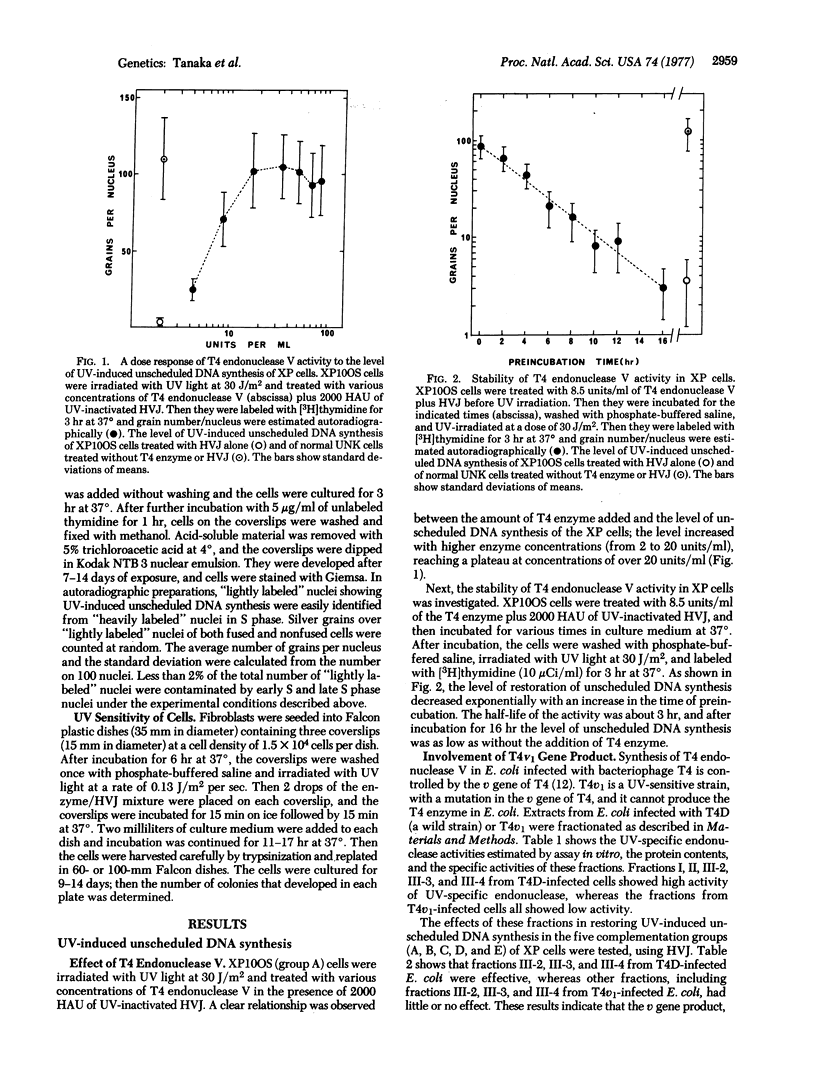
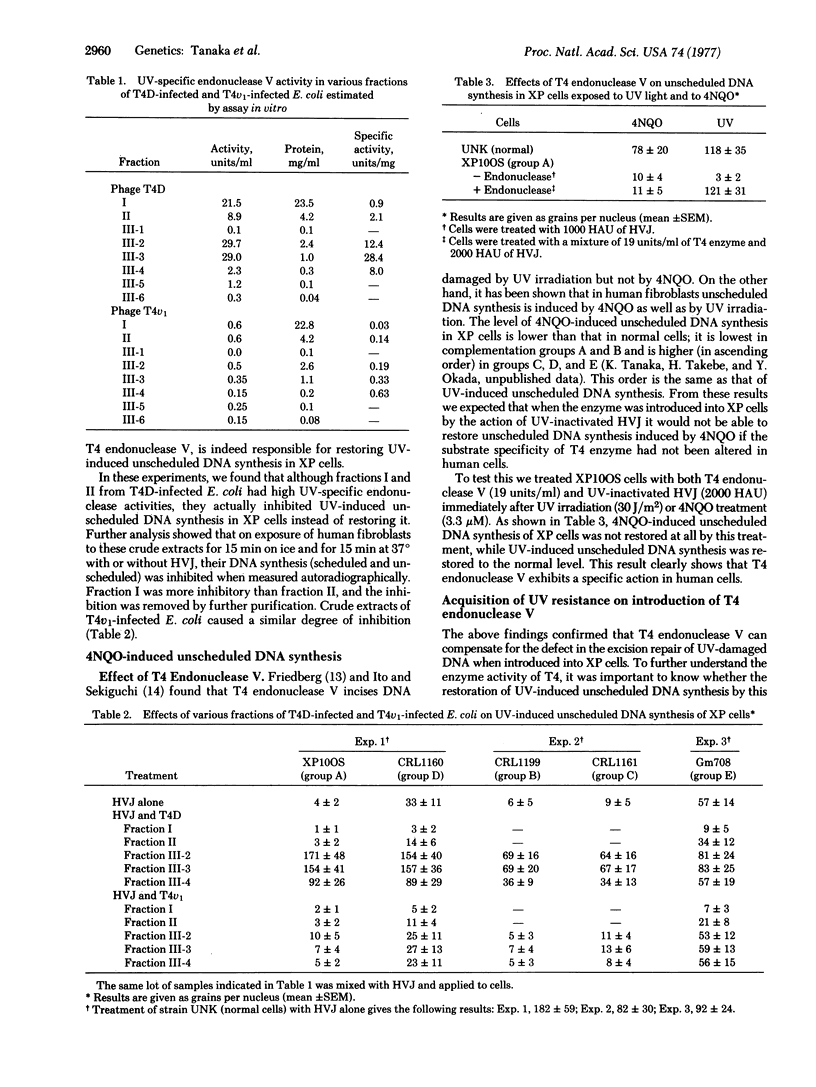
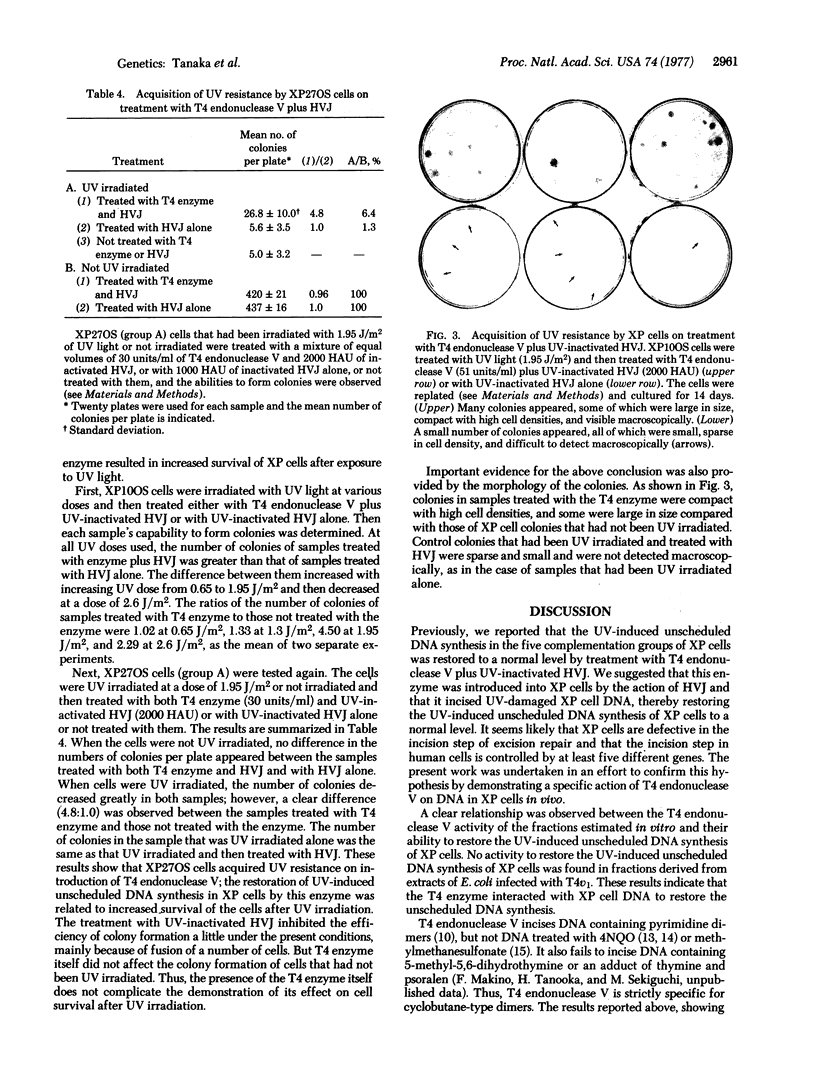
Abstract
The specific action of T4 endonuclease V on damaged DNA in xeroderma pigmentosum cells was examined using an in vivo assay system with hemagglutinating virus of Japan (Sendai virus) inactivated by UV light. A clear dose response was observed between the level of UV-induce unscheduled DNA synthesis of xeroderma pigmentosum cells and the amount of T4 endonuclease V activity added. The T4 enzyme was unstable in human cells, and its half-life was 3 hr. Fractions derived from an extract of Escherichia coli infected with T4V1, a mutant defective in the endonuclease V gene, showed no ability to restore the UV-induced unscheduled DNA synthesis of xeroderma pigmentosum cells. However, fractions derived from an extract of T4D-infected E. coli with endonuclease V activity were effective. The T4 enzyme was effective in xeroderma pigmentosum cells on DNA damaged by UV light but not in cells damaged by 4-nitroquinoline 1-oxide. The results of these experiments show that the T4 enzyme has a specific action on human cell DNA in vivo. Treatment with the T4 enzyme increased the survival of group A xeroderma pigmentosum cells after UV irradiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd Human cells repair DNA damaged by nitrous acid. Mutat Res. 1975 Mar;27(3):407–409. doi: 10.1016/0027-5107(75)90298-5. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Epstein J. H., Fukuyama K., Reed W. B., Epstein W. L. Defect in DNA synthesis in skin of patients with xeroderma pigmentosum demonstrated in vivo. Science. 1970 Jun 19;168(3938):1477–1478. doi: 10.1126/science.168.3938.1477. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Studies on the substrate specificity of the T 4 excision repair endonuclease. Mutat Res. 1972 Jun;15(2):113–123. doi: 10.1016/0027-5107(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Furusawa M., Nishimura T., Yamaizumi M., Okada Y. Injection of foreign substances into single cells by cell fusion. Nature. 1974 May 31;249(456):449–450. doi: 10.1038/249449a0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., De Weerd-Kastelein E. A., Robbins J. H., Keijzer W., Barrett S. F., Petinga R. A., Bootsma D. Five complementation groups in xeroderma pigmentosum. Mutat Res. 1975 Dec;33(2-3):327–340. doi: 10.1016/0027-5107(75)90208-0. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. W., Dipple A. Removal of bound carcinogen during DNA repair in nondividing human lymphocytes. Cancer Res. 1972 Sep;32(9):1855–1860. [PubMed] [Google Scholar]
- Loyter A., Zakai N., Kulka R. G. "Ultramicroinjection" of macromolecules or small particles into animal cells. A new technique based on virus-induced cell fusion. J Cell Biol. 1975 Aug;66(2):292–304. doi: 10.1083/jcb.66.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher V. M., Birch N., Otto J. R., MacCormick J. J. Cytotoxicity of carcinogenic aromatic amides in normal and xeroderma pigmentosum fibroblasts with different DNA repair capabilities. J Natl Cancer Inst. 1975 Jun;54(6):1287–1294. doi: 10.1093/jnci/54.6.1287. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Yasuda S., Sekiguchi M. Repair of DNA damaged by methyl methanesulfonate in bacteriophage T4. Biochim Biophys Acta. 1976 Aug 18;442(2):208–215. doi: 10.1016/0005-2787(76)90491-3. [DOI] [PubMed] [Google Scholar]
- Okada Y., Koseki I., Kim J., Maeda Y., Hashimoto T. Modification of cell membranes with viral envelopes during fusion of cells with HVJ (Sendai virus). Exp Cell Res. 1975 Jul;93(2):368–378. doi: 10.1016/0014-4827(75)90462-0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Mayhew E. Cellular uptake of cyclic AMP captured within phospholipid vesicles and effect on cell-growth behaviour. Biochim Biophys Acta. 1974 Sep 23;363(3):404–418. doi: 10.1016/0005-2736(74)90079-0. [DOI] [PubMed] [Google Scholar]
- Slor H. Induction of unscheduled DNA synthesis by the carcinogen 7-bromomethylbenz(a)anthracene and its removal from the DNA of normal and xeroderma pigmentosum lymphocytes. Mutat Res. 1973 Aug;19(2):231–235. doi: 10.1016/0027-5107(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Rice M., Wagner E. K. Xeroderma pigmentosum cells contain low levels of photoreactivating enzyme. Proc Natl Acad Sci U S A. 1975 Jan;72(1):103–107. doi: 10.1073/pnas.72.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe H., Miki Y., Kozuka T., Furuyama J. I., Tanaka K. DNA repair characteristics and skin cancers of xeroderma pigmentosum patients in Japan. Cancer Res. 1977 Feb;37(2):490–495. [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Yamaizumi M., Okada Y. Reassembled HVJ (Sendai virus) envelopes containing non-toxic mutant proteins of diphtheria toxin show toxicity to mouse L cell. Nature. 1977 Apr 28;266(5605):839–840. doi: 10.1038/266839a0. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. Further purification and characterization of T4 endonuclease V. Biochim Biophys Acta. 1976 Aug 18;442(2):197–207. doi: 10.1016/0005-2787(76)90490-1. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. Mechanism of repair of DNA in bacteriophage. II. Inability of ultraviolet-sensitive strains of bacteriophage in inducing an enzyme activity to excise pyrimidine dimers. J Mol Biol. 1970 Jan 28;47(2):243–255. doi: 10.1016/0022-2836(70)90343-8. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]