, 57, 118). Due to their unique expression levels, it is presumed that the relative strength of each sRNA in controlling quorum-sensing regulated genes via LuxR/HapR follows the same order. Although the sequences of the Qrr sRNAs required for targeting the luxR/hapR mRNAs are identical in both V. harveyi and V. cholerae and the overall sequences of the mRNAs are extraordinarily similar (>80% identity) the sRNAs nonetheless function by different means in the two Vibrio species.
In V. cholerae, Qrr1-4 function redundantly to regulate quorum sensing. That is, any single Qrr sRNA is sufficient to destabilize the hapR mRNA and prevent production of HapR. Therefore, the simultaneous deletion of the four qrr sRNA genes is required to alter quorum sensing (57). Functional redundancy of the V. cholerae four Qrr sRNAs stems from qrr gene dosage compensation. Transcription of a particular qrr gene is affected by the amount of the other three Qrr sRNAs present (118). For example, in the absence of three Qrr sRNAs, transcription of the remaining qrr sRNA gene is increased. qrr gene dosage compensation maintains the Qrr sRNA pool to within a specific range. Dosage compensation depends on two feedback loops called the HapR-Qrr feedback loop and the LuxO-Qrr feedback loop, which are discussed below.
In stark contrast to how the Qrrs function in V. cholerae, in V. harveyi, the five Qrr sRNAs work additively to control quorum sensing. Thus, deletion of any single qrr gene results in a quorum-sensing phenotypic change (124). As mentioned, the strength of each Qrr sRNA in controlling quorum sensing mirrors its respective expression level (i.e. Qrr4 > Qrr2 > Qrr3 > Qrr1 >Qrr5). Therefore, quorum-sensing behavior is nearly wild-type in a mutant possessing only qrr4, whereas in a mutant possessing only qrr5 quorum-sensing behavior is nearly non-existent (124). The two feedback loops analogous to those in V. cholerae, in this case called the LuxR-Qrr feedback loop and the LuxO-Qrr feedback loop, also exist and function in V. harveyi (125, 126). For reasons that are not yet clear, V. harveyi Qrr sRNA mutants do not fully dosage compensate which results in the observed alterations in their quorum-sensing phenotypes. One hypothesis is that transcription of each V. harveyi qrr gene is subject to additional controls that allow fine-tuning of LuxR production under different environmental conditions in addition to the autoinducer inputs.
Processing the Information Contained in Multiple Autoinducers in V. harveyi and V. cholerae
It is not uncommon for a single bacterial species to produce and detect multiple autoinducers (AHLs or peptides) and/or to produce more than one type of autoinducer (45, 91, 110, 111, 140). In these cases, unique information is contained in each autoinducer, and the bacterium has some mechanism to differentiate between and discretely respond to each signal. For example, P. aeruginosa produces two AHLs, 3OC12-HSL and C4-HSL, by LasI and RhlI, respectively (Figure 1A). The systems are arranged in series such that expression of the Rhl system is activated by the LasR-AHL bound complex. Hence, production of 3OC12HSL precedes production of C4HSL (52, 72, 93); and likewise 3OC12HSL-responsive genes are expressed prior to those responsive to C4HSL. P. aeruginosa also produces a third autoinducer, 2-heptyl-3-hydroxy-4-quinolone, designated the Pseudomonas Quinolone Signal (PQS) (Figure 1E) (92). Production of PQS is influenced both positively and negatively by the LasIR and RhlIR systems, respectively (71, 133). In B. subtilis, the peptide autoinducers ComX and CSF have opposing functions in the control of the competence and sporulation pathways (69, 111). ComX accumulation stimulates ComP-dependent phosphorylation of the ComA response regulator which promotes competence (69). By contrast, high concentration of CSF antagonizes ComX-induced phosphorylation of ComA, which decreases competence development, and instead, favors the sporulation pathway (53). As mentioned, four S. aureus specificity groups exist and each group produces an autoinducer peptide that functions as a quorum-sensing agonist in its own S. aureus group but acts as a quorum-sensing antagonist in the heterologous S. aureus groups, resulting in interference in the latter’s quorum-sensing response (43, 45, 66).
Surprisingly, the sensory information contained in the three V. harveyi autoinducers and likewise the two V. cholerae autoinducers is transduced into the cells via shared phosphorelay cascades. Specifically, in both Vibrio species, the two-component autoinducer receptors channel phosphate to and from a single phosphotransfer protein LuxU (see Figures 4 and 5, and associated text). This network arrangement raises the intriguing question of whether or not V. harveyi and V. cholerae can distinguish between the different autoinducers. While this is not completely understood at present, it is clear that, at a minimum, the different autoinducer signals certainly have different strengths. In V. harveyi, HAI-1 > AI-2 > CAI-1 (37). By contrast, in V. cholerae, CAI-1 > AI-2 (75). The differences in signal strengths are likely due to differences in the relative enzymatic activities of the respective receptor kinases/phosphatases. Differences in the intrinsic receptor signaling parameters (e.g. binding constants, free energy differences between on/off configurations) may also play roles in the strengths of the different autoinducers.
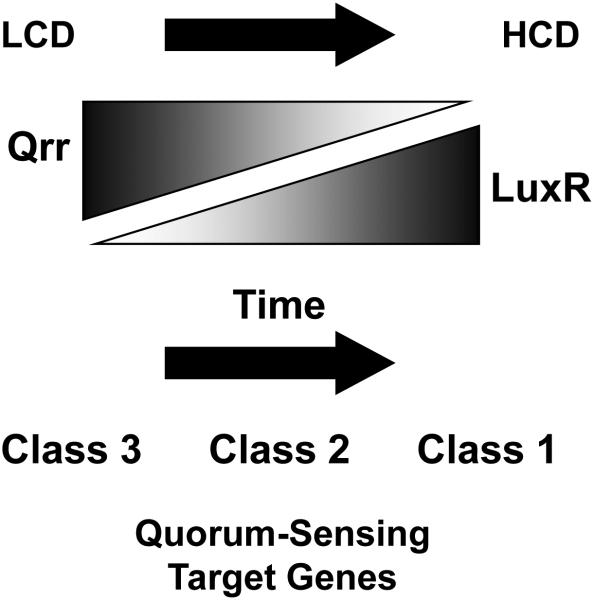
The question of the mechanism underlying signal discrimination in the Vibrios is being most intensively investigated in V. harveyi with respect to how the bacterium distinguishes between its two strongest signals, HAI-1 and AI-2 (60, 80, 135). Several studies, while not yet conclusively solving the problem, have provided insight into how a bacterium can use shared regulatory components and nonetheless respond discretely to the different signals. In one study, expression of over 50 V. harveyi quorum-sensing regulated genes was analyzed in the presence of only HAI-1, only AI-2, or both autoinducers together. Three classes of genes were identified: Class 1 genes show significant regulation only in the simultaneous presence of HAI-1 and AI-2 (i.e. coincidence regulation). Class 2 genes exhibit an alteration in expression when either HAI-1 or AI-2 is present, and expression changes more drastically when both autoinducers are supplied together. Class 3 genes exhibit expression changes in the presence of either HAI-1 alone, or AI-2 alone, and supplying both autoinducers simultaneously does not produce a response that is different from that to one autoinducer alone (135). This report demonstrated that the various combinations of autoinducers produce graded, but inversely correlated changes in Qrr sRNA and LuxR levels (Figure 6). Specifically, low concentrations of autoinducers (i.e., at LCD) lead to high qrr and low luxR expression. High concentrations of autoinducers (i.e., at HCD) result in low qrr and high luxR expression ((135) and see Figures 4 and 6). Thus, the three classes of target-gene responses can be understood in terms of a LuxR promoter affinity model (135). Target genes with promoters that have high affinity for LuxR are regulated in response to the lowest levels of autoinducer, and thus, these genes (Class 3) are the first ones activated/repressed when cells switch into quorum-sensing mode. Genes with promoters that have low affinity for LuxR only respond to the highest autoinducer levels and are regulated at later times in growth (Class 1). Class 2 genes, which respond to mid-level autoinducer concentrations are expressed after Class 3 and prior to Class 1 genes ((135) and Figure 6).
Figure 6.
Reciprocal production of V. harveyi Qrr sRNAs and LuxR leads to temporal control of quorum-sensing target genes. From LCD to HCD, Qrr sRNA levels decrease and LuxR levels increase. As a consequence, Class 3 quorum-sensing target genes, whose promoters have the highest affinity for LuxR, are activated/repressed first, followed by Class 2 genes, and finally Class 1 genes.
To further understand how LuxR regulates genes with different affinities, the LuxR DNA recognition sequence was identified using protein binding microarrays (97). The consensus sequence for LuxR binding contains a 21 bp operator with dyad symmetry, and the critical bases for binding in each half-site are independent of one another. Some LuxR-regulated genes possess multiple binding sites in their promoters, suggesting cooperative binding which may also play a role in the timing and strength of LuxR-dependent regulation (97). Another study with SmcR, the LuxR homolog in Vibrio vulnificus, defined a 22 bp consensus sequence that is highly similar to that found for V. harveyi LuxR (54).
Using single-cell fluorescence microscopy analyses of qrr-gfp, the V. harveyi responses to HAI-1 and AI-2 were quantified. Each autoinducer contributes nearly equally to the total output response (60). Thus, the information from the two distinct autoinducers is combined additively. Based on these analyses, it is proposed that V. harveyi can distinguish between at least three distinct conditions of external autoinducer. First, high qrr-gfp expression occurs when both HAI-1 and AI-2 concentrations are low. Second, low qrr-gfp expression occurs when both HAI-1 and AI-2 concentrations are high. Third, intermediate qrr-gfp expression occurs when one autoinducer concentration is low and the other is high. However, in this final situation, high HAI-1 combined with low AI-2 concentration is indistinguishable from low HAI-1 combined with high AI-2 concentration (60). These recent findings led to the idea that detecting multiple autoinducers allows V. harveyi to monitor the developmental stage of the community. This model assumes that production of each autoinducer follows an invariant temporal order in which high HAI-1/low AI-2 and low HAI-1/high AI-2 are mutually exclusive (60). These findings also suggest that autoinducers allow populations of V. harveyi to monitor the species composition of the community. As mentioned, the different autoinducers specify the relatedness of the members of the community: HAI-1 is only produced by V. harveyi, CAI-1 is produced by many Vibrios, and AI-2 is produced by widely diverse bacterial species. Therefore, the different combinations of autoinducers could reflect the composition and abundance of species in the vicinal community (60). Because the V. harveyi CqsA/CqsS system has not yet been included in these types of investigations and no signal discrimination studies have been performed in V. cholerae, it remains an open question as to whether these findings can be more generally applied.
Feedback Control of Quorum Sensing in V. harveyi and V. cholerae
Positive feedback is a hallmark of quorum-sensing regulatory networks. As mentioned, in canonical LuxI/LuxR quorum-sensing systems, expression of the autoinducer synthase gene is positively controlled by the AHL-bound LuxR type protein (18, 26, 108). The outcome of these feedback loops is accelerated production of AHL autoinducer, which leads to synchrony in quorum-sensing behavior. Positive feedback loops exist in oligopeptide-based Gram-positive quorum-sensing systems (46, 95). In many cases, the genes encoding the peptide signal, the histidine kinase receptor, the cognate response regulator, and its accessory factors form an operon (for example, the agrBDCA and comCDE operons of S. aureus and S. pneumoniae, respectively). Furthermore, typically the response regulator in the system acts as an auto-activator of the operon. This autoregulatory wiring fosters positive feedback through which the levels of the peptide ligand, the membrane receptor, and the response regulator all increase drastically once the autoinducer has accumulated above the initial concentration required for detection.
In V. harveyi and V. cholerae, a different set of feedback loops have recently been identified that ensure precise timing of quorum-sensing transitions (Figure 7). These feedback loops are summarized as follow:
HapR/LuxR auto-repression loop
HapR/LuxR-Qrr feedback loop
LuxO auto-repression loop
LuxO-Qrr feedback loop
Figure 7.
Feedback loops identified in the V. harveyi and V. cholerae quorum-sensing networks. Four different feedback loops are integrated into the V. harveyi and V. cholerae quorum-sensing circuits. Arrows denote activation. T-shape arrows denote repression.
HapR/LuxR auto-repression loop
HapR and LuxR bind to their own promoters and represses transcription (12, 59), which results in a steady increase in HapR/LuxR levels as cell density increases. Thus, this feedback loop, by preventing runaway expression of LuxR/HapR, minimizes the chances of premature commitment of the cells to population-wide changes in gene expression.
HapR/LuxR-Qrr feedback loop
During the transition from LCD to HCD, LuxO-P levels decrease as autoinducer levels increase resulting in decreased qrr transcription and, in turn, increased HapR/LuxR production (Figures 4 and 5). HapR/LuxR feeds back as a transcriptional activator of the qrr genes (Figure 7) (119, 126). When cells switch from LCD to HCD, the HapR/LuxR-Qrr feedback loop prolongs the production of Qrr sRNAs and delays the entry into HCD mode. By contrast, when cells switch from HCD to LCD, the HapR/LuxR-Qrr feedback loop dramatically increases expression of the qrr genes and accelerates the transition out of quorum-sensing mode and into individual-cell behavior (119, 126). Interestingly, HapR (V. cholerae) acts indirectly on qrr1-4 in the HapR-Qrr feedback loop whereas LuxR (V. harveyi) binds directly to qrr promoters in the feedback mechanism. However, in V. harveyi, qrr2, qrr3, and qrr4 are subject to LuxR feedback control but qrr1 and qrr5 are not (119, 126).
LuxO auto-repression loop
The qrr1 gene and the luxO gene lie adjacent to one another in the genome and are transcribed divergently (57). The LuxO-binding site required for qrr1 expression overlaps with the -35 site in the luxO promoter. This unique organization allows LuxO to simultaneously activate expression of qrr1 and repress its own transcription via blocking access to RNAP for transcription (125). Although LuxO requires phosphorylation to act as a transcriptional activator, LuxO-autorepression does not require that LuxO be phosphorylated (118, 125). The LuxO auto-repression loop limits the amount of LuxO to within a narrow window, the consequence of which is to carefully control production of the Qrr sRNAs. The LuxO auto-repression loop is critical for ensuring precise timing of the quorum-sensing transition (125).
LuxO-Qrr feedback loop
Analogous to the way the Qrr sRNAs act on the luxR/hapR mRNAs, the Qrr sRNAs bind to and destabilize the mRNA encoding LuxO and stimulate its degradation. This prevents LuxO protein production. The LuxO-Qrr feedback loop works synergistically with the LuxO auto-repression loop, to restrict Qrr sRNA levels to only a narrow range by limiting fluctuations in LuxO levels (118, 125). Also, as discussed above, the other function of the LuxO-Qrr feedback loop is in qrr gene dosage compensation (118).
Why are so many feedback loops involved in controlling the V. harveyi and V. cholerae quorum-sensing responses? As discussed above, the quorum sensing receptors are insensitive to small perturbations in signal suggesting that the V. harveyi and V. cholerae networks are tuned to ignore sudden fluctuations in the environment. Presumably then, a sudden surge in any component in the quorum sensing network would be detrimental. Indeed, all of the studies of the V. harveyi and V. cholerae feedback loops point to their functioning together to minimize fluctuations in the levels of individual components in the quorum-sensing circuits. The LuxR/HapR auto-repression loop prevents sudden increases in the level of the master quorum-sensing regulator LuxR/HapR (12, 59). The LuxR/HapR-Qrr feedback loop also delays production of LuxR/HapR (119, 126). Finally, the LuxO-Qrr feedback loop together with the LuxO auto-repression loop limit fluctuations in LuxO levels, which prevents surges of Qrr sRNA levels (118, 125). We note that feedback loops similar to those described in these two quorum-sensing systems are common network motifs in biological circuits (99). Feedback loops are known to reduce variations in the steady state levels of regulatory components. In the case of the V. harveyi and V. cholerae quorum-sensing networks, by minimizing alterations in cytoplasmic quorum-sensing regulatory components, the feedback loops, in turn, limit cell-to-cell variations in behavior. In so doing, the feedback loops impose synchrony on the population-wide quorum sensing response which is imperative for successful collective behaviors.
Outlook and Future Directions
A major goal of studying the quorum-sensing systems of V. harveyi and V. cholerae is to understand at the molecular, cellular, and population levels the process used by bacteria for cell-cell communication. Fundamental questions pertaining to this goal remain. Multiple quorum-sensing signals are channeled into a single circuit; yet different signal inputs lead to differential gene expression outputs. Particularly critical is to understand what governs the relative signal strengths of LuxM/LuxN, LuxS/LuxPQ, and CqsA/CqsS. How does CqsS detect and respond to both CAI-1 and amino-CAI-1, and do LuxPQ and CqsS receptors have signaling parameters similar to those of LuxN? Moreover, the networks employ both RNA-based and protein-based regulatory factors. Until recently, sRNA-mediated gene regulation was under-appreciated in bacteria (30, 136). Thus, it remains to be investigated why sRNAs are optimal for quorum-sensing regulation and what advantages multiple sRNAs provide the circuit. For instance, are sRNA regulators more precise than protein regulators in controlling gene expression because they are less prone to fluctuation? Do Qrr sRNAs have different affinities for their targets (e.g. luxO, luxR/hapR, and the recently discovered vca0939 (33)), and if so, does this impinge on the dynamics of the quorum-sensing transitions? What is the molecular mechanism underpinning the differential expression of the qrr genes? What is probably most remarkable when one ponders the V. harveyi and V. cholerae quorum-sensing systems is that they employ a set of nearly identical constituent components, yet, the functioning of these components and the behaviors of the two systems are dramatically different.
Finally, many open questions remain regarding the evolutionary forces that shape quorum-sensing systems, and how quorum-sensing behaviors of individual cells translate into the collective properties of bacterial groups (82, 138). Recent work has shown that populations of P. aeruginosa, which use quorum sensing to up-regulate virulence at HCD, can be invaded both by mutants that do not produce autoinducers and by mutants that do not respond to autoinducers (16, 102). When mixed with wild type cells in equal proportion, exploitative P. aeruginosa quorum-sensing mutants decreased the virulence profiles of both acute and chronic P. aeruginosa infections in a mouse model (101). In contrast to Pseudomonas and many other systems, the Vibrios activate virulence factor expression at LCD and repress these traits at HCD. Theory suggests that Vibrios do so in order to efficiently escape from their hosts (83, 147), but these predictions have not yet been tested by experiments. These newest findings make it clear that the evolution of quorum sensing on short time scales must be addressed as the field turns to the development of biotechnological therapies to manipulate quorum sensing. Furthermore, clarifying the ecological pressures that favor particular quorum-sensing regulatory strategies may allow us to better understand how quorum sensing has evolved and continues to evolve in bacterial populations.
HIGHLIGHTS.
Bacteria use extracellular chemical signal molecules called autoinducers for quorum sensing. Quorum sensing is a cell-cell communication process used to monitor cell number and species complexity in a population. The V. harveyi and V. cholerae quorum-sensing networks exhibit similarity to both canonical Gram-negative and Gram-positive quorum-sensing systems.
V. harveyi possesses three quorum-sensing systems (LuxM/LuxN, LuxS/LuxPQ, and CqsA/CqsS). V. cholerae possesses two quorum-sensing systems (LuxS/LuxPQ, CqsA/CqsS). Although components of the two systems are similar, distinctive features greatly alter their biology.
The quorum-sensing receptors in V. harveyi and V. cholerae are organized such that they prevent dramatic changes in response to small perturbations in autoinducer signals. This arrangement stands in contrast to the bacterial chemotaxis receptor system, which is exquisitely sensitive to small changes in ligand concentration.
Small RNAs (sRNAs) lie at the core of both the V. harveyi and V. cholerae quorum-sensing systems. The sRNAs function differently in these two species. In V. harveyi, five sRNAs function additively. In V. cholerae, four sRNAs function redundantly.
Multiple autoinducers are detected and integrated through shared phosphorelay systems in both the V. harveyi and V. cholerae quorum-sensing systems. Differences exist in the strength of each signal.
Multiple feedback loops exist in the V. harveyi and V. cholerae quorum-sensing networks. These feedback loops prevent fluctuations in the amounts of regulatory components in the respective systems, and ensure precise input-output relationships.
ACKNOWLEDGEMENTS
We thank members of the Bassler laboratory for insightful discussions and Mr. Carey Nadell for his input on evolution of group behaviors in bacteria. This work was supported by NRSA postdoctoral fellowship F32GM082061 for W.-L. Ng, HHMI, NIH grants RO1GM065859 and RO1AI054442 and NSF grant MCB0639855 for B. L. Bassler.
GLOSSARY
- Quorum sensing
a cell-cell communication process used by bacteria to coordinate gene expression in response to changes in population density
- Autoinducers
extracellular signal molecules produced and detected by bacteria to monitor cell population density
ACRONYMS
- ACP
Acyl carrier protein
- AHL
Acyl homoserine lactone autoinducer
- AI-2
Autoinducer 2
- CAI-1
V. cholerae autoinducer 1
- DPD
4,5-dihydroxy-2,3-pentanedione
- GFP
Green fluorescent protein
- HAI-1
V. harveyi autoinducer 1
- HCD
High-cell-density
- LCD
Low-cell-density
- LPS
lipopolysaccharide
- RNAP
RNA polymerase
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- SRH
S-ribosylhomocysteine
- sRNAs
small RNAs
LITERATURE CITED
- 1.Albus AM, Pesci EC, Runyen-Janecky LJ, West SE, Iglewski BH. Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3928–35. doi: 10.1128/jb.179.12.3928-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin B, Zhang XH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol. 2006;43:119–24. doi: 10.1111/j.1472-765X.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 3.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–86. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 4.Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–86. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 5.Bassler BL, Wright M, Silverman MR. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol Microbiol. 1994;12:403–12. doi: 10.1111/j.1365-2958.1994.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 6.Blokesch M, Schoolnik GK. Serogroup conversion of Vibrio cholerae in aquatic reservoirs. PLoS Pathog. 2007;3:e81. doi: 10.1371/journal.ppat.0030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blokesch M, Schoolnik GK. The extracellular nuclease Dns and its role in natural transformation of Vibrio cholerae. J Bacteriol. 2008;190:7232–40. doi: 10.1128/JB.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottomley MJ, Muraglia E, Bazzo R, Carfi A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem. 2007;282:13592–600. doi: 10.1074/jbc.M700556200. [DOI] [PubMed] [Google Scholar]
- 9.Callahan SM, Dunlap PV. LuxR- and acyl-homoserine-lactone-controlled non-lux genes define a quorum-sensing regulon in Vibrio fischeri. J Bacteriol. 2000;182:2811–22. doi: 10.1128/jb.182.10.2811-2822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao JG, Meighen EA. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–6. [PubMed] [Google Scholar]
- 11.Case RJ, Labbate M, Kjelleberg S. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. Isme J. 2008;2:345–9. doi: 10.1038/ismej.2008.13. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee J, Miyamoto CM, Meighen EA. Autoregulation of luxR: the Vibrio harveyi lux-operon activator functions as a repressor. Mol Microbiol. 1996;20:415–25. doi: 10.1111/j.1365-2958.1996.tb02628.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–9. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 14.Croxatto A, Chalker VJ, Lauritz J, Jass J, Hardman A, et al. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J Bacteriol. 2002;184:1617–29. doi: 10.1128/JB.184.6.1617-1629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croxatto A, Pride J, Hardman A, Williams P, Camara M, Milton DL. A distinctive dual-channel quorum-sensing system operates in Vibrio anguillarum. Mol Microbiol. 2004;52:1677–89. doi: 10.1111/j.1365-2958.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 16.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–4. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 17.Dunlap PV. Quorum regulation of luminescence in Vibrio fischeri. J Mol Microbiol Biotechnol. 1999;1:5–12. [PubMed] [Google Scholar]
- 18.Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–81. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 19.Engebrecht J, Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984;81:4154–8. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–14. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Federle MJ, Bassler BL. Interspecies communication in bacteria. J Clin Invest. 2003;112:1291–9. doi: 10.1172/JCI20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fidopiastis PM, Miyamoto CM, Jobling MG, Meighen EA, Ruby EG. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol Microbiol. 2002;45:131–43. doi: 10.1046/j.1365-2958.2002.02996.x. [DOI] [PubMed] [Google Scholar]
- 23.Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–77. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 24.Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–49. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 25.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–68. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 26.Fuqua WC, Winans SC. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisinger E, George EA, Muir TW, Novick RP. Identification of ligand specificity determinants in AgrC, the Staphylococcus aureus quorum-sensing receptor. J Biol Chem. 2008;283:8930–8. doi: 10.1074/jbc.M710227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisinger E, Muir TW, Novick RP. agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc Natl Acad Sci U S A. 2009;106:1216–21. doi: 10.1073/pnas.0807760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilson L, Kuo A, Dunlap PV. AinS and a new family of autoinducer synthesis proteins. J Bacteriol. 1995;177:6946–51. doi: 10.1128/jb.177.23.6946-6951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottesman S. The small RNA regulators of Escherichia coli: roles and mechanisms. Annu Rev Microbiol. 2004;58:303–28. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- 31.Gould TA, Schweizer HP, Churchill ME. Structure of the Pseudomonas aeruginosa acyl-homoserinelactone synthase LasI. Mol Microbiol. 2004;53:1135–46. doi: 10.1111/j.1365-2958.2004.04211.x. [DOI] [PubMed] [Google Scholar]
- 32.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation inVibrio cholerae. Mol Microbiol. 2003;50:101–4. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 33.Hammer BK, Bassler BL. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A. 2007;104:11145–9. doi: 10.1073/pnas.0703860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanzelka BL, Parsek MR, Val DL, Dunlap PV, Cronan JE, Jr., Greenberg EP. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J Bacteriol. 1999;181:5766–70. doi: 10.1128/jb.181.18.5766-5770.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1995;92:11140–4. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henke JM, Bassler BL. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J Bacteriol. 2004;186:3794–805. doi: 10.1128/JB.186.12.3794-3805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004;186:6902–14. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins DA, Kelly RC, Pomianek ME, Lu W, Semmelhack MF, et al. 2009. In preparation.
- 39.Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–6. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- The CAI-1 autoinducer from V. cholerae was purified and its chemical structure was determined in this study. CAI-1 belongs to a new class of autoinducer, and is suggested to be used for inter-Vibrio quorum sensing.
- 40.Hoch JA, Silhavy TJ. Two-Component Signal Transduction. ASM Press; Washington, DC: 1995. p. 488. [Google Scholar]
- 41.Hwang I, Li PL, Zhang L, Piper KR, Cook DM, et al. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci U S A. 1994;91:4639–43. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inouye M, Dutta R. Histidine kinases in signal transduction. Academic Press; London: 2003. p. 520. [Google Scholar]
- 43.Jarraud S, Lyon GJ, Figueiredo AM, Gerard L, Vandenesch F, et al. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J Bacteriol. 2000;182:6517–22. doi: 10.1128/jb.182.22.6517-6522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen RO, Winzer K, Clarke SR, Chan WC, Williams P. Differential recognition of Staphylococcus aureus quorum-sensing signals depends on both extracellular loops 1 and 2 of the transmembrane sensor AgrC. J Mol Biol. 2008;381:300–9. doi: 10.1016/j.jmb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–30. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 46.Ji G, Beavis RC, Novick RP. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci U S A. 1995;92:12055–9. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jobling MG, Holmes RK. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26:1023–34. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 48.Jung K, Odenbach T, Timmen M. The quorum-sensing hybrid histidine kinase LuxN of Vibrio harveyi contains a periplasmically located N terminus. J Bacteriol. 2007;189:2945–8. doi: 10.1128/JB.01723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–4. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keymer JE, Endres RG, Skoge M, Meir Y, Wingreen NS. Chemosensing in Escherichia coli: two regimes of two-state receptors. Proc Natl Acad Sci U S A. 2006;103:1786–91. doi: 10.1073/pnas.0507438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo A, Callahan SM, Dunlap PV. Modulation of luminescence operon expression by N-octanoyl-L-homoserine lactone in ainS mutants of Vibrio fischeri. J Bacteriol. 1996;178:971–6. doi: 10.1128/jb.178.4.971-976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–46. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 53.Lazazzera BA, Solomon JM, Grossman AD. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–25. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 54.Lee DH, Jeong HS, Jeong HG, Kim KM, Kim H, Choi SH. A consensus sequence for binding of SmcR, a Vibrio vulnificus LuxR homologue, and genome-wide identification of the SmcR regulon. J Biol Chem. 2008;283:23610–8. doi: 10.1074/jbc.M801480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee MS, Morrison DA. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol. 1999;181:5004–16. doi: 10.1128/jb.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenz DH, Bassler BL. The small nucleoid protein Fis is involved in Vibrio cholerae quorum sensing. Mol Microbiol. 2007;63:859–71. doi: 10.1111/j.1365-2958.2006.05545.x. [DOI] [PubMed] [Google Scholar]
- 57.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Lilley BN, Bassler BL. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol Microbiol. 2000;36:940–54. doi: 10.1046/j.1365-2958.2000.01913.x. [DOI] [PubMed] [Google Scholar]
- 59.Lin W, Kovacikova G, Skorupski K. Requirements for Vibrio cholerae HapR binding and transcriptional repression at the hapR promoter are distinct from those at the aphA promoter. J Bacteriol. 2005;187:3013–9. doi: 10.1128/JB.187.9.3013-3019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long T, Tu KC, Wang Y, Mehta P, Ong NP, et al. Quantifying the Integration of Quorum-Sensing Signals with Single-Cell Resolution. PLoS Biology. 2009 doi: 10.1371/journal.pbio.1000068. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo P, Li H, Morrison DA. ComX is a unique link between multiple quorum sensing outputs and competence in Streptococcus pneumoniae. Mol Microbiol. 2003;50:623–33. doi: 10.1046/j.1365-2958.2003.03714.x. [DOI] [PubMed] [Google Scholar]
- 62.Luo P, Morrison DA. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J Bacteriol. 2003;185:349–58. doi: 10.1128/JB.185.1.349-358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo ZQ, Su S, Farrand SK. In situ activation of the quorum-sensing transcription factor TraR by cognate and noncognate acyl-homoserine lactone ligands: kinetics and consequences. J Bacteriol. 2003;185:5665–72. doi: 10.1128/JB.185.19.5665-5672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lupp C, Ruby EG. Vibrio fischeri LuxS and AinS: comparative study of two signal synthases. J Bacteriol. 2004;186:3873–81. doi: 10.1128/JB.186.12.3873-3881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lupp C, Urbanowski M, Greenberg EP, Ruby EG. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol Microbiol. 2003;50:319–31. doi: 10.1046/j.1365-2958.2003.t01-1-03585.x. [DOI] [PubMed] [Google Scholar]
- 66.Lyon GJ, Wright JS, Christopoulos A, Novick RP, Muir TW. Reversible and specific extracellular antagonism of receptor-histidine kinase signaling. J Biol Chem. 2002;277:6247–53. doi: 10.1074/jbc.M109989200. [DOI] [PubMed] [Google Scholar]
- 67.Lyon GJ, Wright JS, Muir TW, Novick RP. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry. 2002;41:10095–104. doi: 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- 68.Maddock JR, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–23. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 69.Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–16. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 70.Mayville P, Ji G, Beavis R, Yang H, Goger M, et al. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 1999;96:1218–23. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGrath S, Wade DS, Pesci EC. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS) FEMS Microbiol Lett. 2004;230:27–34. doi: 10.1016/S0378-1097(03)00849-8. [DOI] [PubMed] [Google Scholar]
- 72.Medina G, Juarez K, Diaz R, Soberon-Chavez G. Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology. 2003;149:3073–81. doi: 10.1099/mic.0.26282-0. [DOI] [PubMed] [Google Scholar]
- 73.Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–7. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 74.Meighen EA. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991;55:123–42. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–14. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 76.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, et al. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15:677–87. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 77.Milton DL. Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol. 2006;296:61–71. doi: 10.1016/j.ijmm.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 78.Milton DL, Chalker VJ, Kirke D, Hardman A, Camara M, Williams P. The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl)homoserine lactone and N-hexanoylhomoserine lactone. J Bacteriol. 2001;183:3537–47. doi: 10.1128/JB.183.12.3537-3547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minogue TD, Wehland-von Trebra M, Bernhard F, von Bodman SB. The autoregulatory role of EsaR, a quorum-sensing regulator in Pantoea stewartii ssp. stewartii: evidence for a repressor function. Mol Microbiol. 2002;44:1625–35. doi: 10.1046/j.1365-2958.2002.02987.x. [DOI] [PubMed] [Google Scholar]
- 80.Mok KC, Wingreen NS, Bassler BL. Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. Embo J. 2003;22:870–81. doi: 10.1093/emboj/cdg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.More MI, Finger LD, Stryker JL, Fuqua C, Eberhard A, Winans SC. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–8. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 82.Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol Rev. 2009;33:206–24. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 83.Nadell CD, Xavier JB, Levin SA, Foster KR. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 2008;6:e14. doi: 10.1371/journal.pbio.0060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell. 2005;18:507–18. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 85.Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ninfa EG, Atkinson MR, Kamberov ES, Ninfa AJ. Mechanism of autophosphorylation of Escherichia coli nitrogen regulator II (NRII or NtrB): trans-phosphorylation between subunits. J Bacteriol. 1993;175:7024–32. doi: 10.1128/jb.175.21.7024-7032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–64. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 88.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–30. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 90.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1995;92:1490–4. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96:11229–34. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–32. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pestova EV, Havarstein LS, Morrison DA. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–62. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 95.Peterson S, Cline RT, Tettelin H, Sharov V, Morrison DA. Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J Bacteriol. 2000;182:6192–202. doi: 10.1128/jb.182.21.6192-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piper KR, Beck von Bodman S, Farrand SK. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–50. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 97.Pompeani AJ, Irgon JJ, Berger MF, Bulyk ML, Wingreen NS, Bassler BL. The Vibrio harveyi master quorum-sensing regulator, LuxR, a TetR-type protein is both an activator and a repressor: DNA recognition and binding specificity at target promoters. Mol Microbiol. 2008;70:76–88. doi: 10.1111/j.1365-2958.2008.06389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rickard AH, Palmer RJ, Jr., Blehert DS, Campagna SR, Semmelhack MF, et al. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol. 2006;60:1446–56. doi: 10.1111/j.1365-2958.2006.05202.x. [DOI] [PubMed] [Google Scholar]
- 99.Rosenfeld N, Elowitz MB, Alon U. Negative autoregulation speeds the response times of transcription networks. J Mol Biol. 2002;323:785–93. doi: 10.1016/s0022-2836(02)00994-4. [DOI] [PubMed] [Google Scholar]
- 100.Ruby EG. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu Rev Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. [DOI] [PubMed] [Google Scholar]
- 101.Rumbaugh KP, Diggle SP, Watters CM, Ross-Gillespie A, Griffin AS, West SA. Quorum sensing and the social evolution of bacterial virulence. Curr Biol. 2009;19:341–5. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 102.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci U S A. 2007;104:15876–81. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–9. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- This is the first study to show that bacteria use the extracellular fatty acid pool (e.g. p-coumaric acid) for AHL biosynthesis. This finding implies the possibility of bacteria using AHL-mediated quorum sensing for inter-kingdom signaling between bacteria and plants.
- 104.Schaefer AL, Val DL, Hanzelka BL, Cronan JE, Jr., Greenberg EP. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci U S A. 1996;93:9505–9. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–76. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 106.Schuster M, Greenberg EP. LuxR-Type Proteins in Pseudomonas aeruginosa Quorum Sensing: Distinct Mechanisms with Global Implications. In: Winans SC, Bassler BL, editors. Chemical Communication among Bacteria. ASM Press; Washington, DC: 2008. pp. 133–44. [Google Scholar]
- 107.Schuster M, Urbanowski ML, Greenberg EP. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci U S A. 2004;101:15833–9. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seed PC, Passador L, Iglewski BH. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–9. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simon MI, Crane BR, Crane A. Two-component Signaling Systems. Academic Press; San Diego: 2007. [Google Scholar]
- 110.Solomon JM, Lazazzera BA, Grossman AD. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–24. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 111.Solomon JM, Magnuson R, Srivastava A, Grossman AD. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–58. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 112.Sourjik V, Berg HC. Functional interactions between receptors in bacterial chemotaxis. Nature. 2004;428:437–41. doi: 10.1038/nature02406. [DOI] [PubMed] [Google Scholar]
- 113.Spirig T, Tiaden A, Kiefer P, Buchrieser C, Vorholt JA, Hilbi H. The Legionella autoinducer synthase LqsA produces an alpha-hydroxyketone signaling molecule. J Biol Chem. 2008;283:18113–23. doi: 10.1074/jbc.M801929200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stevens AM, Dolan KM, Greenberg EP. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc Natl Acad Sci U S A. 1994;91:12619–23. doi: 10.1073/pnas.91.26.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Studdert CA, Parkinson JS. Insights into the organization and dynamics of bacterial chemoreceptor clusters through in vivo crosslinking studies. Proc Natl Acad Sci U S A. 2005;102:15623–8. doi: 10.1073/pnas.0506040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sung CK, Morrison DA. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J Bacteriol. 2005;187:3052–61. doi: 10.1128/JB.187.9.3052-3061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A. 1999;96:1639–44. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Svenningsen SL, Tu KC, Bassler BL. Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. Embo J. 2009;28:429–39. doi: 10.1038/emboj.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Svenningsen SL, Waters CM, Bassler BL. A negative feedback loop involving small RNAs accelerates Vibrio cholerae's transition out of quorum-sensing mode. Genes Dev. 2008;22:226–38. doi: 10.1101/gad.1629908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swem LR, Swem DL, Wingreen NS, Bassler BL. Deducing receptor signaling parameters from in vivo analysis: LuxN/AI-1 quorum sensing in Vibrio harveyi. Cell. 2008;134:461–73. doi: 10.1016/j.cell.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Using mutagenesis, mathematical modeling, and antagonist analyses, signaling parameters of the membrane-bound quorum-sensing receptor LuxN in V. harveyi were determined. This approach could be useful for studying other membrane-bound signal receptors for which structures are not available.
- 121.Sztajer H, Lemme A, Vilchez R, Schulz S, Geffers R, et al. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J Bacteriol. 2008;190:401–15. doi: 10.1128/JB.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tiaden A, Spirig T, Carranza P, Bruggemann H, Riedel K, et al. Synergistic contribution of the Legionella pneumophila lqs genes to pathogen-host interactions. J Bacteriol. 2008;190:7532–47. doi: 10.1128/JB.01002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tiaden A, Spirig T, Weber SS, Bruggemann H, Bosshard R, et al. The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell Microbiol. 2007;9:2903–20. doi: 10.1111/j.1462-5822.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 124.Tu KC, Bassler BL. Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev. 2007;21:221–33. doi: 10.1101/gad.1502407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tu KC, Svenningsen SL, Long T, Wingreen NS, Bassler BL. 2009. In preparation. [DOI] [PMC free article] [PubMed]
- 126.Tu KC, Waters CM, Svenningsen SL, Bassler BL. A small-RNA-mediated negative feedback loop controls quorum-sensing dynamics in Vibrio harveyi. Mol Microbiol. 2008;70:896–907. doi: 10.1111/j.1365-2958.2008.06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urbanowski ML, Lostroh CP, Greenberg EP. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J Bacteriol. 2004;186:631–7. doi: 10.1128/JB.186.3.631-637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, et al. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. Embo J. 2002;21:4393–401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3:383–96. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 130.von Bodman SB, Ball JK, Faini MA, Herrera CM, Minogue TD, et al. The quorum sensing negative regulators EsaR and ExpR(Ecc), homologues within the LuxR family, retain the ability to function as activators of transcription. J Bacteriol. 2003;185:7001–7. doi: 10.1128/JB.185.23.7001-7007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.von Bodman SB, Farrand SK. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J Bacteriol. 1995;177:5000–8. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.von Bodman SB, Majerczak DR, Coplin DL. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc Natl Acad Sci U S A. 1998;95:7687–92. doi: 10.1073/pnas.95.13.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, et al. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol. 2005;187:4372–80. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 135.Waters CM, Bassler BL. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 2006;20:2754–67. doi: 10.1101/gad.1466506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–28. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watson WT, Minogue TD, Val DL, von Bodman SB, Churchill ME. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol Cell. 2002;9:685–94. doi: 10.1016/s1097-2765(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 138.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 139.Williams P, Winzer K, Chan WC, Camara M. Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci. 2007;362:1119–34. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Winson MK, Camara M, Latifi A, Foglino M, Chhabra SR, et al. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1995;92:9427–31. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 2003;4:253–63. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 142.Wright JS, 3rd, Lyon GJ, George EA, Muir TW, Novick RP. Hydrophobic interactions drive ligand-receptor recognition for activation and inhibition of staphylococcal quorum sensing. Proc Natl Acad Sci U S A. 2004;101:16168–73. doi: 10.1073/pnas.0404039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xavier KB, Bassler BL. LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol. 2003;6:191–7. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 144.Yang Y, Inouye M. Intermolecular complementation between two defective mutant signal-transducing receptors of Escherichia coli. Proc Natl Acad Sci U S A. 1991;88:11057–61. doi: 10.1073/pnas.88.24.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yao Y, Martinez-Yamout MA, Dickerson TJ, Brogan AP, Wright PE, Dyson HJ. Structure of the Escherichia coli quorum sensing protein SdiA: activation of the folding switch by acyl homoserine lactones. J Mol Biol. 2006;355:262–73. doi: 10.1016/j.jmb.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 146.Zhang RG, Pappas T, Brace JL, Miller PC, Oulmassov T, et al. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–4. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 147.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–56. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 148.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002;99:3129–34. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu J, Winans SC. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci U S A. 1999;96:4832–7. doi: 10.1073/pnas.96.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci U S A. 2001;98:1507–12. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]