Abstract
Context
Adipose tissue inflammation has been implicated in the pathogenesis of obesity related co-morbidities. Adiponectin, an anti-inflammatory protein, improves insulin sensitivity and lipid levels systemically. Because adiponectin is secreted by adipocytes, it may also act locally to counteract insulin resistance and dyslipidemia worsened by inflammation.
Objective
To determine whether associations between adiponectin and insulin sensitivity and lipids are stronger with increasing adiposity.
Design
A cross-sectional study of participants in the “The Princeton School District Study.”
Setting
Princeton City schools (Cincinnati, OH, 2001–2002 school year).
Participants
1196 non-Hispanic white and black students in grades 5–12.
Main Outcome Measure
The relationships between adiponectin and high-density lipoprotein cholesterol (HDL), triglycerides, and insulin. To test our hypothesis we: 1) compared correlation and regression coefficients of lean and non-lean individuals and 2) incorporated an adiponectin by adiposity interaction in regression models.
Results
For HDL and triglycerides, the relationship with adiponectin, while present among lean adolescents, strengthened with increasing adiposity. However, with insulin, a relationship with adiponectin was only present among non-lean adolescents.
Conclusions
These analyses suggest that adiponectin’s relationship with insulin and lipids strengthens with increasing adiposity, such that heavier adolescents have a greater benefit from high levels of adiponectin than their lean counterparts.
Keywords: Adipokines, obesity, interactions, metabolic syndrome
INTRODUCTION
Obesity has reached epidemic proportions in the United States, and is now affecting individuals at younger ages. This is problematic because even in adolescents overweight is associated with increased dyslipidemia, hypertension, and insulin resistance (1). However, not all obese individuals suffer from these co-morbidities. Thus, identification of factors that confer increased risk is essential to developing strategies to prevent or limit obesity-associated morbidity.
The pro-inflammatory environment of obesity has been implicated in the pathogenesis of obesity related co-morbidities. With increasing adiposity, adipose tissue undergoes marked morphologic and physiologic changes including the infiltration of macrophages and the release of pro-inflammatory cytokines (2). In physiologic studies, these pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), decrease insulin sensitivity and increase lipolysis (3, 4). Thus, adipose tissue changes may contribute to insulin resistance and dyslipidemia.
In such an environment, anti-inflammatory factors such as adiponectin may play a central role in modulating obesity related co-morbidities. Although produced exclusively in adipose tissue, plasma adiponectin levels are paradoxically lower in obesity (5), for reasons that have not been fully elucidated. Adiponectin is a major circulating protein in blood and has important endocrine functions, including improving hepatic insulin sensitivity and lipid levels (6–8). Adiponectin also has strong anti-inflammatory properties, as it inhibits macrophage activation and TNF-α action (9–11). Because adiponectin is secreted by adipocytes, it may have the opportunity to act within adipose tissue to counteract the pro-inflammatory cytokines associated with insulin resistance and dyslipidemia.
We hypothesized that adiponectin may have a more important role in improving insulin sensitivity and lipid profiles in obese individuals, despite their lower plasma adiponectin levels. Our objective was to explore these relationships in a large epidemiologic cohort of adolescents and test whether associations between adiponectin and both insulin sensitivity and blood lipids are stronger with increased adiposity.
METHODS
Data collection
Sample
We randomly selected 1236 non-Hispanic white and black students from 2501 students participating in a prospective, urban-suburban school-based study of carbohydrate metabolism (PSD Study)(12). In this larger study, students in grades five through twelve in 2001–2002 in the Princeton City School District (Cincinnati, OH) were invited to participate. Exclusion criteria included chronic disease, medication use known to affect carbohydrate metabolism, and pregnancy. Our cohort consisted of 1196 students with complete data. This cohort did not differ from the overall PSD Study population with regard to age, sex, race, adiposity or family history of diabetes (data not shown). The Institutional Review Boards of Cincinnati Children’s Hospital Medical Center and the University of Cincinnati approved this study. Written informed consent was obtained from all participants ≥ 18 years of age or from the parents/guardian with written assent obtained from participants < 18 years of age.
History and Physical Examination
Parents and/or students completed a medical history documenting chronic disease, medication use and history of menarche for the girls. Trained study personnel conducted physical examinations in school facilities, behind portable screens. Height and weight were measured in street clothes without shoes and with empty pockets. Participants were weighed to the nearest 0.01 kg (Seca 770 scale) and height was measured to the nearest 0.1 cm (Road Rod stadiometer). Waist circumference was measured at the level of the umbilicus to nearest 0.1 cm after an overnight fast. Two measurements were taken (by the same study personnel), and the average was used in the analysis. Axillary hair was documented in boys as none, minimal, or adult distribution (13–15).
Laboratory Measurements
After a minimum 10-hour fast, venipuncture was performed. Laboratory methods for assaying insulin, testosterone, estradiol, HDL, and triglycerides (TG) were previously described (12, 16). Plasma adiponectin levels were measured using a commercial RIA kit (Linco, St. Louis MO), with a sensitivity of 0.5μg/ml and intra- and inter-assay CVs of 5% and 15%, respectively.
Puberty
Pubertal status determination was described previously (12). Briefly, sex hormone cut points for testosterone and estradiol were established to distinguish pre-puberty (Tanner I) from puberty (Tanner II–IV) using data from two large Cincinnati-based adolescent cohorts with full Tanner staging (17, 18). Post-puberty was defined in girls with menarche duration ≥2 years and in boys with an adult distribution of axillary hair.
Calculated Variables
Body mass index (BMI) was calculated (weight (kg)/height (m)2). BMI percentile (BMI %) and z-score (BMI-Z) were determined using Centers for Disease Control and Prevention (CDC) growth charts, which take age and sex into consideration (19). Lean (<85th BMI %) and non-lean (≥ 85th BMI %) categories of adolescents were defined, consistent with the clinical threshold of risk for overweight in children (20).
As nationally representative age- and sex-specific waist z-scores have not been established, we calculated waist z-scores (waist-Z) based on our population distribution of waist circumference by age and sex (n=1196).
Statistical Analyses
Analyses were conducted using SAS, version 9.1 (SAS, Cary, NC). As the outcomes are HDL, TG, and insulin, we used Bonferroni-adjusted p-values (0.05/3) resulting in a significance level of 0.017.
Data preparation
All continuous variables were examined for normality, and natural logarithm (ln) transformations of insulin and TG were used in the analysis.
Correlation analysis
Partial correlations were calculated, using race, sex and puberty as partial variables for the full sample and subsets of lean and non-lean adolescents. A partial correlation is the correlation of two variables while controlling for the covariate relationship between variables. For our sample, the ability to remove covariate effects on the relationship is crucial, as sex, puberty, and race may all influence adiponectin, insulin, and lipids. Thus, these covariates may alter the level of correlation. Significant differences between Pearson correlation coefficients in various subgroups were ascertained using Fisher’s Z-transformation. The differences between Z-transformed scores for the subsets were compared with a standard normal distribution, and two-tailed probabilities were calculated.
Linear regression in lean and non-lean subsets
Linear regression models for each of the three outcome variables were evaluated in lean and non-lean subsets. Sex, puberty, age, race, and adiponectin were considered in the models, with male, pre-puberty, and non-Hispanic white as reference categories. To identify the most parsimonious model, we first used best subsets selection with adjusted R2 as the criterion. Variables not reaching marginal significance (p > 0.10) were eliminated. Additionally, as many variables were correlated, variables exhibiting high collinearity (variance inflation factor > 10) were identified and the one with the higher p-value was removed. Then, variables in the model were exchanged for correlated variables that had been eliminated from the model (e.g., age might be substituted for puberty). The Bayesian Information Criteria (BIC) from these models were compared and the model with the lowest BIC was selected. BIC differences greater than 6 were considered “strong” evidence for the model with the lower BIC (21). Differences in BIC values less than 2 were considered equally likely models and all models within this window were combined to create the most parsimonious model. Regression coefficients are reported as β ± standard error (SE).
To compare the regression coefficients for adiponectin in lean versus non-lean groups, the following equation was used:
Where bi is the regression coefficient for adiponectin and MSerror is the sum of the sum of squares of the error term in the two groups divided by the sum of the degrees of freedom for the error term in the two groups. This statistic is distributed as a Student’s t with ntotal – 4 degrees of freedom (22).
Testing interaction terms in the whole cohort
To evaluate interactions, models including the whole cohort for the three outcome variables were developed allowing for interactions between adiponectin and adiposity (waist-Z, BMI-Z, and non-lean) to enter the model. Model selection followed the criteria described above, except the interaction terms were evaluated separately due to issues with collinearity, and non-significant main effects were retained in the model if interactions were significant.
Interpretation of interaction terms is an important consideration in this study. The β estimate of an interaction between a categorical and a continuous variable indicates the difference in slope of the continuous variable between the non-reference and reference categories. The β estimate of an interaction between two continuous variables indicates that as one continuous variable increases (e.g., BMI-Z), the relationship between the other variable and the outcome (e.g., adiponectin and HDL) changes.
RESULTS
Descriptive statistics of the study population, as well as lean and non-lean subsets, are reported in table 1. Adiponectin and HDL concentrations are higher in the lean group, while insulin and triglycerides are higher in the non-lean group (all p < 0.0001). Lean subjects are more likely to be Non-Hispanic white than non-lean subjects (p < 0.0001).
Table 1.
Characteristics of the study population. Categorical data are reported as percentages, continuous data are reported as means ± SD.
| Total | Lean | Non-Lean | |
|---|---|---|---|
| N | 1196 | 754 | 442 |
| Age (years) | 14.3 ± 2.2 | 14.4 ± 2.2 | 14.2 ± 2.2 |
| Sex (% male) | 49.9 | 50.3 | 49.3 |
| Race (% Non-Hispanic | 53.2 | 58.2 | 44.6* |
| Puberty stage (% Pre, Peri, Post) | 12/ 39/ 49 | 12/ 39/ 49 | 12/ 39/ 49 |
| BMI (kg/m2) | 23.0 ± 5.6 | 19.8 ± 2.4 | 28.3 ± 5.5 |
| BMI Z-score | 0.67 ± 1.02 | 0.05 ± 0.69 | 1.73 ± 0.47 |
| Waist (cm) | 77.3 ± 13.8 | 70.0 ± 6.9 | 89.7 ± 13.8 |
| Adiponectin (μg/mL) | 9.42 ± 4.10 | 10.33 ± 4.04 | 7.87 ± 3.73* |
| Insulin (μU/mL) | 21.49 ± 19.76 | 15.96 ± 11.77 | 30.91 ± 26.07* |
| HDL (mg/dL) | 47.03 ± 11.22 | 49.15 ± 11.08 | 43.40 ± 10.52* |
| Triglycerides (mg/dL) | 78.65 ± 38.17 | 72.16 ± 31.33 | 89.71 ± 45.58* |
significantly different from lean (p < 0.0001)
BMI, BMI Z-score, and waist were not compared between lean and non-lean individuals. To convert insulin, HDL, and triglycerides to SI units, multiply the level by 6.0, 0.02586, and 0.01129, respectively.
Correlation analyses
In the full dataset after accounting for the effects of sex, race and puberty, plasma adiponectin was significantly negatively correlated with ln(insulin) and ln(TG), and positively correlated with HDL cholesterol (Table 2).
Table 2.
Partial correlations between adiponectin and insulin and blood lipids adjusted for sex, race and puberty stage.
| Full cohort | Lean | Non-Lean | |
|---|---|---|---|
| N | 1196 | 754 | 442 |
| Ln (insulin) | −0.24 | −0.06 | −0.28* |
| HDL | 0.29 | 0.17 | 0.34* |
| Ln (TG) | −0.26 | −0.13 | −0.31* |
All correlations in the full cohort, lean and non-lean subgroups were significantly different from 0 (p<0.0004), except ln(insulin) in leans.
significantly different from lean (p < 0.002)
After stratifying by lean versus non-lean status, all partial correlations, except ln(insulin) in leans, were significantly different from 0 (Table 2). In addition, partial correlations between adiponectin and ln(insulin), HDL, and ln(TG) were significantly higher among non-lean than lean adolescents (p <0.002 for all), indicating a significantly stronger relationship between adiponectin and these outcomes in the non-lean subset.
Regression Analysis
To further explore this finding, linear regression models were created separately in lean and non-lean subsets.
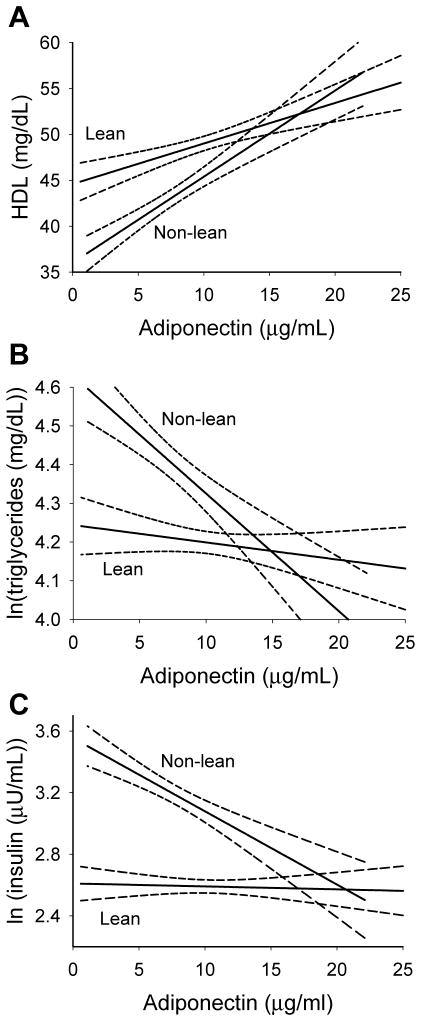
After adjusting for puberty, age, sex, and race, adiponectin was significantly associated with HDL in both lean and non-lean subsets (β ± SE: 0.47 ± 0.01 and 0.94 ± 0.13, respectively, both p<0.0001). In addition, the relationship between adiponectin and HDL was significantly stronger in non-lean versus lean adolescents (p = 0.0013, figure 1A), confirming our partial correlation findings.
Figure.
Relationship between adiponectin and HDL (A), ln(triglycerides) (B), and ln(insulin) (C) in lean and non-lean adolescents (not adjusted for other factors). Solid lines represent the predicted regression lines; dashed lines represent 95% confidence limits on the regression lines. To convert insulin, HDL, and triglycerides to SI units, multiply the level by 6.0, 0.02586, and 0.01129, respectively.
Similarly, after adjusting for puberty, sex, and race, adiponectin was associated with ln(TG) in both lean and non-lean subsets (β ± SE: −0.05 ± 0.01 and −0.036 ± 0.005, both p < 0.0001). Also supporting our partial correlation findings, the relationship was significantly stronger in non-lean versus lean adolescents (p < 0.0001, figure 1B).
By contrast, after adjusting for puberty, sex, and race, adiponectin was significantly associated with ln(insulin) in the non-lean subset (β ± SE: −0.050 ± 0.008, p < 0.0001) but not in the lean subset (β ± SE: −0.008 ± 0.005, p = 0.13). These regression coefficients were significantly different from each other (p < 0.0001, figure 1C), again supporting the partial correlation analyses.
Regression Analysis with Interaction
To test for interaction between adiponectin and adiposity in the whole cohort, linear regression models were developed for HDL, ln(TG), and ln(insulin) including adiponectin by adiposity interaction terms. The best models for HDL included either adiponectin*BMI-Z (BIC = 5505) or adiponectin*non-lean (BIC = 5506), which were better than the model without an interaction (BIC = 5510). The best models for ln(TG) included either adiponectin*non-lean (BIC = −2307) or adiponectin*waist-Z (BIC = −2305), which were better than the model without an interaction (BIC = −2300). The best models for ln(insulin) included adiponectin*non-lean (BIC = −1375) or adiponectin*BMI-Z (BIC = −1375), which were superior to the model without an interaction (BIC = −1368). Table 3 reports the model for each outcome with the lowest BIC value. For each outcome, the adiponectin by adiposity interaction acts to increase adiponectin’s effect with increasing adiposity. However, given the similarities in BIC values, we are not able to address whether this is a function of central (waist) or overall (BMI) adiposity.
Table 3.
Adiponectin by Adiposity Interactions in Regression Models for Insulin and Lipids
| HDL-cholesterol | Ln Triglycerides | Ln Insulin | ||||
|---|---|---|---|---|---|---|
| β ± SE | p-value | β ± SE | p-value | β ± SE | p-value | |
| Intercept | 51.24 ± 2.88 | <0.0001 | 4.48 ± 0.04 | <0.0001 | 2.37 ± 0.08 | <0.0001 |
| Female | 1.10 ± 0.62 | 0.07 | 0.07 ± 0.02 | 0.004 | 0.30 ± 0.03 | <0.0001 |
| Peri-puberty | −3.27 ± 1.03 | 0.002 | -- | -- | 0.30 ± 0.05 | <0.0001 |
| Post-puberty | −3.87 ± 1.37 | 0.005 | −0.05 ± 0.02 | 0.018 | 0.17 ± 0.05 | 0.002 |
| Age | −0.51 ± 0.21 | 0.016 | -- | -- | -- | -- |
| Non-Hispanic black | 4.91 ± 0.61 | <0.0001 | −0.27 ± 0.02 | < 0.0001 | 0.13 ± 0.03 | <0.0001 |
| Non-lean | -- | -- | 0.19 ± 0.06 | 0.004 | 0.31 ± 0.10 | 0.002 |
| Adiponectin | 0.45 ± 0.09 | <0.0001 | −0.01 ± 0.004 | 0.002 | −0.005 ± 0.005 | 0.36 |
| Adiponectin by non-lean | -- | -- | −0.02 ± 0.006 | 0.002 | −0.03 ± 0.01 | 0.0008 |
| BMI-Z | −2.66 ± 0.94 | 0.005 | -- | -- | 0.06 ± 0.03 | 0.07 |
| Adiponectin by BMI-Z | 0.19 ± 0.07 | 0.009 | -- | -- | -- | -- |
| Waist-Z | −2.11 ± 0.56 | 0.0002 | 0.11 ± 0.02 | <0.0001 | 0.25 ± 0.03 | <0.0001 |
| Adjusted R2 | 21.5 | 21.2 | 34.5 | |||
| BIC | 5505 | −2307 | −1375 | |||
To convert insulin, HDL, and triglycerides to SI units, multiply the level by 6.0, 0.02586, and 0.01129, respectively.
DISCUSSION
Previous research has demonstrated an association between adiponectin and adiposity, blood lipids, and insulin sensitivity in adults (6, 23, 24) and children (25–27). Our results are consistent with these findings. Adiponectin levels were lower in non-lean individuals and adiponectin was positively correlated with HDL and negatively correlated with insulin and triglycerides.
However, our results go beyond the concept that the level of adiponectin, insulin, and lipids are different in lean and non-lean adolescents. Rather, these data suggest that the relationship between adiponectin and insulin and lipids strengthens with increasing adiposity. This is the first report of the relationship of adiponectin with insulin and lipids being conditional on adiposity. Weiss and colleagues found that adiponectin’s association with intramyocellular lipid content was present only in their non-lean group (28), however, they did not formally test for interactions.
The relationship between adiponectin and HDL and ln(TG) were present in both lean and non-lean adolescents but were strengthened with increasing adiposity. The existence of an effect in both groups was supported by the partial correlations and the regression coefficients for adiponectin, which were significant in both lean and non-lean groups. Strengthening of the relationship was supported by statistically higher correlations and regression coefficients for the non-lean adolescents as well as statistically significant interaction terms between adiponectin and adiposity measures, suggesting that as adiposity increases the relationship between adiponectin and lipids strengthens.
Adiponectin’s relationship with insulin resistance is complex. We demonstrated a strengthening of the association with increasing adiposity, as the partial correlations and regression coefficients were significantly higher in non-lean than lean adolescents. Additionally, the adiponectin by adiposity interaction was highly significant. However, our results from the partial correlations and regression subsets suggest that adiponectin and insulin are only significantly associated in the non-lean group. Additionally, the main effect for adiponectin became insignificant after including the interaction term. Our non-significant results in the lean group are not due to low power given that there were nearly twice as many lean individuals as non-lean individuals. The lack of an effect of adiponectin on insulin sensitivity in the lean state has been noted in adiponectin knockout mice. On the standard diet, these mice fail to exhibit insulin resistance, but insulin resistance can be induced on a high fat diet (7).
We speculate that adiponectin’s associations with lipids and insulin are strengthened in non-lean adolescents because of the pro-inflammatory, macrophage-rich environment associated with obesity (29). This pro-inflammatory state has been implicated in the pathogenesis of type 2 diabetes and dyslipidemia (30–32). Adiponectin, by contrast, has strong anti-inflammatory properties, including suppression of pro-inflammatory cytokines and their actions in adipose tissue and the inhibition of macrophage accumulation and activity (9–11, 29). Thus, in overweight individuals, the anti-inflammatory, macrophage-inhibiting actions of adiponectin are likely very important in influencing insulin sensitivity and lipid metabolism given the pro-inflammatory adipose tissue milieu. However, in lean individuals, the absence of adipose tissue inflammation attenuates the impact of adiponectin on insulin and lipids.
The current study has several limitations. First, the proportion of the variability accounted for in our models is modest, indicating that other unmeasured factors also impact insulin and lipid profiles. In particular, genetic and environmental influences are likely, some of which we will be considering in future analyses, and some of which (e.g., diet, physical activity) were not measured in the current study. While adiponectin may not account for the majority of variability in HDL, TG or insulin in our population, it remains a strong independent factor, and one that improves the model R2 and BIC. Second, the use of epidemiologic and anthropometric measures may limit our ability to precisely characterize the physiologic mechanism involved. However, large epidemiologic studies such as this provide important clues to interactions between factors that are not discernable in smaller cohorts or in vitro studies. Alternate study designs and populations should thus validate the proposed mechanisms suggested by our data.
In conclusion, we have provided novel evidence to suggest that the relationships between adiponectin and insulin and blood lipids (e.g., HDL and TG) are strengthened with increasing adiposity, representing a paradigm shift. The current philosophy with adiponectin is that higher levels are associated with better lipid and insulin profiles regardless of adiposity. Our data suggest that heavier individuals have a greater benefit from high levels of adiponectin than their lean counterparts. This could have important diagnostic and therapeutic implications, which should be explored in future research.
Acknowledgments
We would like to thank the Princeton City School District and participants and their families without which this research would not have been possible. We would also like to acknowledge Walter Banach, Tamara Rausch and the PSD Study team: Tara Hamann, RN, Stacey Poe, MS, Amy Cline, RN, Elena Strickland, RN, Tara Schafer-Kalkhoff, Sang Sam, Michelle Hull, and Julie Schwarber. This work was supported by a grant from the American Diabetes Association (7-03-CD-06) and the NIH grants DK59183, HD-41527, NIEHS T32-ES 10957, and M01 RR 08084.
References
- 1.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among u.s. Adolescents, 1999–2000. Diabetes Care. 2004;27:2438–43. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929–35. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4854–8. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Haring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–43. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 7.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 8.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 9.Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171:5091–9. doi: 10.4049/jimmunol.171.10.5091. [DOI] [PubMed] [Google Scholar]
- 10.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 11.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 12.Dolan L, Bean J, D’Alessio D, Cohen R, Morrison J, Goodman E, Daniels S. The frequency of abnormal carbohydrate intolerance and diabetes in a population based screening of adolescents. Journal of Pediatrics. doi: 10.1016/j.jpeds.2005.01.045. In press. [DOI] [PubMed] [Google Scholar]
- 13.Macias-Tomei C, Lopez-Blanco M, Espinoza I, Vasquez-Ramirez M. Pubertal Development in Caracas Upper-Middle-Class Boys and Girls in a Longitudinal Context. American Journal of Human Biology. 2000:88–96. doi: 10.1002/(SICI)1520-6300(200001/02)12:1<88::AID-AJHB10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas CC. The Diagnosis and Treatment of Endocrine Disroders in Childhood and Adolescence. 3. Springfield, IL: 1965. [Google Scholar]
- 16.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–51. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 17.Morrison JA, Sprecher DL, Biro FM, Hansen CA, Lucky AW, Wride K. Sex hormones and lipoproteins in adolescent male offspring of parents with premature coronary heart disease and a control group. J Pediatr. 1998;133:526–32. doi: 10.1016/s0022-3476(98)70062-9. [DOI] [PubMed] [Google Scholar]
- 18.Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health. 1992;82:1613–20. doi: 10.2105/ajph.82.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center for Disease Control. [Accessed on December 2002];Center for Disease Control and Prevention: A SAS Program for the CDC Growth Charts. 2002 www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm.
- 20.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. Jama. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 21.Raftery A. Bayesian Model Selection in Social Research. In: Marsden P, editor. Sociological Methodology 1995. Blackwells; Cambridge MA: 1995. pp. 111–195. [Google Scholar]
- 22.Sokal RR, Rohlf FJ. Biometry. 2. W.H. Freeman and Company; New York: 1981. [Google Scholar]
- 23.Yamamoto Y, Hirose H, Saito I, Tomita M, Taniyama M, Matsubara K, Okazaki Y, Ishii T, Nishikai K, Saruta T. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond) 2002;103:137–142. doi: 10.1042/cs1030137. [DOI] [PubMed] [Google Scholar]
- 24.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–9. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 25.Stefan N, Bunt JC, Salbe AD, Funahashi T, Matsuzawa Y, Tataranni PA. Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J Clin Endocrinol Metab. 2002;87:4652–6. doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- 26.Huang KC, Lue BH, Yen RF, Shen CG, Ho SR, Tai TY, Yang WS. Plasma adiponectin levels and metabolic factors in nondiabetic adolescents. Obes Res. 2004;12:119–24. doi: 10.1038/oby.2004.16. [DOI] [PubMed] [Google Scholar]
- 27.Nemet D, Wang P, Funahashi T, Matsuzawa Y, Tanaka S, Engelman L, Cooper DM. Adipocytokines, body composition, and fitness in children. Pediatr Res. 2003;53:148–52. doi: 10.1203/00006450-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE, Shulman G, Caprio S. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab. 2003;88:2014–8. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 29.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 30.Jonkers IJ, Mohrschladt MF, Westendorp RG, van der Laarse A, Smelt AH. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: reversal with bezafibrate therapy in a randomized controlled trial. Am J Med. 2002;112:275–80. doi: 10.1016/s0002-9343(01)01123-8. [DOI] [PubMed] [Google Scholar]
- 31.Popa C, Netea MG, Radstake T, Van Der Meer JW, Stalenhoef AF, Van Riel PL, Barrera P. Influence of anti-TNF treatment on the cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004 doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]