Abstract
Methylation of cytosines in DNA is important for the regulation of expression of many genes. During carcinogenesis, normal patterns of gene methylation can be altered. Oxygen radical injury, shown to damage DNA in a variety of ways associated with cancer development and other conditions, has been suggested to affect DNA methylation, but a mechanism has not been demonstrated. Using oligonucleotides containing the common oxygen radical adduct 8-hydroxyguanine to replace guanine, we found that the enzymatic methylation of adjacent cytosines is profoundly altered. Furthermore, there is a high degree of positional specificity with respect to this effect. Thus, free radical injury may explain some of the altered methylation observed during carcinogenesis.
Full text
PDF
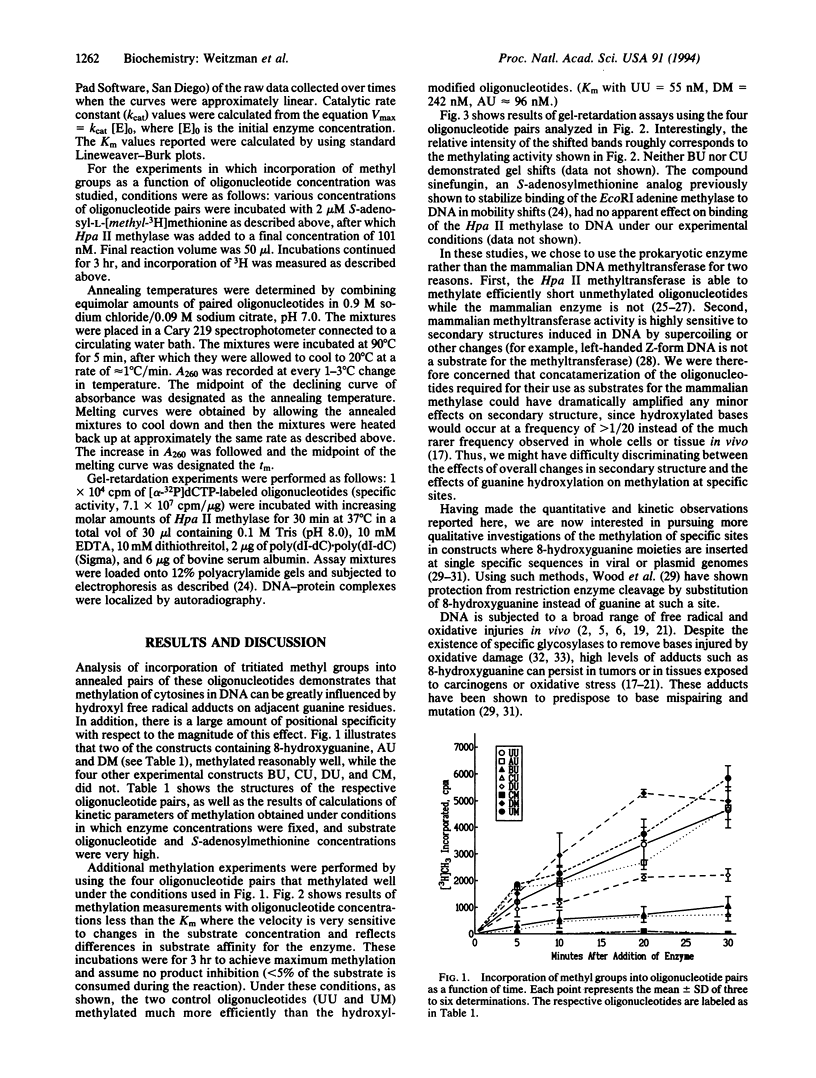
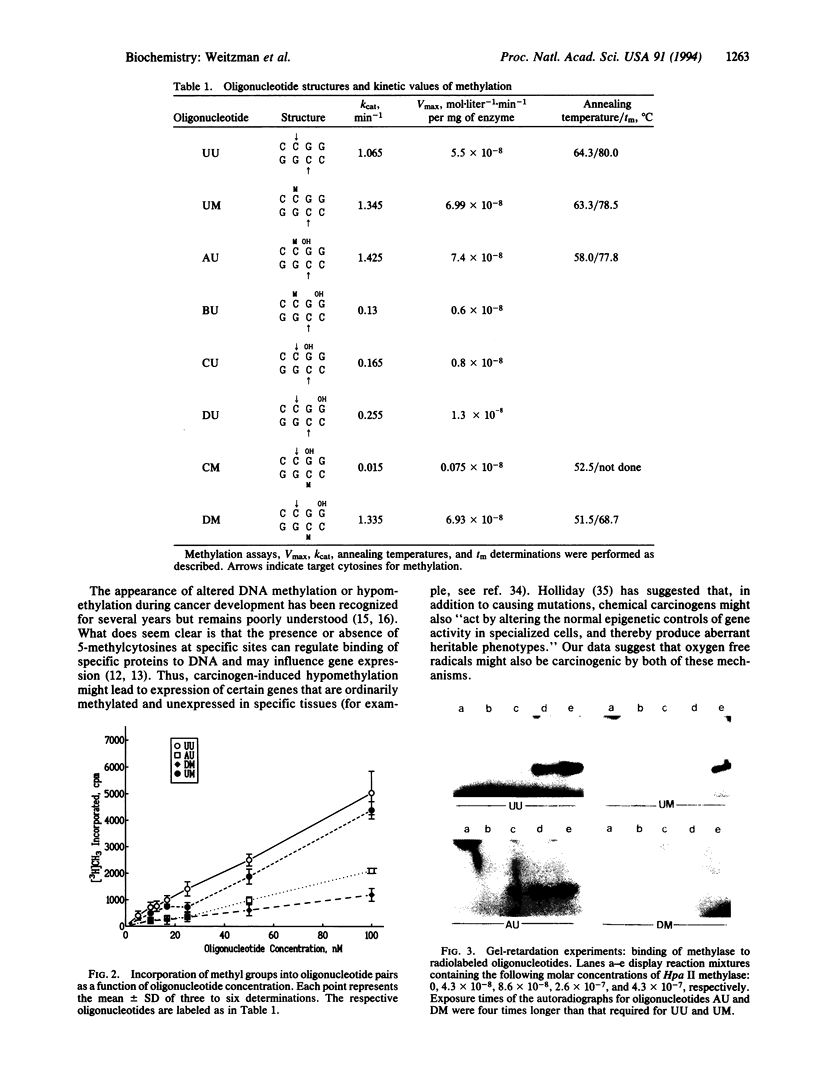

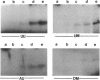
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. DNA methylation. The effect of minor bases on DNA-protein interactions. Biochem J. 1990 Jan 15;265(2):309–320. doi: 10.1042/bj2650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N. Oxygen radicals and 8-hydroxyguanine in DNA. Jpn J Cancer Res. 1991 Dec;82(12):1460–1461. [PubMed] [Google Scholar]
- Baylin S. B., Makos M., Wu J. J., Yen R. W., de Bustros A., Vertino P., Nelkin B. D. Abnormal patterns of DNA methylation in human neoplasia: potential consequences for tumor progression. Cancer Cells. 1991 Oct;3(10):383–390. [PubMed] [Google Scholar]
- Bestor T. H., Ingram V. M. Two DNA methyltransferases from murine erythroleukemia cells: purification, sequence specificity, and mode of interaction with DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5559–5563. doi: 10.1073/pnas.80.18.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor T. Supercoiling-dependent sequence specificity of mammalian DNA methyltransferase. Nucleic Acids Res. 1987 May 11;15(9):3835–3843. doi: 10.1093/nar/15.9.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow J. J. DNA replication. Methyltransferases in foci. Nature. 1993 Feb 25;361(6414):684–685. doi: 10.1038/361684a0. [DOI] [PubMed] [Google Scholar]
- Cannon-Carlson S. V., Gokhale H., Teebor G. W. Purification and characterization of 5-hydroxymethyluracil-DNA glycosylase from calf thymus. Its possible role in the maintenance of methylated cytosine residues. J Biol Chem. 1989 Aug 5;264(22):13306–13312. [PubMed] [Google Scholar]
- Cedar H., Razin A. DNA methylation and development. Biochim Biophys Acta. 1990 May 24;1049(1):1–8. doi: 10.1016/0167-4781(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A., Trump B. F. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991 Jan;3(1):1–7. [PubMed] [Google Scholar]
- Doerfler W., Toth M., Kochanek S., Achten S., Freisem-Rabien U., Behn-Krappa A., Orend G. Eukaryotic DNA methylation: facts and problems. FEBS Lett. 1990 Aug 1;268(2):329–333. doi: 10.1016/0014-5793(90)81280-2. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Cedar H., Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982 Feb 18;295(5850):620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr 9;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Holliday R. DNA methylation and epigenetic defects in carcinogenesis. Mutat Res. 1987 Dec;181(2):215–217. doi: 10.1016/0027-5107(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Buckley J. D. The role of DNA methylation in cancer. Adv Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- Kamiya H., Miura K., Ishikawa H., Inoue H., Nishimura S., Ohtsuka E. c-Ha-ras containing 8-hydroxyguanine at codon 12 induces point mutations at the modified and adjacent positions. Cancer Res. 1992 Jun 15;52(12):3483–3485. [PubMed] [Google Scholar]
- Kasai H., Okada Y., Nishimura S., Rao M. S., Reddy J. K. Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following long-term exposure to a peroxisome proliferator. Cancer Res. 1989 May 15;49(10):2603–2605. [PubMed] [Google Scholar]
- Klein J. C., Bleeker M. J., Saris C. P., Roelen H. C., Brugghe H. F., van den Elst H., van der Marel G. A., van Boom J. H., Westra J. G., Kriek E. Repair and replication of plasmids with site-specific 8-oxodG and 8-AAFdG residues in normal and repair-deficient human cells. Nucleic Acids Res. 1992 Sep 11;20(17):4437–4443. doi: 10.1093/nar/20.17.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. Major alterations in the nucleotide structure of DNA in cancer of the female breast. Cancer Res. 1991 Oct 1;51(19):5430–5432. [PubMed] [Google Scholar]
- Moriya M., Ou C., Bodepudi V., Johnson F., Takeshita M., Grollman A. P. Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat Res. 1991 May;254(3):281–288. doi: 10.1016/0921-8777(91)90067-y. [DOI] [PubMed] [Google Scholar]
- Olinski R., Zastawny T., Budzbon J., Skokowski J., Zegarski W., Dizdaroglu M. DNA base modifications in chromatin of human cancerous tissues. FEBS Lett. 1992 Sep 7;309(2):193–198. doi: 10.1016/0014-5793(92)81093-2. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Why is the hydroxyl radical the only radical that commonly adds to DNA? Hypothesis: it has a rare combination of high electrophilicity, high thermochemical reactivity, and a mode of production that can occur near DNA. Free Radic Biol Med. 1988;4(4):219–223. doi: 10.1016/0891-5849(88)90043-3. [DOI] [PubMed] [Google Scholar]
- Reich N. O., Mashhoon N. Inhibition of EcoRI DNA methylase with cofactor analogs. J Biol Chem. 1990 May 25;265(15):8966–8970. [PubMed] [Google Scholar]
- Roy D., Floyd R. A., Liehr J. G. Elevated 8-hydroxydeoxyguanosine levels in DNA of diethylstilbestrol-treated Syrian hamsters: covalent DNA damage by free radicals generated by redox cycling of diethylstilbestrol. Cancer Res. 1991 Aug 1;51(15):3882–3885. [PubMed] [Google Scholar]
- Shacter E., Beecham E. J., Covey J. M., Kohn K. W., Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988 Dec;9(12):2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Kan J. L., Baker D. J., Kaplan B. E., Dembek P. Recognition of unusual DNA structures by human DNA (cytosine-5)methyltransferase. J Mol Biol. 1991 Jan 5;217(1):39–51. doi: 10.1016/0022-2836(91)90609-a. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S. A., Gordon L. I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990 Aug 15;76(4):655–663. [PubMed] [Google Scholar]
- Weitzman S. A., Lee R. M., Ouellette A. J. Alterations in c-abl gene methylation in cells transformed by phagocyte-generated oxidants. Biochem Biophys Res Commun. 1989 Jan 16;158(1):24–30. doi: 10.1016/s0006-291x(89)80171-8. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Smith R. A., Longoria J., Liotta M. A., Harper C. M., Harris C. C. Chemical carcinogen-induced decreases in genomic 5-methyldeoxycytidine content of normal human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3298–3301. doi: 10.1073/pnas.84.10.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- Zimmerman R., Cerutti P. Active oxygen acts as a promoter of transformation in mouse embryo C3H/10T1/2/C18 fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2085–2087. doi: 10.1073/pnas.81.7.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]