Abstract
Chemokines secreted by astrocytes play multiple roles in the pathology of Alzheimer’s disease, a chronic inflammation disorder of central nervous system. The level of chemokines in serum, cerebrospinal fluid and brain tissue and their receptors both significantly changed in patients with Alzheimer’s disease. In this review, we briefly summarized the involvement of astrocytes and chemokines in Alzheimer’s disease, and the role of chemokine/chemokine receptors in the occurrence and development of Alzheimer’s disease. Clarification of the involvement of chemokines and their receptors, such as MCP-1/CCR2, fractalkine/CX3CR1, SDF-1α/CXCR4, MIP-1α/CCR5, IP-10/CXCR3, IL-8/CXCR1, CXCR2, and RANTES/CCR1, CCR3, CCR5, will provide a new strategy and more specific targets for the treatment of Alzheimer’s disease.
Keywords: Alzheimer’s disease, inflammation, astrocytes, chemokines, chemokine receptors
Introduction
Alzheimer’s disease (AD), a progressive and irreversible neurodegenerative disease, is now the most common cause of dementia of old people. In the patients with AD, memory and cognitive functions are gradually destroyed, and eventually develop into a comprehensive cognitive dysfunction. The major neuropathologic hallmarks of AD include senile plaques (SP), which are formed by extracellular deposition of amyloid β-protein (Aβ), intracellular neurofibrillary tangles, which are composed of the tau protein, and the lack of neurons and synapses.
The pathogenesis of AD is quite complex, however, a growing number of researches proved that AD could be considered as a chronic inflammation disorder of central nervous system (CNS). The inflammatory cytokines and chemokines may play a vital role in the occurrence and development of AD. Immunogens formed by abnormal deposition of Aβ in AD patients, resulting in the activation of microglia, astrocytes (AC), complement and release of inflammatory cytokines, lead to neurons damage through the direct or indirect toxic effects by chronic immune response [1]. Recently, migration of neutrophils targeting amyloid plaques in AD mouse model has been reported [2], which demonstrated a new molecular process underlying the pathophysiology of AD.
Inflammatory components associated to AD neuroinflammation include brain cells such as microglia and AC, the classic and alternate pathways of the complement system, the pentraxin acute-phase proteins, neuronal-type nicotinic acetylcholine receptors (AChRs), peroxisomal proliferators-activated receptors (PPARs), as well as cytokines and chemokines [3].
AC participates in the occurrence and development of AD
AC, the most abundant type of glial cells in the CNS, performs many functions including biochemical support of endothelial cells that form the blood-brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, and a role in the repairing and scarring process of the brain and spinal cord after traumatic injuries. AC, which greatly outnumber microglia in the brain, is suggested to have a more important and sustained role than microglia in the enduring neuroinflammatory [4,5], and in contrast to microglia, astrocytes are able to remove and degrade Aβ without mediators or stimuli such as opsonins or cytokines [6-8]. In response to injury, neurons produce adhesion molecules and trophic factors that recruit microglia cells and AC. AC could participate in the ongoing process of damage and repairment. In addition to glial cells, the microvasculature also participates in this process. Neurodegeneration is concomitant with astrogliosis, microgliosis, and microvascular remodeling. Though the trophic factors released initially by AC during astrogliosis are benefit to tissue repair, they also amplify the inflammatory response, augment vascular permeability, and result in increased microglial activation and release of more cytokines and chemokines. In states of prolonged inflammation, continual activation and recruitment of effector cells can establish a feedback loop that perpetuates inflammation and ultimately results in neuronal injury [9].
AC mediates CNS inflammation of AD by taking on some roles of immune cells, releasing cytokines and chemokines to influence effector cells, modulating the blood-brain barrier and forming glial scars [10]. Astrocytosis is a typical morphological feature of the AD brain and represents either proliferation of astrocytes in an effort to replace dying neurons or a reaction to degrade the increasing amounts of toxic Aβ peptides [6]. Massive amounts of AC were found around SP in AD patients’ autopsy [11]. Glial fibrillary acidic protein (GFAP) is a specific marker of AC, and it is recently proposed that the transcript levels of different isoforms of GFAP were different in AD [12]. AC participates in the occurrence and development of AD mainly by upregulating the expression of proinflammatory cytokines and chemokines and regulating the generation, internalization and degradation of Aβ [13-15]. On the other hand, Aβ could elevate the expression of cytokines and chemokines in AC, thus in turn cause AC to be reactivated [16,17]. Besides, AC is closely associated with the oxidative stress response in AD, and it has been proved that activation of AC is one of the reasons of intracellular neurofibrillary tangles [18,19]. A recent research reported that forebrain engraftment of human glial progenitor cells enhanced synaptic plasticity and learning in adult mice [20], suggests that AC may play useful roles in the improvement in learning, cognition and behavior.
Chemokines and chemokine receptors
Chemokines are small heparin-binding proteins, some of them are considered to be pro-inflammatory and can be induced during an immune response to recruit cells of the immune system to a site of infection, while others are considered to be homeostatic and are involved in controlling the migration of cells during normal processes of tissue maintenance or development.
According to the primary structure of protein, the chemokine family is subdivided into four groups: α (CXC), β (CC), γ (CX3C) and δ (C) based on the number of amino acids separating two cysteine residues. Chemokine receptors are classified similarly according to which group of chemokines they bind and are designated CXCR1-CXCR6, CCR1-CCR11, CX3CR1 and XCR [21]. Most chemokines bind to more than one receptors and most receptors conjugate to several chemokines [22].
Evidence is emerging that chemokines play a role in the physiology of the nervous system, including neuronal migration, cell proliferation, and synaptic activity, besides mediating neuroinflammation. Upon stimulation by pathogens or abnormal cells, immune cells as well as cells of the nervous system such as microglia, AC, oligodendrocytes, myelinating cells of the CNS, and Schwann cells in the peripheral nervous system (PNS), endothelial cells of the brain microvasculature, and even neurons can release cytokines and chemokines [23]. For the distribution of chemokine receptors, neurons express CXCR2, CXCR3 and CXCR4, microglias express CCR2, CCR5 and CX3CR1, AC express CXCR2, CXCR4, CCR1, CCR2, CCR3, CCR5, CCR10, CCR11 and CX3CR1 [24,25], and those receptors could bind to their ligands, which are also produced by AC and other cells. Chemokines produced by AC and their receptors were listed in Table 1.
Table 1.
Chemokines produced by AC and their receptors
| Branches | Chemokines | Receptors | Function | Ref. | |
|---|---|---|---|---|---|
|
| |||||
| Original | Systematic | ||||
| α (CXC) | |||||
| CXCL1 | GRO-α/MGSA-α | CXCR1, CXCR2 | Inflammatory | [42-44] | |
| CXCL3 | GRO-γ/MGSA-γ | CXCR2 | Inflammatory | [43,44] | |
| CXCL5 | ENA-78 | CXCR2 | Inflammatory | [45] | |
| CXCL6 | GCP-2 | CXCR2 | Inflammatory | [44,46,47] | |
| CXCL7 | NAP-2 | CXCR2 | Inflammatory | [44,48] | |
| CXCL8 | IL-8 | CXCR1, CXCR2 | Inflammatory | [44,47-49] | |
| CXCL9 | Mig | CXCR3 | Dual-function | [47,49] | |
| CXCL10 | IP-10 | CXCR3 | Dual-function | [24,50,51] | |
| CXCL11 | I-TAC | CXCR3 | Dual-function | [49,52] | |
| CXCL12 | SDF-1α/β | CXCR4 | Homeostatic | [47,53,54] | |
| β (CC) | |||||
| CXCL2 | MCP-1/MCAF/TDCF | CCR2 | Inflammatory | [49,54,55] | |
| CXCL3 | MIP-1α/LD78α | CCR1, CCR2, CCR5 | Inflammatory | [45,54,56] | |
| CXCL4 | MIP-1β | CCR3, CCR5 | Inflammatory | [54,57] | |
| CXCL5 | RANTES | CCR1, CCR3, CCR5 | Inflammatory | [54,58,59] | |
| CXCL7 | MCP-3 | CCR1, CCR2 | Inflammatory | [60-62] | |
| CXCL19 | MIP-3β/ELC/exodus-3 | CCR7 | Homeostatic | [47,54] | |
| CXCL20 | MIP-3α/LARC/exodus-3 | CCR6 | Dual-function | [54,63] | |
| CXCL22 | MDC/STCP-1 | CCR4 | Dual-function | [47,64] | |
| γ (CX3C) | |||||
| CX3CL1 | Fractalkine | CX3CR1 | Inflammatory | [49,65] | |
| δ (C) | |||||
| -- | -- | -- | -- | ||
Changes of chemokines and their receptors in AD
Significant differences of chemokines and their receptors’ level in serum, cerebrospinal fluid (CSF) and brain tissue have been proved between patients with AD and normal people. Chemokines and their receptors, represented by MCP-1 and it’s receptor CCR2, are regarded as biomarkers to monitor the progression of AD, since it is possible that the severity of AD could be correlated with the expression of chemokines [26,27]. Clinical studies had shown that prodromal AD patients with the highest tertile of CSF MCP-1 exhibited a significantly faster cognitive decline and developed dementia within a shorter time period compared to those that with the lowest tertile [28]. Plasma expression of CCR2, was decreased while plasma MCP-1 levels were significantly increased and were related to the degree of monocyte/macrophage activation [29]. Also it had been reported that leukocyte CCR2 expression was associated with mini-mental state examination score (MMSE) in older adults [30].
Studies showed serum chemokines changed in patients with AD included increased expression of MCP-1 [29] and IL-8 [31] (weaker sensitivity compared to other biomarkers), and decreased expression of soluble fractalkine (CX3CL1) [32], stromal cell-derived factor-1 (SDF-1, CXCL12) [33] and regulated upon activation normal T-cell expressed and secreted (RANTES, CCL5) [34]. CSF chemokines changed in patients with AD mainly include the increased expression of MCP-113 and IL-8 [35], and the decreased expression of SDF-1 [33]. However, the changes of IP-10 (CXCL10) were controversial. It had been reported that IP-10 serum level or gene was not increased in mild cognitive impairment (MCI) and AD [36,37], suggesting IP-10 does not seem to be a risk factor of AD. Meanwhile, another research showed CSF IP-10 concentration was significantly increased in patients with MCI and mild AD but not in patients with severe AD, and correlation between IP-10 level and age has not been found [38].
Changes of chemokine levels in AD brain tissues should also be concerned. In a study of ten patients with AD, Liao et al. [39] found levels of NFκB, MCP-1, MIP-1α in hippocampus, temporal and frontal cortices were all higher than that in age-matched normal controls, and an increased number of AC stained with GFAP were observed to extensively distribute around the SP in AD brains. Sokolova et al. [40] simultaneously quantified 17 cytokines and chemokines in brain tissue from controls and patients with and without genetic forms of AD, group comparisons accounting for multiple testing revealed that MCP-1, IL-6 and IL-8 were consistently upregulated in AD brain tissue. Immunohistochemistry for MCP-1, IL-6 and IL-8 confirmed this increase and determined localization of these factors in neurons (MCP-1, IL-6, IL-8), AC (MCP-1, IL-6) and plaque pathology (MCP-1, IL-8). Logistic linear regression modeling demonstrated that MCP-1 was the most reliable predictor of disease. Besides, elevated expression of RANTES was shown in the cerebral microcirculation of AD patients [41]. Changes of chemokines in patients with AD were summarized in Table 2.
Table 2.
Changes of chemokines in patients with AD
| Chemokines | Serum | CSF | Brain tissue | Ref. |
|---|---|---|---|---|
| MCP-1 | Increased | Increased | increased | [16,29,39,40] |
| Fractalkine | Decreased | Not reported | not reported | [32] |
| SDF-1 | Decreased | Decreased | not reported | [33] |
| MIP-1 | not reported | Not reported | increased | [39] |
| IP-10 | not reported | Increased | not reported | [36-38] |
| IL-8 | Increased | Increased | increased | [31,35] |
| RANTES | Dereased | Not reported | increased | [34,41] |
Chemokines and chemokine receptors play multiple roles in the occurrence and development of AD
Activate AC and microglia and induce inflammatory cascade
The inflammatory chemokines, as showed in Table 1, are formed under pathological conditions (pro-inflammatory stimuli, such as IL-1, TNF-α, LPS, or viruses) and actively participate in the inflammatory response attracting immune cells to the site of inflammation. Chemokines play an important role in AD, which is considered as a chronic inflammation, since they regulate both the amplitude and duration of the immune/inflammatory response.
AC and microglia can both be activated by Aβ [66-68]. Reactive microglia may contribute to neuronal damage by the generation of free oxygen radicals and nitric oxide (NO), which forms the particularly aggressive peroxynitrites, and by the release of potentially neurotoxic cytokines such as tumor necrosis factor-alpha (TNF-α). The pathologically stimulated release of interleukin-1beta (IL-1β) from microglia triggers secondary activation of AC, which are forced to proliferate and to exit their differentiated state [69]. Thus cascading glial cell activation further regulates the synthesis of βAPP and accelerates Aβ deposition, while Aβ stimulates the production of chemokines including MCP-1, macrophage inflammatory protein 1-alpha (MIP-1α, CCL4), IL-8, IFN-γ-inducible protein-10 (IP-10). Aβ is also able to induce expression of adhesion molecules. The production of adhesion molecules and interaction of CD40-CD40 ligand (CD40L) further increase the Aβ-induced expression of adhesion molecules in these same cells [70-75]. Over expression of chemokines and cytokines induces growth of dystrophic neuritis expressing elevated levels of phosphorylated tau and neurofilament protein [76]. Different chemokines may also interact with each other, for example, the combination of SDF-1α and CXCR4 in astroglioma cell could induce expression of the MCP-1, IL-8 and IP-10 through ERK signaling cascade [77].
Cyclically, cytokines and chemokines themselves can also activate microglia and AC [78], thus leads to a further over expression of cytokines and chemokines. As the phagocyte in CNS, reactivated microglia could engulf the debris of damaged tissue and the deposition of Aβ. Reactivated AC also plays an important role as introduced in Part 2.
Induction of cell migration
Acting as a chemoattractant to guide the migration of cells, chemokines regulate the migration of microglia, AC, neurons and neural progenitors to sites of neuroinflammation [14,79-82], and induce migration of monocytes and peripheral T cells from blood to brain (the latter is microglial TNF-α dependent, also mediated by MIP-3α produced by AC) [83-86]. It is remarkable that microglia and AC response differently to the chemokines even when they express the same receptor. Peterson et al. [87] observed that all of MIP-1α, MIP-1β and MCP-2 could induce migration of microglia but not AC in vitro. Flynn et al. [47] reported only microglia but not AC responded to IP-10, while Biber [52] showed that IP-10 could enhance the migration of both microglia and AC.
Participating in APP process
It’s well known that Aβ could stimulate chemokine production, including MCP-1 [23], fractalkine [88], IP-10 [75] and IL-8 [89]. Effects of chemokines on Aβ are controversial, some are shown to aggravate Aβ deposition, for example, MIP-1α [73], and some are supposed to reduce Aβ deposition, for example, SDF-1α/β [90]. Those would be introduced further in Part 6. Besides, the inflammation cascade leads to over releasing of cytokines, especially IL-1, which increase the maturation of APP and cause enhanced processing of the full length APP isoforms and secretion of APP [91].
Direct/indirect effects on neurons
Some chemokines are considered to have effects on neurons, as introduced in Part 6. Cytokines involved in the inflammatory cascade are also supposed to have direct effects on neurons, for example, IL-1β could increase neuronal vulnerability to Aβ toxicity [92], and IL-6 has been shown to selectively enhance the calcium response of neurons to excitotoxic stimuli [93]. Roles chemokines and their receptors play in AD were introduced in Figure 1.
Figure 1.
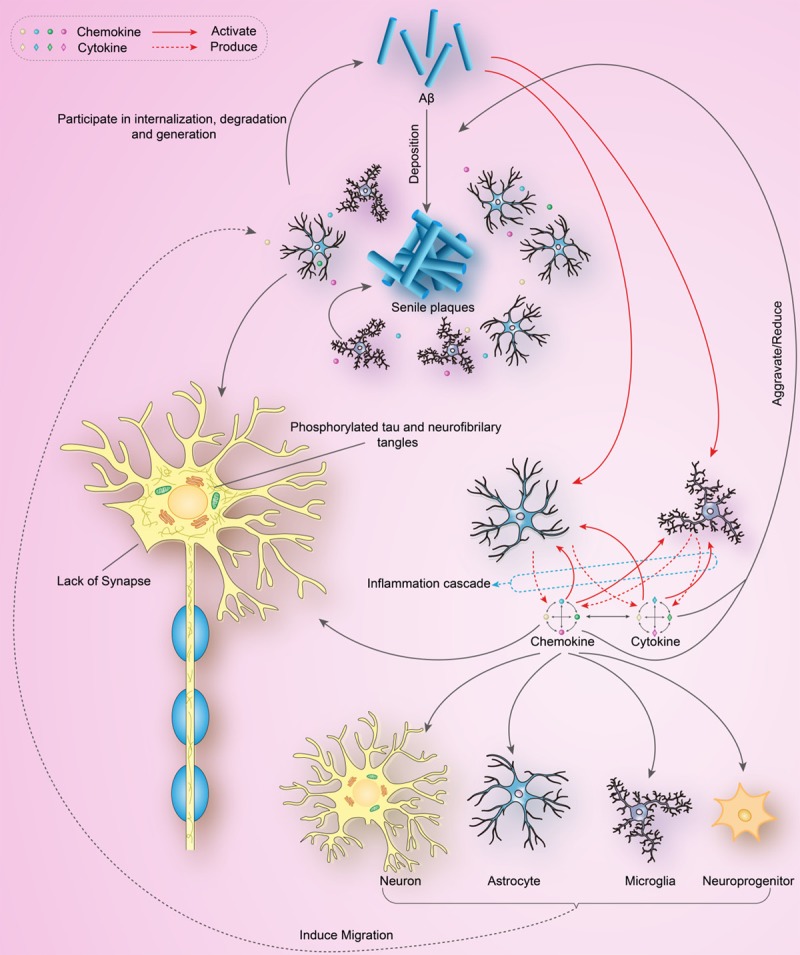
Roles chemokines and their receptors play in AD.
Chemokines and their receptors in AD
MCP-1/CCR2
Apart from being synthesized and secreted by monocytes/macrophages and endothelial cells, MCP-1 is also secreted by AC, the secretion is mediated by the stimulation of Aβ, and dependent on physical contact between monocytes and AC [72,94]. MCP-1 presents a significant chemotactic activity, and its synthesis is increased by stimulation of inflammatory factors such as TNF-α. MCP-1 could recruit monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.
Numerous clinical data suggest that MCP-1 in both serum and CSF are increased in patients with AD. Vukic et al. [95] found expression of MCP-1 induced by Aβ in human brain endothelial cells and in AD’s brain was mediated by the JNK-AP1 signaling pathway. Also, CCR2 is strongly upregulated in AD in a subpopulation of neuritic plaques, and one of the CXCR2 ligand GROα/KC can be a potent trigger for the ERK1/2 and PI-3 kinase pathways, as well as tau hyperphosphorylation in the mouse primary cortical neurons [96]. MCP-1/CCR2 is also involved in the occurrence and development of AD. Firstly, as an initiating factor in inflammation network, MCP-1/CCR2 mediates the progress of inflammation by triggering inflammatory responses, regulating the production and release of other inflammatory mediators, and thus triggers the inflammatory cascade. MCP-1/CCR2 recruits macrophages into the brain, while macrophages differentiate into microglia and further expand the inflammatory response. MCP-1 transgene expression accelerates deficits in spatial and working memory and hippocampal synaptic transmission in APP transgenic AD mouse model as early as 2-3 months of age, positing that MCP-1 facilitates Aβ oligomer formation in microglia and proposing such events accelerate memory dysfunction by affecting Aβ seeding in the brain [97].
On the other hand, macrophages are involved in the clearance of SP, thus suggests MCP-1/CCR2 deficiency may also aggravate the development of AD. Kiyota et al. [98] reported MCP-1 deficiency influences behavioral abnormalities and disease progression in APP/PSI transgenic AD mouse model. Khoury et al. [99] reported that CCR2 deficiency accelerates early disease progression and markedly impairs microglial accumulation in Tg2576 transgenic AD mouse model. AD mice deficient in CCR2 accumulated Aβ earlier and died prematurely, in a manner that correlated with CCR2 gene dosage, indicating that absence of early microglial accumulation leads to decreased Aβ clearance and increased mortality. Naert et al. [100] found that CCR2 deficiency aggravates amnesic deficits and amyloid pathology in APP (Swe)/PS1 transgenic mice, indeed, memory impairment was accelerated and enhanced in APP (Swe)/PS1/CCR2 (-/-) mice. Apparition of cognitive decline occurred earlier and correlated with intracellular accumulation of soluble oligomeric forms of Aβ. Memory deficits worsened with age and were aggravated in APP (Swe)/PS1/CCR2 (-/-) mice compared with their respective control groups. Soluble Aβ assemblies increased significantly in APP (Swe)/PS1 mice in a context of CCR2 deficiency, whereas the plaque load remained relatively similar in the brain of aging APP (Swe)/PS1 and APP (Swe)/PS1/CCR2 (-/-) mice. Also, AC-derived MCP-1 mediates neuroprotective effects of noradrenalin [101].
Fractalkine/CX3CR1
Clinical studies showed that serum soluble fractalkine level is decreased in patients with AD, and the decline is correlated with MMSE score [32], thus suggests fractalkine/CX3CR1 may participate in the regulating of AD behavior. In fact, fractalkine/CX3CR1 plays multiple roles in the development of AD, which are mainly by regulating microglia’s abnormal activation and cytokines, but not by acting on SP. Knocking out CX3CR1 leaded to the decreased activation of microglia and reduced neuronal loss [102,103]. Soluble fractalkine over expression using adeno-associated viral vectors significantly reduced tau pathology in the rTg4510 mouse model of tau deposition. Furthermore, this treatment could also reduce microglial activation and appeared to prevent neurodegeneration. An upregulation of fractalkine in the cerebral cortex and hippocampus was recently reported, and the highly expression of cytokines in Aβ burdened neurons confirmed the occurrence of a proinflammatory process preceding amyloid plaque deposition [104]. However, it is also reported in contrast to studies with fractalkine receptor null mice, parallel studies in an APP/PS1 model found no effect of increased fractalkine signaling on amyloid deposition [105]. Ablation of CX3CR1 in APP mice enhanced tau pathology and exacerbated the depletion of calbindin in the dentate gyrus, up-regulated TNF-α, and significantly decreased learning and memory abilities [106]. Meanwhile, it has also been reported recently that suppressing CX3CR1 signaling with CX3CR1 small interfering RNA (siRNA) in rats injected with Aβ1-40 fibrils blunted Aβ1-40 induced CX3CR1 upregulation, microglial activation, interleukin-1beta expression, restored basal glutamatergic strength and electric stimuli-induced long-term potentiation, and cognitive capacities [88], thus proved the fractalkine/CX3CR1’s regulation of microglia and cytokines, and suggested further studies are needed to examine the role of fractalkine/CX3CR1 in the occurrence and development in AD.
SDF-1α/CXCR4
SDF-1α is widely concerned since it’s proposed to regulate neuronal excitability and synaptic transmission. Both plasma and CSF levels of SDF-1α were found to be decreased in patients with early AD, and they were significantly inversely correlated with CSF tau protein levels and positively correlated with changes of cognitive functions over the time period of 15 months [33,107]. Parachikova et al. [108-110] reported SDF-1α’s mRNA, protein and its receptor (CXCR4) were downregulated in Tg2576 mouse model of AD coinciding with cognitive deficits. Also, they found out chronically treated young non-transgenic mice with an antagonist to CXCR4 showed selectively impaired learning and memory, thus suggests a potential role for this chemokine in cognitive functioning. Briefly, SDF-1α/CXCR4 enhances glutamate release from AC and regulates neuronal excitability, signal propagation within glial networks and synaptic transmission, and reduces the deposition of Aβ via activating microglia [90,111-113], thus provides a promising therapy target of AD.
MIP-1α/CCR5
MIP-1α/CCR5 could participate in the regulation of learning and cognition and the inflammation process of AD by activating microglia and AC, recruiting and accumulating microglia in SP, promoting T cells transendothelial migration through Rho/ROCK pathway [84,114,115]. Subcutaneous injection of CCR5 antagonist reduced the number of activated microglia and astrocytes up-regulated by lipopolysaccharide [115], suggests MIP-1α/CCR5 is involved in neuroinflammation associated with AD, and CCR5 antagonists may attenuate this effect. Lee [73] reported that long-term and spatial memory functions were impaired in CCR5 knockout (CCR5 (-/-)) mice. The expression of CCR5 was observed in CCR5 (+/+) astrocytes, but was reduced in the CCR5 (-/-) astrocytes even though the expression of GFAP was much higher, the Aβ level was higher in the brains of CCR5 (-/-) mice than that of CCR5 (+/+) mice paralleling with the activation of astorcytes. Activation of CCR2 causes the activation of AC that leads to Aβ deposition and memory dysfunction in CCR5 (-/-) mice. In CCR5 (-/-) mice, CCR2 expression was high and co-localized with GFAP. These findings suggest that the absence of CCR5 increases expression of CCR2, which leads to the activation of astrocytes causing Aβ deposition, and thereby impairs memory function. Though it is hypothesized that individuals carrying a 32-base pair deletion in the CCR5 gene would show a reduced risk of AD, a case-control study in 376 Spanish AD patients and 369 healthy controls ruled out the association [114].
IP-10/CXCR3
Although the plasma level of IP-10 in patients with AD is controversial, as introduced in Part 5, its expression in AC was widely recognized to be increased. In mouse model, secretion of IP-10 by AC was significantly increased after injecting Aβ [75], clinical research also proved that IP-10 was markedly elevated in AC in AD brains, many IP-10 positive AC were associated with SP and had an apparently coordinated upregulation of MIP-1β [116]. Lai [75] identified 19 up-regulated secreted proteins after Aβ1-42 treatment by SILAC labeling and LC-MS/MS analyses, and validated the role played by IP-10 in promoting AC aggregation around amyloid plagues through in vitro cell migration analysis. Moreover, IP-10/CXCR3 is able to activate ERK1/2 pathway in mouse cortical neurons, suggesting a novel mechanism of neuronal-glial interaction.
IL-8/CXCR1, CXCR2
IL-8 is the first confirmed presenced chemokine in human brain. Upon Aβ and/or pro-inflammatory cytokine stimulation, microglia, AC and neurons were all capable to produce IL-8 in vitro [74], while CXCR2, a receptor of IL-8, has been found to exist in the neuritic portion of plaques surrounding amyloid deposits in pathologic brain tissues of AD patients [48]. IL-8 protects neurons possibly by paracrine or autocrine loop and regulates neuronal functions. Although IL-8 alone did not alter neuronal survival, it did inhibit Aβ-induced neuronal apoptosis and increase production of neuronal brain-derived neurotrophic factor (BDNF), and be involved in the neuronal damage and astrogliosis caused by Aβ [74,117]. Further, IL-8 might be involved in the intervention of the complement (C) system, as its significant upregulation in AC caused by neuroprotective anaphylatoxins, which are released by C activities [118,119]. Therefore, IL-8 may play a protective role in the AD pathogenesis.
RANTES/CCR1, CCR3, CCR5
RANTES is a powerful leukocyte activator, a feature potentially relevant in a range of inflammatory disorders [120] but it’s suggested to be neuroprotective in AD, since treatment of neurons in vitro with RANTES resulted in an increase in cell survival and a neuroprotective effect against the toxicity of thrombin and sodium nitroprusside [41]. Curcumin, a kind of herb extracts, is reported to enhance neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in AC [121]. In the CNS, RANTES are mainly produced by AC, and AC RANTES/CCL5 has been shown to be induced by IL-1, with interferon-gamma (IFN-γ) as a primer. IFN-β also plays a positive regulatory role in the expression of RANTES/CCL5 in human AC through several distinct mechanisms [58].
Conclusions
AC plays multiple roles in the occurrence and development of AD. On one side, AC could clean up debris by the process of phagocytosis, provide nourishment and be benefit to control the chemical composition of fluid surrounding neurons. On the other side, AC mediates inflammation reaction, and is involved in the formation of oxygen free radicals and intracellular neurofibrillary tangles, thus exacerbates the development of AD. Effects of AC on Aβ are more complicated and controversial, since it’s involved both in the internalization, degradation and generation of Aβ. The role of AC in AD remains to be further studied in order to deepen the understanding of AD pathology, exploring the feasibility of AC as a target for the treatment of AD.
The involvement of chemokines and their receptors in the occurrence and development of AD could be summarized as activating AC and microglia and inducing inflammatory cascade, inducing migration of cells, being involved in APP process, and providing direct/indirect effects on neurons. Chemokines and their receptors significantly changed in serum and CSF in AD patients, and the changes were correlated with the development of AD pathology, thus providing the feasibility of monitoring AD progression. Changes of chemokine levels may be the pathological basis of chronic inflammatory reactions. Represented by MCP-1, the increased levels of some toxic chemokines may be related to AC stimulation by over-expressed Aβ. Up-regulated chemokines further induced the inflammatory cascade, accelerated Aβ deposition, and aggravated the progression of AD. Represented by SDF-1, the decreased levels of some protective chemokines also lead to aggravating the progression of AD. Researches indicated that interfering chemokines by using chemokine receptor antagonist, siRNA or other methods could affect pathology of AD, thus suggests chemokines and their receptors may be considered as a new target for the treatment of AD. Recently, chemokine receptor antagonist has been put into the treatment of some diseases, such as painful diabetic polyneuropathy [122] and HIV [123]. It is worth looking forward to the application of chemokines and/or chemokine receptor antagonist to treat AD and other CNS diseases. As immune and inflammatory responses play a key role in the pathogenesis of AD, clarification of the involvement of chemokine and their receptors will provide a new strategy and more specific targets for the treatment of AD.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (No. 81102670 and 31400838), and the National Basic Science Personal Training Foundation of China (No. J1103607).
Disclosure of conflict of interest
None.
References
- 1.Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 2.Baik SH, Cha MY, Hyun YM, Cho H, Hamza B, Kim DK, Han SH, Choi H, Kim KH, Moon M, Lee J, Kim M, Irimia D, Mook-Jung I. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, Chui D, Hoi Yu AC. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- 5.Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience. 2000;96:195–203. doi: 10.1016/s0306-4522(99)00538-2. [DOI] [PubMed] [Google Scholar]
- 6.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 7.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 8.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloidbeta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 9.Gendelman HE. Neural immunity: Friend or foe? J Neurovirol. 2002;8:474–479. doi: 10.1080/13550280290168631. [DOI] [PubMed] [Google Scholar]
- 10.Puffer BA, Sharron M, Coughlan CM, Baribaud F, McManus CM, Lee B, David J, Price K, Horuk R, Tsang M, Doms RW. Expression and coreceptor function of APJ for primate immunodeficiency viruses. Virology. 2000;276:435–444. doi: 10.1006/viro.2000.0557. [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Gondo T, Hoshii Y, Takahashi M, Yamada M, Ishihara T. Confocal observation of senile plaques in Alzheimer’s disease: senile plaque morphology and relationship between senile plaques and astrocytes. Pathol Int. 1998;48:332–340. doi: 10.1111/j.1440-1827.1998.tb03915.x. [DOI] [PubMed] [Google Scholar]
- 12.Kamphuis W, Middeldorp J, Kooijman L, Sluijs JA, Kooi EJ, Moeton M, Freriks M, Mizee MR, Hol EM. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol Aging. 2014;35:492–510. doi: 10.1016/j.neurobiolaging.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P, Grubeck-Loebenstein B. Costimulatory effects of interferon-γ and interleukin-1β or tumor necrosis factor α on the synthesis of Aβ1-40 and Aβ1-42 by human astrocytes. Neurobiol Dis. 2000;7:682–689. doi: 10.1006/nbdi.2000.0321. [DOI] [PubMed] [Google Scholar]
- 14.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 15.Pihlaja R, Koistinaho J, Malm T, Sikkilä H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited β-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56:154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 16.Forloni G, Mangiarotti F, Angeretti N, Lucca E, De Simoni MG. β-amyloid fragment potentiates IL-6 and TNF-α secretion by LPS in astrocytes but not in microglia. Cytokine. 1997;9:759–762. doi: 10.1006/cyto.1997.0232. [DOI] [PubMed] [Google Scholar]
- 17.GE YW, Lahiri D. Regulation of promoter activity of the APP gene by cytokines and growth factors. Ann N Y Acad Sci. 2002;973:463–467. doi: 10.1111/j.1749-6632.2002.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 18.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha RN, Pahan K. Signals for the induction of nitric oxide synthase in astrocytes. Neurochem Int. 2006;49:154–163. doi: 10.1016/j.neuint.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–335. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 22.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Ann Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesselgesser J, Horuk R. Chemokine and chemokine receptor expression in the central nervous system. J Neurovirol. 1999;5:13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- 25.Dorf ME, Berman MA, Tanabe S, Heesen M, Luo Y. Astrocytes express functional chemokine receptors. J Neuroimmunol. 2000;111:109–121. doi: 10.1016/s0165-5728(00)00371-4. [DOI] [PubMed] [Google Scholar]
- 26.Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Todarello O, Pellicani V, Capurso SA, Pietrarossa G, Santamato V, Capurso A. Circulating biomarkers of cognitive decline and dementia. Clin Chim Acta. 2006;364:91–112. doi: 10.1016/j.cca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Tarawneh R, Holtzman DM. Biomarkers in translational research of Alzheimer’s disease. Neuropharmacology. 2010;59:310–322. doi: 10.1016/j.neuropharm.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westin K, Buchhave P, Nielsen H, Minthon L, Janciauskiene S, Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS One. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Miller RG, Madison C, Jin X, Honrada R, Harris W, Katz J, Forshew DA, McGrath MS. Systemic immune system alterations in early stages of Alzheimer’s disease. J Neuroimmunol. 2013;256:38–42. doi: 10.1016/j.jneuroim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harries LW, Bradley-Smith RM, Llewellyn DJ, Pilling LC, Fellows A, Henley W, Hernandez D, Guralnik JM, Bandinelli S, Singleton A, Ferrucci L, Melzer D. Leukocyte CCR2 expression is associated with mini-mental state examination score in older adults. Rejuvenation Res. 2012;15:395–404. doi: 10.1089/rej.2011.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsadany MA, Shehata HH, Mohamad MI, Mahfouz RG. Histone deacetylases enzyme, copper, and IL-8 levels in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2013;28:54–61. doi: 10.1177/1533317512467680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, Lee CU. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Laske C, Stellos K, Stransky E, Seizer P, Akcay O, Eschweiler GW, Leyhe T, Gawaz M. Decreased plasma and cerebrospinal fluid levels of stem cell factor in patients with early Alzheimer’s disease. J Alzheimers Dis. 2008;15:451–460. doi: 10.3233/jad-2008-15311. [DOI] [PubMed] [Google Scholar]
- 34.Kester MI, van der Flier WM, Visser A, Blankenstein MA, Scheltens P, Oudejans CB. Decreased mRNA expression of CCL5 [RANTES] in Alzheimer’s disease blood samples. Clin Chem Lab Med. 2012;50:61–65. doi: 10.1515/CCLM.2011.731. [DOI] [PubMed] [Google Scholar]
- 35.Li K, Liu S, Yao S, Wang B, Dai D, Yao L. Interaction between interleukin-8 and methylenetetrahydrofolate reductase genes modulates Alzheimer’s disease risk. Dement Geriatr Cogn Disord. 2009;27:286–291. doi: 10.1159/000204766. [DOI] [PubMed] [Google Scholar]
- 36.Venturelli E, Galimberti D, Fenoglio C, Lovati C, Finazzi D, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Forloni G, Bresolin N, Scarpini E. Candidate gene analysis of IP-10 gene in patients with Alzheimer’s disease. Neurosci Lett. 2006;404:217–221. doi: 10.1016/j.neulet.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 37.Galimberti D, Venturelli E, Fenoglio C, Lovati C, Guidi I, Scalabrini D, Mariani C, Bresolin N, Scarpini E. IP-10 serum levels are not increased in mild cognitive impairment and Alzheimer’s disease. Eur J Neurol. 2007;14:e3–4. doi: 10.1111/j.1468-1331.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 38.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Venturelli E, Pijnenburg YA, Bresolin N, Scarpini E. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2006;66:146–147. doi: 10.1212/01.wnl.0000191324.08289.9d. [DOI] [PubMed] [Google Scholar]
- 39.Liao Y, Guan ZZ, Ravid R. [Changes of nuclear factor and inflammatory chemotactic factors in brain of patients with Alzheimer’s disease] . Zhonghua Bing Li Xue Za Zhi. 2011;40:585–589. [PubMed] [Google Scholar]
- 40.Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer’s disease brain: A possible neuroprotective role. Neurobiol Aging. 2010;31:8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 43.Puma C, Danik M, Quirion R, Ramon F, Williams S. The chemokine interleukin-8 acutely reduces Ca(2+) currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J Neurochem. 2001;78:960–971. doi: 10.1046/j.1471-4159.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 44.Lu W, Maheshwari A, Misiuta I, Fox SE, Chen N, Zigova T, Christensen RD, Calhoun DA. Neutrophil-specific chemokines are produced by astrocytic cells but not by neuronal cells. Brain Res Dev Brain Res. 2005;155:127–134. doi: 10.1016/j.devbrainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia M, Qin S, McNamara M, Mackay C, Hyman BT. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am J Pathol. 1997;150:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 48.Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- 49.Croitoru-Lamoury J, Guillemin GJ, Boussin FD, Mognetti B, Gigout LI, Chéret A, Vaslin B, Le Grand R, Brew BJ, Dormont D. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNFα and IFNγ in CXCR4 and CCR5 modulation. Glia. 2003;41:354–370. doi: 10.1002/glia.10181. [DOI] [PubMed] [Google Scholar]
- 50.Ransohoff R, Hamilton T, Tani M, Stoler M, Shick H, Major J, Estes M, Thomas D, Tuohy V. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 51.Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75:7067–7077. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biber K, Dijkstra I, Trebst C, De Groot CJ, Ransohoff RM, Boddeke HW. Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience. 2002;112:487–497. doi: 10.1016/s0306-4522(02)00114-8. [DOI] [PubMed] [Google Scholar]
- 53.Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 1999;73:2348–2357. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- 54.Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- 55.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger O, Gan X, Gujuluva C, Burns AR, Sulur G, Stins M, Way D, Witte M, Weinand M, Said J. CXC and CC chemokine receptors on coronary and brain endothelia. Mol Med. 1999;5:795. [PMC free article] [PubMed] [Google Scholar]
- 57.Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, Doms RW, González-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim MO, Suh HS, Brosnan CF, Lee SC. Regulation of RANTES/CCL5 expression in human astrocytes by interleukin-1 and interferon-beta. J Neurochem. 2004;90:297–308. doi: 10.1111/j.1471-4159.2004.02487.x. [DOI] [PubMed] [Google Scholar]
- 59.Tanabe S, Heesen M, Berman MA, Fischer MB, Yoshizawa I, Luo Y, Dorf ME. Murine astrocytes express a functional chemokine receptor. J Neurosci. 1997;17:6522–6528. doi: 10.1523/JNEUROSCI.17-17-06522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong X, Gong W, Kuhns DB, Ben-Baruch A, Howard OZ, Wang JM. Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J Biol Chem. 1997;272:11682–11685. doi: 10.1074/jbc.272.18.11682. [DOI] [PubMed] [Google Scholar]
- 61.Andjelkovic AV, Kerkovich D, Shanley J, Pulliam L, Pachter JS. Expression of binding sites for β chemokines on human astrocytes. Glia. 1999;28:225–235. [PubMed] [Google Scholar]
- 62.Renner NA, Ivey NS, Redmann RK, Lackner AA, MacLean AG. MCP-3/CCL7 production by astrocytes: implications for SIV neuroinvasion and AIDS encephalitis. J Neurovirol. 2011;17:146–152. doi: 10.1007/s13365-010-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ambrosini E, Columba-Cabezas S, Serafini B, Muscella A, Aloisi F. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3α/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia. 2003;41:290–300. doi: 10.1002/glia.10193. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida H, Imaizumi T, Fujimoto K, Matsuo N, Kimura K, Cui XF, Matsumiya T, Tanji K, Shibata T, Tamo W. Synergistic stimulation, by tumor necrosis factor-α and interferon-γ, of fractalkine expression in human astrocytes. Neurosci Lett. 2001;303:132–136. doi: 10.1016/s0304-3940(01)01699-8. [DOI] [PubMed] [Google Scholar]
- 66.Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 67.Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Amyloid-β peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem. 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 69.Schubert P, Rudolphi K. Interfering with the pathologic activation of microglial cells and astrocytes in dementia. Alzheimer Dis Assoc Disord. 1998;12(Suppl 2):S21–28. [PubMed] [Google Scholar]
- 70.Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M. Alzheimer’s β-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- 72.Smits HA, Rijsmus A, van Loon JH, Wat JW, Verhoef J, Boven LA, Nottet HS. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J Neuroimmunol. 2002;127:160–168. doi: 10.1016/s0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- 73.Lee YK, Kwak DH, Oh KW, Nam SY, Lee BJ, Yun YW, Kim YB, Han SB, Hong JT. CCR5 deficiency induces astrocyte activation, Abeta deposit and impaired memory function. Neurobiol Learn Mem. 2009;92:356–363. doi: 10.1016/j.nlm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Ashutosh , Kou W, Cotter R, Borgmann K, Wu L, Persidsky R, Sakhuja N. CXCL8 protects human neurons from amyloid-beta-induced neurotoxicity: relevance to Alzheimer’s disease. Biochem Biophys Res Commun. 2011;412:565–571. doi: 10.1016/j.bbrc.2011.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai W, Wu J, Zou X, Xie J, Zhang L, Zhao X, Zhao M, Wang Q, Ji J. Secretome analyses of Abeta (1-42) stimulated hippocampal astrocytes reveal that CXCL10 is involved in astrocyte migration. J Proteome Res. 2013;12:832–843. doi: 10.1021/pr300895r. [DOI] [PubMed] [Google Scholar]
- 76.Sheng J, Zhu S, Jones R, Griffin W, Mrak R. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol. 2000;163:388–391. doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh JW, Drabik K, Kutsch O, Choi C, Tousson A, Benveniste EN. CXC chemokine receptor 4 expression and function in human astroglioma cells. J Immunol. 2001;166:2695–2704. doi: 10.4049/jimmunol.166.4.2695. [DOI] [PubMed] [Google Scholar]
- 78.Allan Butterfield D, Griffin S, Munch G, Pasinetti GM. Amyloid β-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. J Alzheimers Dis. 2002;4:193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- 79.Bolin LM, Murray R, Lukacs NW, Strieter RM, Kunkel SL, Schall TJ, Bacon KB. Primary sensory neurons migrate in response to the chemokine RANTES. J Neuroimmunol. 1998;81:49–57. doi: 10.1016/s0165-5728(97)00158-6. [DOI] [PubMed] [Google Scholar]
- 80.Xia MQ, Hyman BT. Chemokines/chemokine receptors in the central nervous system and Alzheimer’s disease. J Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- 81.Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- 82.Noda M, Suzumura A. Sweepers in the CNS: microglial migration and phagocytosis in the alzheimer disease pathogenesis. Int J Alzheimers Dis. 2012;2012:891087. doi: 10.1155/2012/891087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, Hama S, Way D, Weinand M, Witte M. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood--brain barrier model. Mol Med. 1998;4:480. [PMC free article] [PubMed] [Google Scholar]
- 84.Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, Fang WG, Zhu L, Chen YH. Peripheral T cells overexpress MIP-1α to enhance its transendothelial migration in Alzheimer’s disease. Neurobiol Aging. 2007;28:485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Liu YJ, Guo DW, Tian L, Shang DS, Zhao WD, Li B, Fang WG, Zhu L, Chen YH. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-α-dependent. Neurobiol Aging. 2010;31:175–188. doi: 10.1016/j.neurobiolaging.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M, Iwakura Y, Mizuno T, Suzumura A. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J Immunol. 2011;186:4415–4421. doi: 10.4049/jimmunol.1003307. [DOI] [PubMed] [Google Scholar]
- 87.Peterson PK, Hu S, Salak-Johnson J, Molitor TW, Chao CC. Differential production of and migratory response to β chemokines by human microglia and astrocytes. J Infect Dis. 1997;175:478–481. doi: 10.1093/infdis/175.2.478. [DOI] [PubMed] [Google Scholar]
- 88.Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M. Suppression of central chemokine fractalkine receptor signaling alleviates amyloid-induced memory deficiency. Neurobiol Aging. 2013;34:2843–2852. doi: 10.1016/j.neurobiolaging.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Meda L, Bonaiuto C, Szendrei GI, Ceska M, Rossi F, Cassatella MA. beta-Amyloid( 25-35) induces the production of interleukin-8 from human monocytes. J Neuroimmunol. 1995;59:29–33. doi: 10.1016/0165-5728(95)00021-s. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q, Xu Y, Chen JC, Qin YY, Liu M, Liu Y, Xie MJ, Yu ZY, Zhu Z, Wang W. Stromal cell-derived factor 1alpha decreases beta-amyloid deposition in Alzheimer’s disease mouse model. Brain Res. 2012;1459:15–26. doi: 10.1016/j.brainres.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 91.Dash PK, Moore AN. Enhanced processing of APP induced by IL-1 beta can be reduced by indomethacin and nordihydroguaiaretic acid. Biochem Biophys Res Commun. 1995;208:542–548. doi: 10.1006/bbrc.1995.1372. [DOI] [PubMed] [Google Scholar]
- 92.Fagarasan MO, Aisen PS. IL-1 and anti-inflammatory drugs modulate A beta cytotoxicity in PC12 cells. Brain Res. 1996;723:231–234. doi: 10.1016/0006-8993(96)00259-4. [DOI] [PubMed] [Google Scholar]
- 93.Qiu Z, Parsons KL, Gruol DL. Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. J Neurosci. 1995;15:6688–6699. doi: 10.1523/JNEUROSCI.15-10-06688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilliland CT, Salanga CL, Kawamura T, Trejo J, Handel TM. The chemokine receptor CCR1 is constitutively active, which leads to G protein-independent, beta-arrestin-mediated internalization. J Biol Chem. 2013;288:32194–32210. doi: 10.1074/jbc.M113.503797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, Romero IA, Weksler B, Stanimirovic DB, Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xia M, Hyman BT. GROα/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer’s disease? J Neuroimmunol. 2002;122:55–64. doi: 10.1016/s0165-5728(01)00463-5. [DOI] [PubMed] [Google Scholar]
- 97.Kiyota T, Yamamoto M, Xiong H, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009;4:e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kiyota T, Gendelman HE, Weir RA, Higgins EE, Zhang G, Jain M. CCL2 affects beta-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 100.Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J Neurosci. 2009;29:263–267. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010;13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hanzel CE, Pichet-Binette A, Pimentel LSB, Iulita MF, Allard S, Ducatenzeiler A, Do Carmo S, Cuello AC. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2249–62. doi: 10.1016/j.neurobiolaging.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 105.Nash KR, Lee DC, Hunt JB Jr, Morganti JM, Selenica ML, Moran P, Reid P, Brownlow M, Guang-Yu Yang C, Savalia M, Gemma C, Bickford PC, Gordon MN, Morgan D. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging. 2013;34:1540–1548. doi: 10.1016/j.neurobiolaging.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laske C, Stellos K, Eschweiler GW, Leyhe T, Gawaz M. Decreased CXCL12 (SDF-1) plasma levels in early Alzheimer’s disease: a contribution to a deficient hematopoietic brain support? J Alzheimers Dis. 2008;15:83–95. doi: 10.3233/jad-2008-15107. [DOI] [PubMed] [Google Scholar]
- 108.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28:143–153. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 112.Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 114.Combarros O, Infante J, Llorca J, Pena N, Fernandez-Viadero C, Berciano J. The chemokine receptor CCR5-Delta32 gene mutation is not protective against Alzheimer’s disease. Neurosci Lett. 2004;366:312–314. doi: 10.1016/j.neulet.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 115.Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia MQ, Bacskai BJ, Knowles RB, Qin SX, Hyman BT. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer’s disease. J Neuroimmunol. 2000;108:227–235. doi: 10.1016/s0165-5728(00)00285-x. [DOI] [PubMed] [Google Scholar]
- 117.Franciosi S, Ryu JK, Choi HB, Radov L, Kim SU, McLarnon JG. Broad-spectrum effects of 4-aminopyridine to modulate amyloid β1-42-induced cell signaling and functional responses in human microglia. J Neurosci. 2006;26:11652–11664. doi: 10.1523/JNEUROSCI.2490-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mukherjee P, Pasinetti GM. The role of complement anaphylatoxin C5a in neurodegeneration: implications in Alzheimer’s disease. J neuroimmunol. 2000;105:124–130. doi: 10.1016/s0165-5728(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 119.Jauneau AC, Ischenko A, Chan P, Fontaine M. Complement component anaphylatoxins upregulate chemokine expression by human astrocytes. FEBS Lett. 2003;537:17–22. doi: 10.1016/s0014-5793(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 120.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 121.Lin MS, Hung KS, Chiu WT, Sun YY, Tsai SH, Lin JW, Lee YH. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog NeuroPsychopharmacol Biol Psychiatry. 2011;35:931–938. doi: 10.1016/j.pnpbp.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 122.Kalliomäki J, Jonzon B, Huizar K, O’Malley M, Andersson A, Simpson DM. Evaluation of a novel chemokine receptor 2 (CCR2)-antagonist in painful diabetic polyneuropathy. Scandinavian J Pain. 2013;4:77–83. doi: 10.1016/j.sjpain.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 123.Murata M, Furusyo N, Hayashi J. P273 Beneficial effect of C-C chemokine receptor type 5 antagonist, as an add-on treatment for HIV-1 patients with virological response but no immunological response. Intl J Antimicrobial Agents. 2013;42(Suppl 2):S128. [Google Scholar]


