Abstract
Background
Postshock mesenteric lymph (PSML) is the mechanistic link between splanchnic ischemia reperfusion (IR) and remote organ injury. We hypothesize that an unbiased inspection of the proteome of PSML will reveal previously unrecognized aberrations in systems biology provoked by hemorrhage-induced mesenteric IR injury in vivo.
Methods
Shock was induced in male Sprague-Dawley rats by controlled hemorrhage, and the mesenteric duct was cannulated for lymph collection. Preshock and postshock lymph were collected for differential in-gel electrophoresis (DIGE)-based proteomics. Proteins that increased or decreased in relative concentration ≥1.5-fold were selected for trypsin digestion and analysis by mass spectrometry (MS).
Results
Evidence of tissue injury was detected by an increase in cell/tissue proteins in PSML. Components of coagulation were depleted, whereas products of hemolysis were increased. Haptoglobin was decreased, which supports an early postshock hemolytic process. Interestingly, several protective protease inhibitors were decreased in PSML. The unexpected findings were an increase in α-enolase (a key glycolitic enzyme and cell-surface plasminogen binding receptor, +2.4-fold change) and increased major urinary protein (MUP, a sex-specific lipid-binding protein, +17.1-fold change) in PSML.
Conclusion
A proteomic evaluation of PSML revealed evidence of several shock-associated processes: protein release from tissue injury, depletion of coagulation factors and evidence of hemolysis, depletion of protective protease inhibitors, and an increase in abundance of lipid carriers. These results suggest that constitutive changes in the proteome of PSML may provide novel insights into the complex pathophysiology of postshock systems biology.
Multiple organ failure (MOF) remains the leading cause of post-traumatic death after the first 24 h following injury.1,2 Mesenteric ischemia reperfusion (IR), subsequent to trauma (T)/hemorrhagic shock (HS), is central in the pathogenesis of postinjury organ dysfunction. 3 However, the molecular processes involved are not well understood, and to date the identification of culprit mediators remains elusive. The failure of advances in medical therapy to impact significantly the late mortality associated with trauma is partly because of our incomplete understanding of the complex mechanisms by which T/HS contributes to remote organ dysfunction.
Experimental work has demonstrated that post-shock mesenteric lymph (PSML) serves as the conduit by which causative agents, which are contained in exudates from these stressed splanchnic beds, are conveyed to the systemic circulation.4,5 In animal models, the diversion of mesenteric lymphatics prior to T/HS attenuates postshock neutrophil priming, pulmonary neutrophil sequestration, endothelial adhesion molecule expression, and remote organ injury.5–7 We have shown previously that the concentration of proteins, cholesterol, triglycerides, and high-density lipoprotein (HDL) in mesenteric lymph are altered after T/HS.8 We have also found that gelsolin, which is an actin scavenger and lipid binding protein, is depleted in PSML.9 However, each of these studies resulted from hypothesis-driven biochemical approaches. A mass spectrometry (MS)-based proteomic analysis can identify how a tableau of proteins might change at the quantitative and qualitative level. The role of PSML as a conduit for the transport of responsible mediators makes it an attractive target for MS investigations directed at elucidating the altered proteome after shock. Consequently, we hypothesize that an unbiased inspection of the proteome of PSML will reveal previously unrecognized aberrations in systems biology provoked by hemorrhage-induced mesenteric IR injury in vivo.
In this study, we used differential in-gel electrophoresis (DIGE) and MS in an animal model of T/HS to determine the early constitutive changes in the proteome of PSML. The results of this study reveal evidence of proteomic changes involved in several shock-associated processes, which include tissue injury, evidence of hemolyis, depletion of coagulation factors, depletion of protective protease inhibitors, and potentially enhanced bioavailability of proinflammatory lipids because of an increased abundance of lipid carriers.
MATERIALS AND METHODS
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver. Pentobarbital sodium was purchased from Abbott Labs (Chicago, IL). Intramedic polyethylene tubing was acquired from Fisher Scientific (Pittsburgh, PA), and heparin was purchased from American Pharmaceutical Partner (Schaumburg, IL). The reagents used for DIGE experiments were obtained from GE Healthcare (Piscataway, NJ). All other reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO) unless otherwise specified.
Hemorrhagic shock
Sprague-Dawley rats weighing 218 mg to 351 mg (Colorado State University, Fort Collins, CO) were housed in a climate-controlled barrier facility with 12-h light/dark cycles and free access to food and water for a period of at least 1 week prior to experimental procedures. Anesthesia was given with intraperitoneal injection of 50 mg/kg sodium pentobarbital. Local anesthesia was performed with subcutaneous injection of 1% lidocaine. The femoral artery and vein were then cannulated with PE 50 tubing, and blood pressure was monitored using a ProPaq invasive monitoring device (Welch-Allyn, Skaneateles Falls, NY). A separate skin puncture was created to tunnel the catheters prior to closure of the groin incision. A 3-cm midline laparotomy was performed to mimic tissue injury with trauma. The bowel was eviscerated and rotated to the left, and the mesenteric duct and accessory duct (located adjacent to the superior mesenteric artery) were isolated by blunt dissection. The main lymphatic duct was cannulated with PE 100 tubing and secured with 7-0 prolene suture; the catheter was tunneled posteriorly through the skin. The accessory duct was then ligated with suture. After a 1-h period of preshock lymph collection, shock was induced by controlled hemorrhage to a mean arterial pressure of 30 mm Hg, which was sustained for 40 min by withdrawing or returning shed blood (SB). Body temperature was monitored rectally, and euthermia was maintained with a heat lamp. Resuscitation was performed by infusing 2 × SB volume in NS over 30 min, followed by ½ SB volume over 30 min, then completed with 2 × SB volume in NS over 60 min via the femoral vein. Lymph collection continued for 1 h after the completion of resuscitation (3rd-h lymph). The animals were then euthanized via pentobarbital overdose. Preshock, shock, and postshock lymph samples were collected each 30 min during the procedure and centrifuged at 5,000 g for 10 min. The supernatant were collected and frozen in liquid nitrogen prior to storage at −80°C. Protein quantification was performed using the bicinchoninic acid protein analysis method with bovine serum albumin standards to create a regression analysis for estimating overall protein concentration for each hourly sample.
Lymph sample preparation and protein isolation
Equal fractions of lymph from 3 animals (preshock and postshock) were pooled for proteomic analysis. Samples were methanol-chloroform precipitated, and the resulting protein pellet was resuspended in room-temperature rehydration buffer overnight.10 Protein concentration was quantified using the Bradford assay as previously described.
Cy dye labeling
Three analytical gels (50 µg protein per sample) were run (each representing 1 animal) to compare differentially the preshock with postshock (3rd-h) lymph. Additionally, a preparative gel was run using 500 µg of protein from pooled preshock lymph and 500 µg of protein from pooled postshock lymph from the 3 animals. The dyes were swapped between analytical and preparative gels, so that preshock and postshock lymph samples were alternatively labeled with either Cy3 or Cy5 to control for any dye-specific labeling artifacts. All Cy labeling was performed according to the manufacturer’s protocol, where 200 pmol of dye was used to label 50 mg of protein under standard minimal dye labeling conditions.12
Two-dimensional (2-D) electrophoresis
After combining the Cy-labeled preshock and post-shock proteins, the mixture was diluted to a total volume of 450 µL with reaction buffer. Samples for both analytical and preparative gels were passively rehydrated with Immobiline DryStrips (GE Healthcare) 24 cm pH3-10 overnight or for at least 18 h, followed by isoelectric focusing (IEF) using an IPGPhor IEF unit. IEF and 2D electrophoresis for analytical and preparative gels was performed using 9–16% trisglycine gels (Jules Gels, Milford, CT) run at 20 W per gel on the Ettan Dalt System for approximately 4–6 h as previously described.13
Gel imaging
Imaging was performed on a Typhoon 9400 Variable Mode Laser Imager (GE Healthcare).14 The initial preparative gel was then fixed for 1 h in 7.5% acetic acid/10% methanol and stained overnight with Sypro Ruby protein gel stain (Invitrogen/Molecular Probes, Eugene, OR). After destaining (7.5% acetic acid/30% methanol), gels were reimaged at 100-µm resolution (laser excitation 532 nm, emission 560 nm, LP Gen. Purple).
Data analysis
DeCyder software v 6.5 (Amersham Pharmacia, Piscataway, NJ; GE Healthcare) was used for image analysis. The differential in-gel analysis module was used to merge Cy3 and Cy5 images and to detect spot boundaries. Dust, scratches, and other nonprotein features were eliminated prior to calculation of normalized spot volume as previously described.15 The preparative image of the gel set was then loaded into the biologic variance module, where spots that changed in relative abundance greater than 1.5-fold were identified for spot picking and trypsin digestion.
Spot picking
Proteins of interest were robotically excised from the preparative gel using a robotic spot picker (Ettan SpotPicker software v 1.10; GE Healthcare) and then transferred to an Ettan Digester (software v 1.10; GE Healthcare). Excised spots were washed twice with 100 µL of 50 mmol/L ammnonium bicarbonate, once with 100 µL of 75% acetonitrile and once with 100 µL of 100% acetonitrile and left to dry at room temperature.
Tryptic digestion
Excised spots of interest, from the preparative gel, were incubated for 30 min at 4°C with sequencing grade modified trypsin in 25 mmol/L ammonium bicarbonate (1:50 wt/wt; Promega, Madison, WI). Tryptic digestion of proteins, within the excised spot, proceeded for approximately 16 h at room temperature. Samples were then stored at −80°C prior to analysis.
Mass spectrometry
For MS analysis, equal parts of sample and α-cyano-4-hydroxycinnamic acid solution (7%) were manually spotted onto 100-well or 384-well stainless steel target plates (Applied Biosystems, Foster City, CA). Matrix Assisted Laser-Desportion Tandem Time-of-Flight (MALDI TOF-TOF) analysis was carried out on either an Applied Biosystems 4700 MS or Applied Biosystems 4800 MS. All spectra were processed in Data Explorer v 5.0 (Applied Biosystems) and internally calibrated to a minimum of 3 monoisotopic trypsin autolysis peaks. Spectra were then used to interrogate sequences in the Swiss-Prot database using Mascot Daemon software v 2.2.2 (Matrix Science, Boston, MA) running the Mascot server v 2.0. Mass tolerance was set at 100 ppm and 300 ppm for fragment ions and enzyme specificity to trypsin. One missed cleavage was allowed per protein, and carboxyamidomethylation of cysteine was set as a fixed modification. The oxidation of methionine was set as a variable modification. Protein identification, with a P value of <.05, required a Protein Prospector best discriminant score of >0.9 and/ or a Mascot score of >70.
RESULTS
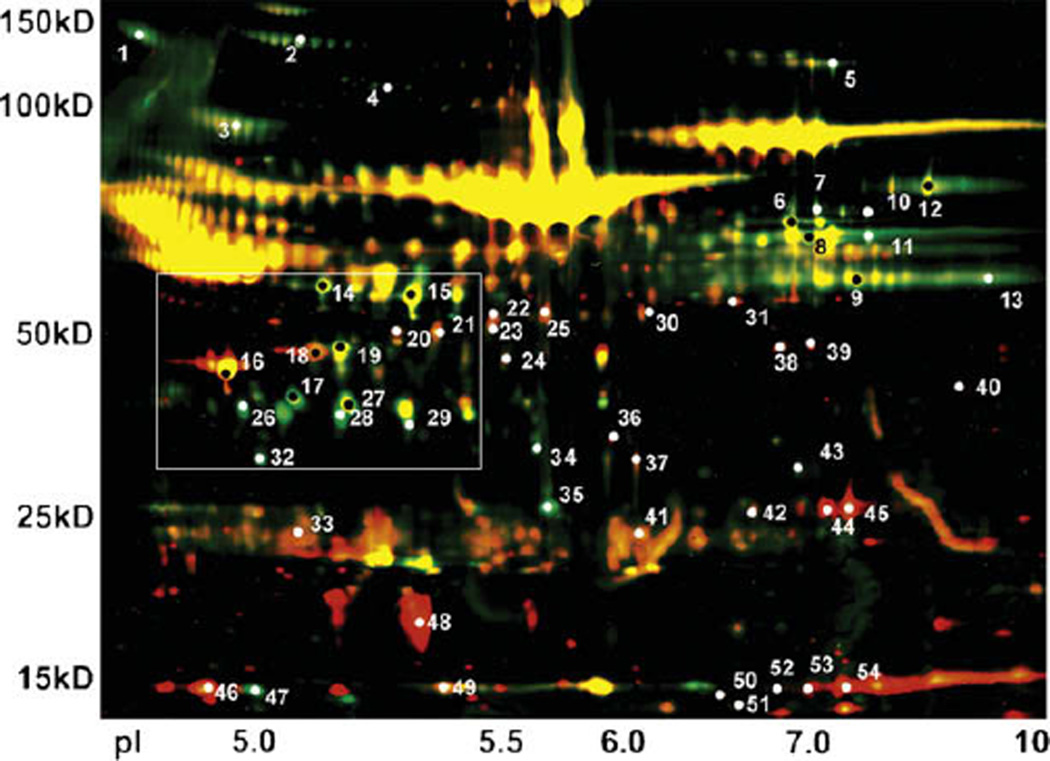
A threshold of 1.5-fold change in protein abundance between preshock and postshock was chosen to evaluate changes in the proteome of PSML. Using differential in-gel image analysis for the 2D gel of Cy3 labeled (green) preshock lymph and Cy5 labeled (red) postshock lymph, we identified 55 spots on the gel where proteins changed in relative abundance at least 1.5-fold after hemorrhagic shock (Fig 1). In 28 of these spots, the protein abundance increased from preshock to postshock, whereas in 27 spots, proteins were depleted. Given that equal amounts of preshock and postshock lymph protein were loaded for electrophoresis, the differences in abundance after hemorrhagic shock suggests selective protein changes in PSML (Fig 2, A and B).
Fig 1.
Two-dimensional–DIGE of preshock and post-shock mesenteric lymph. Two-dimensional–DIGE illustrates the separation of proteins from preshock and PSML, which are loaded at equal protein concentrations, by molecular weight on the y-axis and isoelectric point on the x-axis. Preshock proteins are Cy3 labeled (green), whereas postshock proteins are Cy5 labeled (red). Areas where a given protein was present in approximately the same abundance for both preshock and postshock subsequently appear yellow. Spots on the gel where proteins changed in relative abundance ≥ 1.5-fold after shock, as determined by difference in fluorescent emission from a computerized biologic variance model, were robotically excised for trypsin digestion and MS analysis. Numbers correspond to identifications shown in Tables I and II, and the white box corresponds to the region shown in Fig 2, A and B.
Fig 2.
Matched areas from 2D–DIGE of preshock proteins (A) and postshock proteins (B) showing selective protein changes in PSML. Equal amounts of preshock and postshock lymph protein were loaded for electrophoresis, and a comparison of matched areas from the preparative gel (corresponding to the white box on Fig 1) illustrates that whereas some proteins increased in abundance from preshock to postshock (white arrows), other proteins were depleted (black arrows).
Significant protein identification results (P< .05) by MALDI TOF-TOF MS for the 55 spots of interest are shown in Tables I and II. The apparent molecular weight (MW) was estimated from the gel images. Many proteins such as fibrinogen, creatine kinase, albumin, and complement are known to exist in multiple isoforms, which results in different MWs and/or isoelectric points. Consistent with this, we identified several proteins from multiple spots on the gel. These proteins could result from splice forms, post-translational modifications, and protease cleavage of the translational product listed in the sequence database. Proteins were grouped into physiologic classes (nonsecreted intracellular proteins associated with tissue injury, coagulation, protease inhibitors, etc) based on gene ontology (Table III). Certain proteins such as afamin (P36953, an alpha albumin that carries unknown ligands) or transthyretin (P02767, a prealbumin that can carry thyroxine) were not grouped into physiologic classes because of limited gene ontology for those proteins. Because of the complexity of the complement pathway (as indicated by the numerous complement identifications) and its interaction in multiple physiologic processes, these proteins were not grouped by physiologic class. Although the findings of this study are exploratory, they provide insight into postshock pathophysiology and may be useful in developing investigational hypothesis.
Table I.
Proteins decreased in abundance in PSML
| Spot number |
Protein | Accession number |
Theoretical MW (kDa) |
Apparent MW (kDa) |
*VR (Prep) | Protein prospector: Best disc. score |
Mascot score |
|---|---|---|---|---|---|---|---|
| 1 | Inter-alpha-inhibitor H3 heavy chain | Q63416 | 99 | 150 | −1.8 | 4.81 | 532 |
| 2 | Inter-alpha-inhibitor H4 heavy chain | O35802 | 99 | 150 | −1.6 | 3.75 | 495 |
| 3 | Afamin (Alpha-albumin) | P36953 | 69 | 100 | −1.5 | 1.79 | 116 |
| 4 | Fibrinogen gamma chain | P02680 | 51 | 120 | −12.3 | 1.85 | 163 |
| 5 | Plasminogen | Q01177 | 91 | 140 | −1.9 | 3.22 | 362 |
| 6 | Fibrinogen beta chain | P14480 | 54 | 65 | −1.7 | 5.37 | 530 |
| 7 | Fibrinogen alpha chain | P06399 | 87 | 75 | −2.1 | 4.98 | 631 |
| 8 | Ig gamma-2B chain C region | P20761 | 36 | 65 | −1.5 | 2.09 | 96 |
| 9 | Ig gamma-2A chain C region | P20760 | 35 | 60 | −1.7 | 3.86 | 263 |
| 10 | Complement factor B | Q923K9 | 85 | 75 | −1.7 | 3.43 | 184 |
| 11 | Fibrinogen beta chain | P14480 | 54 | 65 | −2.3 | 3.55 | 465 |
| 12 | Complement C3 | P01026 | 186 | 80 | −1.6 | 5.12 | 710 |
| 13 | Ig gamma-2A chain C region | P20760 | 35 | 60 | −1.6 | 3.19 | 174 |
| 14 | Fibrinogen gamma chain | P02680 | 51 | 60 | −2.1 | 3.13 | 413 |
| 15 | Fibrinogen gamma chain | P02680 | 51 | 55 | −2.0 | 5.92 | 702 |
| 17 | Alpha-2-macroglobulin | P06238 | 166 | 45 | −2.7 | 2.06 | 516 |
| 19 | Complement C3 | P01026 | 186 | 50 | −1.8 | 4.19 | 400 |
| 26 | Haptoglobin | P06866 | 39 | 40 | −6.0 | 2.93 | 421 |
| 27 | Alpha-2-macroglobulin | P06238 | 166 | 40 | −3.0 | 3.88 | 640 |
| 28 | Haptoglobin | P06866 | 39 | 40 | −6.9 | 3.55 | 197 |
| 29 | Haptoglobin | P06866 | 39 | 40 | −4.7 | 2.15 | 312 |
| 35 | Complement C3 | P01026 | 186 | 30 | −7.7 | 5.73 | 495 |
| 32 | Apolipoprotein E | P02650 | 36 | 35 | −3.8 | 6.15 | 775 |
| 34 | Alpha-2-macroglobulin | P06238 | 166 | 37 | −2.2 | 1.59 | 269 |
| 43 | Complement C4 | P08649 | 192 | 30 | −1.2 | 1.97 | 190 |
| 47 | Haptoglobin | P06866 | 39 | 15 | −4.3 | 2.09 | 103 |
| 55 | Alpha-1-inhibitor 3 | P14046 | 164 | 180 | −2.1 | 0.87 | 166 |
Ig, Immunoglobulin; VR (Prep), the volume ratio that corresponds to a fold change in protein abundance between preshock and postshock for a given spot on the 2D–DIGE (Fig 1).
Table II.
Proteins increased in abundance in PSML
| Spot number |
Protein | Accession number | Theoretical MW (kDa) |
Apparent MW (kDa) |
*VR (Prep) | Protein prospector: Best disc. score |
Mascot score |
|---|---|---|---|---|---|---|---|
| 16 | Apolipoprotein A-IV | P02651 | 44 | 45 | 2.7 | 5.17 | 785 |
| 18 | Actin, cytoplasmic 1 (beta-actin) | P60711 | 42 | 47 | 3.2 | 4.29 | 395 |
| 18.b | Complement C3 | P01026 | 186 | 47 | 3.2 | 2.67 | 188 |
| 20 | Creatine kinase B-type | P07335 | 43 | 50 | 1.8 | 1.56 | 142 |
| 21 | Serum albumin | P02770 | 69 | 50 | 1.5 | 3.1 | 621 |
| 22 | Serum albumin | P02770 | 69 | 55 | 2.0 | 2.95 | 256 |
| 23 | Serum albumin | P02770 | 69 | 50 | 1.7 | 4.1 | 198 |
| 24 | Serum albumin | P02770 | 69 | 45 | 1.5 | 0.98 | 57 |
| 25 | Serum albumin | P02770 | 69 | 55 | 1.6 | 4.32 | 477 |
| 30 | Serum albumin | P02770 | 69 | 60 | 1.8 | 3.93 | 200 |
| 31 | Alpha-enolase | P04764 | 47 | 55 | 2.4 | 1.47 | 61 |
| 33 | Apolipoprotein A-I | P04639 | 30 | 25 | 3.5 | 1.33 | 41 |
| 36 | Malate dehydrogenase, cytoplasmic | O88989 | 36 | 30 | 3.6 | 1.97 | 92 |
| 37 | Serum albumin | P02770 | 69 | 30 | 1.8 | 2.77 | 189 |
| 38 | Transthyretin | P02767 | 16 | 47 | 2.5 | 7.13 | 403 |
| 38.b | Creatine kinase, muscle | P00564 | 43 | 47 | 2.5 | — | 106 |
| 39 | Creatine kinase M-type | P00564 | 43 | 45 | 3.2 | 3.17 | 484 |
| 40 | Complement C3 | P01026 | 186 | 40 | 10.8 | 2.88 | 100 |
| 41 | Ig kappa chain C region | P01836 | 12 | 25 | 2.0 | 3.88 | 192 |
| 42 | Serum albumin | P02770 | 69 | 27 | 1.9 | 3.69 | 367 |
| 44 | Carbonic anhydrase 3 | P14141 | 29 | 25 | 5.1 | 4.78 | 369 |
| 45 | Carbonic anhydrase 3 | P14141 | 29 | 27 | 8.3 | 3.5 | 209 |
| 46 | Parvalbumin alpha | P02625 | 12 | 15 | 19.7 | 3.23 | 246 |
| 48 | MUP | P02761 | 21 | 17 | 17.1 | 5.08 | 428 |
| 49 | Transthyretin (prealbumin) | P02767 | 16 | 15 | 1.7 | 3.92 | 92 |
| 50 | Hemoglobin beta chain, major-form | P02091 | 16 | 10 | 9.4 | — | 133 |
| 51 | Hemoglobin alpha-1/2 subunit | P01946 | 15 | 10 | 6.4 | 2.82 | 125 |
| 52 | Hemoglobin beta chain, major-form | P02091 | 16 | 15 | 5.9 | — | 131 |
| 53 | Hemoglobin alpha-1/2 subunit | P01946 | 15 | 15 | 3.4 | 2.3 | 173 |
| 54 | Hemoglobin beta-2 subunit | P11517 | 16 | 15 | 5.5 | 2.96 | 341 |
Ig, Immunoglobulin; VR (Prep), the volume ratio that corresponds to a fold change in protein abundance between preshock and postshock for a given spot on the 2D–DIGE (Fig 1).
Table III.
Proteins by physiologic class
| Tissue injury | Fold change | Hemolysis | Fold change |
|---|---|---|---|
| Parvalbumin-α | +19.7 | Hemoglobin-α chain | +6.4 |
| Carbonic anhydrase 3 | +8.3 | Hemoglobin-β chain | +9.4 |
| Cytoplasmic malate dehydrogenase | +3.6 | Haptoglobin | −6.9 |
| Cytoplasmic β-actin | +3.2 | ||
| Protease inhibitors | |||
| Creatine kinase-M | +3.2 | ||
| Creatine kinase-B | +1.8 | α-2-Macroglobulin | −2.7 |
| α-enolase | +2.4 | α-1-Inhibitor 3 | −2.1 |
| Inter-α-inhibitor H3 | −1.8 | ||
| Inter-α-inhibitor H4 | −1.6 | ||
| Coagulation/fibrinolysis | Lipid carrier | ||
| Fibrinogen-α | −2.1 | ||
| Fibrinogen-β | −2.3 | Major urinary protein | +17.1 |
| Fibrinogen-γ | −12.3 | Apolipoprotein A-I | +3.5 |
| Plasminogen | −1.9 | Apolipoprotein A-IV | +2.7 |
Tissue injury can be inferred from the finding that PSML has an increased abundance of cellular proteins that should not be secreted. These include parvalbumin-α, carbonic anhydrase 3, cytoplasmic malate dehydrogenase, cytoplasmic β-actin, creatine kinase, and α-enolase (Table II). Parvalbumin-α (P02625) is a calcium-binding protein of the EF-hand type, which functions as an intracellular cytoplasmic calcium sink in excitable cells. In the rat, the highest concentrations of this protein are found in muscle and neurons.16 Changes in the extracellular concentration of parvalbumin in response to injury or disease have not previously been described, and the protein does not seem to be secreted. The finding that parvalbumin-α was the protein most increased in PSML (nearly 20-fold) suggests that it may serve as a novel marker of injury severity after hemorrhagic shock. Carbonic anhydrase 3 (EC 4.2.1.1, P14141) belongs to a family of enzymes that function in the conversion of carbon dioxide and water to bicarbonate and protons. This cellular protein was increased 8.3-fold in PSML. In the rat, carbonic anhydrase III (CAIII) is predominately expressed by the liver and type I skeletal muscle fibers with only trace amounts expressed in the heart, erythrocytes, and other tissues.17 This enzyme is regulated by androgenic hormones and is 30-fold greater in male rats than in females.18 In humans, elevated serum CAIII has been reported after acute lower extremity ischemia, as a reflection of deteriorating cellular integrity.19 Given the tissue specificity and cellular release after acute ischemia, the elevation of CAIII after T/HS may serve as a novel biomarker of specific secondary tissue injury. Cytoplasmic-malate dehydrogenase (C-MDH) (EC 1.1.1.37, O88989) was elevated 3.6-fold in PSML. Malate dehydrogenase exists as 2 isozymes, cytoplasmic and mitochondrial, which are crucial to cellular metabolism in the malate-aspartate shuttle and citric acid cycle.20 Interestingly, both the intestine and liver are represented in the rat tissue distribution of C-MDH.21,22 The increased levels of C-MDH in PSML may serve as a marker for the severity of mesenteric and/or hepatic ischemia associated with hemorrhagic shock. Cytoplasmic β-actin (P60170) is one of 2 nonmuscle cytoskeletal actins and was increased 3.2-fold in PSML. Actin monomers may be released from cells during apoptosis or cell turnover, or from cell death associated with tissue injury; the extracellular environment favors polymerization of actin monomers into filaments.23,24 Experimental evidence suggests that if these actin filaments are not removed from the circulation by actin scavengers (GC-globulin and gelsolin) and the reticuloendothelial system, then the increased intravascular abundance of filamentous actin may contribute to the pathophysiology of acute respiratory distress syndrome (ARDS) through microangiopathic mechanisms.24 Previous work by our laboratory has shown that gelsolin is depleted in PSML.9 This previously reported depletion of actin scavengers, taken with the current findings of elevated actin in PSML, suggests a potential contributing mechanism for development of ARDS after trauma/hemorrhagic shock. Creatine kinase-M (CK-M) (EC 2.7.3.2, P00564) and -B (EC 2.7.3.2, P07335) isoforms were found to increase 3.2-fold and 1.8-fold, respectively, in PSML. The measurement of CK and its isoenzymes have been employed in the diagnosis and management of cardiac injury, myopathies, encephalopathies, musculoskeletal trauma, and crush injuries, and these isoenzymes have recently been reported in hemorrhagic shock encephalopathy syndrome.25–27 Note that the myocardial isoform is the CK-MB dimer. The lack of stoichiometric increase in CK-M and CK-B isoenzymes suggests at least some muscle injury other than myocardium. Thus, the assessment of creatine kinase isozymes in PSML may serve as a marker of specific tissue injury associated with T/HS. Finally, α-enolase (EC 4.2.1.11, P04764) was elevated 2.4-fold in PSML. This multifunctional enzyme is highly conserved across species and is best known for its role in the glycolytic pathway. Endothelial cell expression of α-enolase is increased during chronic hypoxia where cellular protection may be conferred by increased aerobic metabolism.28 This enzyme is also expressed on the surface of eukaryotic cells where it serves as a strong plasminogen-binding receptor. Surface expression of α-enolase in neutrophils, monocytes, T-cells and B-cells is dependent on the pathophysiologic condition of the cells and increases after stimulation with lipopolysaccharide.29 This function of α-enolase as a plasminogen-binding receptor and the initiation of fibrinolytic systems have been implicated in several disease processes, which include bacterial infection, tumor formation, and cancer metastasis.30 The impact of circulating α-enolase on coagulation during pathologic states is unknown, but it may have significant implications on patient outcome. Assessment of plasma or cerebrospinal fluid levels of an alternate enolase isozyme, which is called neuron-specific enolase (γ-enolase), has diagnostic or prognostic value in several disease states, including small-cell lung cancer, neuroblastoma, pancreatic endocrine tumors, and medullary thyroid cancer, as well as traumatic and ischemic brain injury.31,32 Similarly, the assessment of α-enolase levels in PSML may be useful as both a marker of injury severity and prognostic factor after T/HS.
Postinjury coagulopathy is critical to the management of the severely injured patient and has prognostic implications for outcome after trauma. Although acidosis, hypothermia, and hypotension are known to accentuate coagulopathy,33,34 the precise physiologic mechanisms remain unknown. Brohi et al35 have reported that acute traumatic coagulopathy may be related to an early protein C activation and the subsequent inhibition of thrombin generation, which is associated with protein-protein interactions in the clotting cascade, as a result of hypoperfusion. In our experimental model, where surgical hemostasis and euthermia are controlled, we observed a depletion of fibrinogen-α, -β, -γ, and plasminogen in PSML (Table II). Curiously, the observed MW of fibrinogen gamma chain (120 kD) and plasminogen (140 kD) seemed to be much larger than the theoretical masses (51 kD and 81 kD, respectively). This suggests substantial modification of these proteins in mesenteric lymph.
We also found evidence for hemolysis in the increase of hemoglobin subunits after T/HS and resuscitation. Three distinguishable spots of hemoglobin beta chain, albeit of similar mass and pI, were increased in PSML 9.4-fold, 5.9-fold, and 5.5-fold, respectively. However, 2 spots for the hemoglobin alpha chain did not increase proportionately. This potentially indicates different degradation rates for the hemoglobin heterotetramer after red cell lysis. In support of this evidence for hemolysis, haptoglobin (Hp) was decreased in PSML. The accession number denotes the primary product of Hp translation, which is a single polypeptide that contains both α and β subunits. This protein is modified cotranslationally by proteolytic activity in the rough endoplasmic reticulum (RER) and by carbohydrate side-chain processing in the RER and golgi, which forms prohaptoglobin. Prohaptoglobin is secreted from cells where additional modification by proteolytic enzymes in the plasma occurs resulting in the native α2, β2 tetramer.36 Haptoglobin functions in the sequestration and processing of free hemoglobin (Hb). Through interaction with the CD163 scavenger receptor on monocytes and macrophages, the Hp-Hb complex is taken up for Hb degradation and iron recycling. Given the role of heme toxicity and iron-generated reactive oxygen species, especially during reperfusion injury, the depletion of haptoglobin reveals a defensive process that may be overwhelmed after T/HS.
Taken with the observation of increased products of hemolysis (hemoglobin α and β) and depletion of haptoglobin, these findings suggest both an early postshock hemolytic process and a depletion of coagulation factors associated with splanchnic hypoperfusion. Understanding the complex interplay between the coagulation pathways, the fibrinolytic system and complement cascades will require more work. However, our findings suggest that PSML may be a novel media for investigating the complex pathophysiology of postshock coagulopathy.
Endogenous proteases are emerging as important mediators of inflammation and disease processes. In addition to their specific proteolytic activity in physiologic pathways (ie, coagulation, digestion, and wound remodeling), proteases such as thrombin, trypsin, and tissue kallikreins may function in disease and inflammation via protease-activated receptors. This alternate function of endogenous proteases has been implicated in several disease states, which include cancer and inflammation of the cardiovascular, respiratory, musculoskeletal, gastrointestinal, and nervous systems.37 Excessive activity of proteases in vivo is counteracted by inhibitors including serine-protease inhibitors (inter-alpha-inhibitor H3 and H4) and alpha-2-macroglobulin.38 In humans with sepsis, a relative depletion of inter-alpha-inhibitors or alpa-2-macroglobulin has been associated with increased disease severity and mortality.38,39 In this study, we observed a depletion of the protease inhibitors alpha-2-macroglobulin, alpha-1-inhibitor 3, and inter-alpha-inhibitor H3 and H4 in PSML (Table III). These findings may implicate the loss of protective protease inhibitors and subsequent unopposed protease activity in the development of postshock inflammation.
Lipid carriers may play an important role in posttraumatic inflammation by contributing to the bioavailability of proinflammatory lipids. Major urinary protein is a fatty-acid-binding protein of the lipocalin family.40 Interestingly, it is found only in the urine of adult males.41 The concentration of other potential lipid carriers was also altered in PSML. We have previously found apolipoprotein A-I in lymph by biochemical assay.8 Analysis by MS clarifies that apolipoprotein A-I and A-IV are increased in PSML 3.5-fold and 5.17-fold, respectively, whereas paradoxically apolipoprotein E is depleted (−3.8). The apolipoprotein A-I and A-IV genes are both located on chromosome 8 and are known components of HDL and chlylomicrons, which are reported to be anti-inflammatory.42 In contrast, apoliprotein E is located on a different chromosome(1) and is involved in low-density lipoprotein metabolism. The increase in various albumin species (with apparent molecular weight comparable with the theoretical weight) may also contribute to altered lipid transport after T/HS.
DISCUSSION
Mesenteric ischemia reperfusion is central in the genesis of postinjury MOF, and PSML serves as the conduit by which causative agents from these stressed splanchnic beds are conveyed to the circulation.3–5 This critical role of PSML in transporting responsible mediators makes it a valuable media for investigating postshock systems biology. The results of the current study suggest that constitutive changes in the proteome of PSML, which are determined by 2D-DIGE and MS proteomics, can provide novel insights into the complex pathophysiology of postshock systems biology.
Research by our laboratory and that of Deitch et al, using animal models of hemorrhagic shock, has demonstrated that PSML can incite acute lung injury through mechanisms of neutrophil priming, endothelial cell inflammation, and epithelial cell apoptosis; in addition, PSML can induce the upregulation of cellular adhesion molecules involved in the systemic posttraumatic inflammatory response.4–7 Diversion of the mesenteric lymphatics prior to hemorrhagic shock attenuates systemic neutrophil priming as well as pulmonary neutrophil sequestration, and it diminishes the severity of postshock lung injury; this supports the role of PSML as a conduit for the transport of mediators responsible for postinjury organ dysfunction.4–7 Investigations to identify the specific causative agents contained in PSML have implicated both lipids and proteins as potential mediators of this process, which include phospholipase-A2 derived lipids43,44 and modified albumin.45,46 However, to date the complex molecular pathophysiology remains to be defined, and no single substance has been identified to account for the downstream pathology that results from delivery of PSML to the systemic circulation.
This study is the first proteomic exploration of PSML, and as such it provides novel findings that may be important to expand the understanding of postshock pathophysiology. In preliminary comparison of preshock and postshock lymph from individual animals, hundreds of proteins are available that change in relative abundance of ≥1.5-fold after T/HS (data not shown). The results presented here are limited to those proteins that consistently changed in abundance from pooled preshock and postshock samples from 3 animals. This finding provides a measure of confidence for the biologic relevance of the proteins we have identified. However, a larger sample size may increase the number of spots with significant fold change and may allow for a more detailed analysis of the postshock physiology. Second, the analysis was restricted to a comparison of lymph sampled before shock and after resuscitation. The inclusion of lymph samples obtained during shock could provide a better temporal distinction between shock and resuscitation. However, the volumes of lymph obtained during the shock period are prohibitively small.8 A comparison of the lymphatic proteome with that of plasma is also required to provide a better understanding of whether proteins that increase in PSML are released from the mesenteric bed or elsewhere systemically. Using the current analytical platform, we could not identify some proteins from low-intensity spots on the gel despite having a significant fold change (≥1.5). Alternative analytical platforms (nLC-MS/MS) should improve these identifications.
Proteomics studies the panel of proteins present in a cell, organ, or organism at a given time,47 and whereas the genome reflects what can happen in a given circumstance, the proteome reflects what actually happens.48 The physiologic response to trauma has been inferred by many specific proteins, which include acute phase components, cytokines, and contents of cellular lysates. The proteome of mesenteric lymph after T/HS is also amenable to such characterization. Leak et al49 have compared the proteome of normal mesenteric lymph with the proteome of normal plasma in an ovine model.49 Their results indicate that in the absence of disease or shock, the proteins present in lymph are quantitatively and qualitatively different from those expressed in plasma and that certain proteins are unique to the lymph. These findings support the premise that lymph is more than simply an ultrafiltrate of plasma. Alterations in the proteome during stress or disease may reflect sequelae of a disease state and be useful as biomarkers; alternatively, specific proteins may be causative in the pathology and thus potential targets for therapeutics. Thus, characterizing the changes in the proteome of mesenteric lymph after T/HS may provide insight into postshock path-ophysiology and may aid in the identification of causative agents.
The novel and intriguing molecular processes after hemorrhagic shock implicated in this exploratory study include tissue injury, coagulation, hemolysis, loss of protease inhibitors, and an increase in lipid carriers (Table II). As anticipated, the evidence of tissue injury was detected by an increase in the abundance of intracellular proteins in PSML. However, although creatine kinase and possibly actin have previously been associated with tissue injury or ischemia, the others (parvalbumin α, carbonic anhydrase 3, malate dehydrogenase, and α-enolase) have not been described in this context. Moreover, apart from the surprising role of α-enolase in coagulation and of actin in endothelial inflammation, the extracellular effects of these proteins remain unexplored. The discovery of increased androgen regulated proteins in PSML (carbonic anhydrase 3 and major urinary protein 1 [MUP1]) was unexpected. It is unclear whether these and other undiscovered sex-linked proteins are related to the recently described sensitivity of males to hemorrhagic shock.50 Finally, the depletion of both the heme scavenger haptoglobin and the antiproteases could lead to new areas of therapeutic intervention to reduce iron toxicity and proteolysis in the setting of hemorrhagic shock.
Both the consequences of changes in specific protein levels and their subsequent contribution to postshock organ dysfunction require additional investigation. However, these unique findings may be useful in the development of future investigational hypothesis. It is important to consider that although therapies directed at single proteins elaborated as distal components of inflammatory cascades may not significantly impact the overall postshock pathophysiology, the identification of pathway activation may lead to therapies directed at key components involved in the initiation of those cascades. Additionally, effective treatment strategies may require the integration of therapeutic efforts against multiple aspects of the postshock proteome to generate clinically significant improvements in patient outcome. Alternatively, an understanding of the proteomic changes that occur after trauma may aid in the development of investigations directed at the epigenetics of trauma and the regulation of gene transcription/translation, which results in the observed changes in the postshock proteome.
In conclusion, by using a DIGE-MS–based proteomic approach to evaluate the constitutive changes in the proteome of PSML, this study provides evidence of several shock-associated processes: protein release from tissue injury, depletion of coagulation factors, and evidence of hemolysis. Other findings, with potentially profound implications, are (1) an increase in α-enolase, a known plasminogen binding receptor and initiator of the fibrinolytic system which may contribute to the early coagulopathy associated with trauma; (2) the loss of intrinsic protection caused by the depletion of protease inhibitors; and (3) potentially enhanced bioavailability of proinflammatory lipids because of the increased abundance of lipid carriers (MUP and apolipoproteins). These results suggest novel pathways are involved in PSML mediated organ injury and postshock systems biology, and they provide a road map for future investigation of postshock pathophysiology.
Acknowledgments
Supported by National Institute of General Medical Sciences Grants T32-GM008315 and P50-GM049222.
Footnotes
Presented at the Society of University Surgeons 4th Annual Academic Surgical Congress, Fort Myers, Florida, February 3–6, 2009.
REFERENCES
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–95. [PubMed] [Google Scholar]
- 3.Biffl WL, Moore EE. Splanchnic ischaemia/reperfusion and multiple organ failure. Br J Anaesth. 1996;77:59–70. doi: 10.1093/bja/77.1.59. [DOI] [PubMed] [Google Scholar]
- 4.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83–88. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez RJ, Moore EE, Ciesla DJ, Nieto JR, Johnson JL, Silliman CC. Post-hemorrhagic shock mesenteric lymph activates human pulmonary microvascular endothelium for in vitro neutrophil-mediated injury: the role of intercellular adhesion molecule-1. J Trauma. 2003;54:219–223. doi: 10.1097/01.TA.0000047807.12644.95. [DOI] [PubMed] [Google Scholar]
- 7.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. doi: 10.1067/msy.2001.109119. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AM, Moore EE, Masuno T, Escobar GA, Sarin EL, Johnson JL, et al. Normal mesenteric lymph blunts the pulmonary inflammatory response to endotoxin. J Surg Res. 2006;136:166–171. doi: 10.1016/j.jss.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Jordan JR, Moore EE, Damle SS, Eckels P, Johnson JL, Roach JP, et al. Gelsolin is depleted in post-shock mesenteric lymph. J Surg Res. 2007;143:130–135. doi: 10.1016/j.jss.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhingra V, Li Q, Allison AB, Stallknecht DE, Fu ZF. Proteomic profiling and neurodegeneration in West-Nile-virus-infected neurons. J Biomed Biotechnol. 2005;2005:271–279. doi: 10.1155/JBB.2005.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 12.Tannu NS, Hemby SE. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat Protoc. 2006;1:1732–1742. doi: 10.1038/nprot.2006.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Boylan MT, Evans CA, Whetton AD, Wright EG. Application of two-dimensional difference gel electrophoresis to studying bone marrow macrophages and their in vivo responses to ionizing radiation. J Proteome Res. 2005;4:1371–1380. doi: 10.1021/pr050067r. [DOI] [PubMed] [Google Scholar]
- 14.Yu KH, Rustgi AK, Blair IA. Characterization of proteins in human pancreatic cancer serum using differential gel electrophoresis and tandem mass spectrometry. J Proteome Res. 2005;4:1742–1751. doi: 10.1021/pr050174l. [DOI] [PubMed] [Google Scholar]
- 15.Gharbi S, Gaffney P, Yang A, Zvelebil MJ, Cramer R, Waterfield MD, et al. Evaluation of two-dimensional differential gel electrophoresis for proteomic expression analysis of a model breast cancer cell system. Mol Cell Proteomics. 2002;1:91–98. doi: 10.1074/mcp.t100007-mcp200. [DOI] [PubMed] [Google Scholar]
- 16.Berchtold MW. Parvalbumin genes from human and rat are identical in intron/exon organization and contain highly homologous regulatory elements and coding sequences. J Mol Biol. 1989;210:417–427. doi: 10.1016/0022-2836(89)90119-8. [DOI] [PubMed] [Google Scholar]
- 17.Shiels A, Jeffery S, Wilson C, Carter N. Radioimmunoassay of carbonic anhydrase III in rat tissues. Biochem J. 1984;218:281–284. doi: 10.1042/bj2180281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiels A, Jeffery S, Phillips IR, Shephard EA, Wilson CA, Carter ND. Sexual differentiation of rat liver carbonic anhydrase III. Biochim Biophys Acta. 1983;760:335–342. doi: 10.1016/0304-4165(83)90370-7. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman C, Eriksson I, Ronquist G, Roxin LE, Venge P, Wistrand P. Muscle ATP and lactate and the release of myoglobin and carbanhydrase III in acute lower-limb ischaemia. Eur J Vasc Surg. 1991;5:407–414. doi: 10.1016/s0950-821x(05)80172-0. [DOI] [PubMed] [Google Scholar]
- 20.Lo AS, Liew CT, Ngai SM, Tsui SK, Fung KP, Lee CY, et al. Developmental regulation and cellular distribution of human cytosolic malate dehydrogenase (MDH1) J Cell Biochem. 2005;94:763–773. doi: 10.1002/jcb.20343. [DOI] [PubMed] [Google Scholar]
- 21.Farooq N, Yusufi ANK, Mahmood R. Effect of fasting on enzymes of carbohydrate metabolism and brush border membrane in rat intestine. Nutr Res. 2004;24:407–416. [Google Scholar]
- 22.Saggerson D, Evans CJ. The activities and intracellular distribution of nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase, phosphoenolpyruvate carboxykinase and pyruvate carboxylase in rat, guinea-pig and rabbit tissues. Biochem J. 1975;146:329–332. doi: 10.1042/bj1460329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandekerckhove J, Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978;126:783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- 24.Haddad JG, Harper KD, Guoth M, Pietra GG, Sanger JW. Angiopathic consequences of saturating the plasma scavenger system for actin. Proc Natl Acad Sci U S A. 1990;87:1381–1385. doi: 10.1073/pnas.87.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajappa M, Sharma A. Biomarkers of cardiac injury: an update. Angiology. 2005;56:677–691. doi: 10.1177/000331970505600605. [DOI] [PubMed] [Google Scholar]
- 26.Malinoski DJ, Slater MS, Mullins RJ. Crush injury and rhabdomyolysis. Crit Care Clin. 2004;20:171–192. doi: 10.1016/s0749-0704(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 27.Brancaccio P, Maffulli N, Buonauro R, Limongelli FM. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1–18. vii. doi: 10.1016/j.csm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson RM, Graven KK, Tucci M, McDonald RJ, Farber HW. Non-neuronal enolase is an endothelial hypoxic stress protein. J Biol Chem. 1995;270:27752–27757. doi: 10.1074/jbc.270.46.27752. [DOI] [PubMed] [Google Scholar]
- 29.Fontan PA, Pancholi V, Nociari MM, Fischetti VA. Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae. J Infect Dis. 2000;182:1712–1721. doi: 10.1086/317604. [DOI] [PubMed] [Google Scholar]
- 30.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser E, Kuzmits R, Pregant P, Burghuber O, Worofka W. Clinical biochemistry of neuron specific enolase. Clin Chim Acta. 1989;183:13–31. doi: 10.1016/0009-8981(89)90268-4. [DOI] [PubMed] [Google Scholar]
- 32.Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RSB. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- 33.Kashuk JL, Moore EE, Millikan JS, Moore JB. Majorabdominal vascular trauma—a unified approach. J Trauma. 1982;22:672–679. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 35.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypo-perfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley JM, Haugen TH, Heath EC. Biosynthesis and processing of rat haptoglobin. J Biol Chem. 1983;258:7858–7869. [PubMed] [Google Scholar]
- 37.Hansen KK, Oikonomopoulou K, Li Y, Hollenberg MD. Proteinases, proteinase-activated receptors (PARs) and the pathophysiology of cancer and diseases of the cardiovascular, musculoskeletal, nervous and gastrointestinal systems. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:377–392. doi: 10.1007/s00210-007-0194-2. [DOI] [PubMed] [Google Scholar]
- 38.de Boer JP, Creasey AA, Chang A, Abbink JJ, Roem D, Eerenberg AJ, et al. Alpha-2-macroglobulin functions as an inhibitor of fibrinolytic, clotting, and neutrophilic pro-teinases in sepsis: studies using a baboon model. Infect Immun. 1993;61:5035–5043. doi: 10.1128/iai.61.12.5035-5043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Pal-ardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–926. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 40.Saito K, Nishikawa J, Imagawa M, Nishihara T, Matsuo M. Molecular evidence of complex tissue- and sex-specific mRNA expression of the rat alpha(2u)-globulin multigene family. Biochem Biophys Res Commun. 2000;272:337–344. doi: 10.1006/bbrc.2000.2694. [DOI] [PubMed] [Google Scholar]
- 41.Vandoren G, Mertens B, Heyns W, Van Baelen H, Rombauts W, Verhoeven G. Different forms of alpha 2u-globulin in male and female rat urine. Eur J Biochem. 1983;134:175–181. doi: 10.1111/j.1432-1033.1983.tb07548.x. [DOI] [PubMed] [Google Scholar]
- 42.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Offner PJ, Silliman CC. Phospholipase A(2)—derived neutral lipids from posthemorrhagic shock mesenteric lymph prime the neutrophil oxidative burst. Surgery. 2001;130:198–203. doi: 10.1067/msy.2001.115824. [DOI] [PubMed] [Google Scholar]
- 44.Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silli-man CC, et al. Arachidonic acid in postshock mesenteric lymph induces pulmonary synthesis of leukotriene B4. J Appl Physiol. 2008;104:1161–1166. doi: 10.1152/japplphysiol.00022.2007. [DOI] [PubMed] [Google Scholar]
- 45.Kaiser VL, Sifri ZC, Senthil M, Dikdan GS, Lu Q, Xu DZ, et al. Albumin peptide: a molecular marker for trauma/ hemorrhagic-shock in rat mesenteric lymph. Peptides. 2005;26:2491–2499. doi: 10.1016/j.peptides.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, et al. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock. 2005;23:417–425. doi: 10.1097/01.shk.0000160524.14235.6c. [DOI] [PubMed] [Google Scholar]
- 47.Zerkowski HR, Grussenmeyer T, Matt P, Grapow M, Engel-hardt S, Lefkovits I. Proteomics strategies in cardiovascular research. J Proteome Res. 2004;3:200–208. doi: 10.1021/pr034079t. [DOI] [PubMed] [Google Scholar]
- 48.Matt P, Carrel T, White M, Lefkovits I, Van Eyk J. Proteomics in cardiovascular surgery. J Thorac Cardiovasc Surg. 2007;133:210–214. doi: 10.1016/j.jtcvs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Leak LV, Liotta LA, Krutzsch H, Jones M, Fusaro VA, Ross SJ, et al. Proteomic analysis of lymph. Proteomics. 2004;4:753–765. doi: 10.1002/pmic.200300573. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh YC, Frink M, Choudhry MA, Bland KI, Chaudry IH. Metabolic modulators following trauma sepsis: sex hormones. Crit Care Med. 2007;35:S621–S629. doi: 10.1097/01.CCM.0000278603.18687.4F. [DOI] [PubMed] [Google Scholar]