Abstract
Macrophages, the major host cells harboring Mycobacterium tuberculosis (M.tb), are a heterogeneous cell type depending on their tissue of origin and host they are derived from. Significant discord in macrophage responses to M.tb exists due to differences in M.tb strains and the various types of macrophages used to study tuberculosis (TB). This review will summarize current concepts regarding macrophage responses to M.tb infection, while pointing out relevant differences in experimental outcomes due to the use of divergent model systems. A brief description of the lung environment is included since there is increasing evidence that the alveolar macrophage (AM) has immunoregulatory properties that can delay optimal protective host immune responses. In this context, this review focuses on selected macrophage immunoregulatory pattern recognition receptors (PRRs), cytokines, negative regulators of inflammation, lipid mediators and microRNAs (miRNAs).
Keywords: macrophages, innate immunity, pattern recognition receptors, microRNAs, lung
1. Introduction
Macrophages serve as the major host cell niche for intracellular growth and persistence of M.tb during all phases of TB, from primary infection with bacillary dissemination, through latency (with bacterial persistence within granulomas) and reactivation TB. In addition, macrophages are responsible for activation of protective immune responses, both innate and acquired, thus playing a critical role in the ongoing cross-talk that is necessary to control or eliminate the infection [1-6].
Our understanding of the great heterogeneity and plasticity of macrophages residing in diverse tissues of different mammals continues to evolve [7-10]. If our goal of finding relevant tissue bio-signatures and therapeutic targets to combat human M.tb is to be achieved, we must pay particular attention to the stage of TB infection being modeled (e.g. primary infection, latency with bacterial persistence, reactivation), source of the macrophage (e.g. primary or cell line, human, or other mammal), and experimental conditions, particularly as they apply to the specific tissue microenvironment being modeled (e.g. media for in vitro studies, organ for in vivo studies, granuloma).
In this review, we will summarize current concepts in macrophage biology as they pertain to M.tb pathogenesis. We place the discussion in the context of lung biology and alveolar macrophages (AMs), given their prominent role in airborne TB. AMs are unique mucosal immunoregulatory cells and there is increasing evidence that they are important in allowing M.tb to replicate for an extended period of time prior to complete activation of protective immune responses [11-16]. We have coined the time period necessary for optimal responses to occur in the lung “the switching time” [11] and provide evidence that M.tb itself can further drive the immunoregulatory activation state of macrophages to enhance its survival [12]. Thus we highlight emerging key macrophage immunoregulatory determinants for M.tb.
2. Airborne M.tb infection and the lung
M.tb nearly always infects humans via inhalation of airborne droplets released by individuals with active TB that are deposited in the lung alveolus, where as few as one to five bacteria can result in infection. Deposited M.tb are engulfed by the resident AMs, which are less able to kill and clear all of the bacteria, ultimately becoming the microbe’s home and allowing for dissemination to occur. Primary M.tb infection is generally self-limited (subclinical), most often resulting in latency and containment of the bacteria that were not eradicated, although complete clearance is possible. During primary infection bacteremia can occur, resulting in bacterial deposition in other organs which serve as a nidus for extrapulmonary reactivation later [17].
Fourteen thousand liters of inhaled air passes through the nose, mouth and trachea each day, where mechanical defenses clear particulates and microbes ≥ 5um in diameter. Smaller inhaled items may pass through the bronchioles and settle in the alveoli where they encounter AMs. The alveoli are delicate grape-like clusters which exchange gases with the surrounding capillary meshwork [18]. Due to the fragility of alveoli and the need for gas exchange, clearance of pathogenic matter without excessive and destructive inflammation is extremely important in this locale.
2.1. Alveolar physiology and cell types
A thin lining of epithelial type I and type II cells surrounds the alveolus. Type I cells are thin, flat and cover 93-97% of the alveolar surface, allowing for efficient gas exchange. Type II epithelial cells are cuboidal with apical microvilli and cytoplasmic lamellar bodies. They produce and secrete pulmonary surfactant lipids and proteins as well as other soluble components of the innate immune system [19]. These substances have widespread immune activity, functioning as opsonins and/or microbial aggregating agents, signaling molecules that shape immune cell phenotypes and microbicides that destroy or destabilize microbial cell walls [20-32]. In addition to AMs, and the epithelium and interstitium which contain capillaries and venules, there are several other innate immune cells including intravascular and interstitial macrophages (IMs), dendritic cells (DCs) and scattered neutrophils [33,34].
2.2. Surfactant Proteins and hydrolases
Pulmonary surfactant is a lipid and protein complex which forms a thin film at the air-liquid alveolar interface for the purpose of reducing surface tension and preventing alveolar collapse during expiration. AMs are bathed in surfactant which is primarily composed of phospholipids such as dipalmitoyl phosphatidylcholine (DPPC) with lesser concentrations of other lipids and cholesterol [35]. There are four surfactant associated proteins, Surfactant protein -A (SP-A), SP-B, SP-C and SP-D. Surfactant lipids adsorb to the air-liquid interface with the assistance of SP-B and SP-C [reviewed in [36, 37]. SP-A and SP-D are large, multimeric and relatively hydrophilic collagenous lectins (collectins) with carbohydrate recognition domains (CRDs) that are important in the Ca2+-dependent recognition of microbes [reviewed in [37]. SP-A and SP-D are key regulators in the pulmonary innate immune response through several mechanisms including microbe binding, agglutination, and direct effects on immune cells [38].
SP-A enhances macrophage phagocytosis of apoptotic cells and various pathogens including M.tb [39-43] through direct interaction with macrophages [44] as well as binding to the bacterial cell wall proteins and lipoglycans [45,46]. SP-A is a major regulator of macrophage phenotype and function with effects on PRRs, the oxidative burst, and negative regulators of inflammation [21,47-49]. By interacting with mannosylated lipoarabinomannan (ManLAM) found on virulent M.tb (and some other pathogenic mycobacteria) [22], SP-D agglutinates M.tb and decreases macrophage phagocytosis while enhancing phagolysosomal fusion and killing of the bacilli that are phagocytosed [22,23,50]. Type II cells produce lamellar bodies which are packed with surfactant phospholipids and hydrolytic enzymes and hydrolases in the extracellular lining of the lung [51,52]. Hydrolases can alter the outer cell wall of M.tb with the potential to change the macrophage-microbe interaction and host immune response [20].
3. Macrophage phenotype and functional diversity
Macrophages are phenotypically heterogeneous, having diverse functions in different tissues. Some studies indicate that the initial interaction of macrophages with specific cytokines determines their functional phenotype, while others have shown that macrophages can be continuously altered as the environment changes [53-55]. Macrophage heterogeneity has a direct impact on M.tb interactions in different tissue environments. Macrophages have been categorized into two major groups: the pro-inflammatory, “classically” activated M1 type macrophage and the immunoregulatory, “alternatively” activated M2 type macrophage [56,57]. However, it is increasingly clear that macrophage function represents a spectrum, with biological activities varying greatly among mammalian species and experimental stimuli [7,58-65]. For example, mouse macrophages express unique phenotypic markers for M1/M2, not expressed by human macrophages [57,61].
3.1. Classically activated macrophage (CAM)
Once primed by IFNγ, followed by a second signal such as TNFα or LPS, the CAM mediates more efficient antigen presentation, increased synthesis and release of pro-inflammatory mediators and more efficient phagocytosis [57]. One of the most reliable markers of mouse CAMs is robust nitric oxide (NO) production; however, human macrophages produce limited NO even after activation [66], an observation which holds relevance for TB research, discussed below.
3.2. Alternatively activate macrophages (AAMs)
AAMs are generated by IL-4, and IL-13, which are produced by T helper 2 (Th2) cells, and partially share receptor complexes [67]. AAMs generally mediate Th2 type immune responses [57]. In antithesis to CAMs, AAMs are generally anti-inflammatory, producing high levels of IL-10 and TGF-β and are less efficient antigen presenting cells due to reduced MHC class II expression [56,57,60]. AAMs also demonstrate impaired killing of intracellular pathogens, but play an important role in controlling extracellular parasites [68]. In general, AAMs are believed to promote resolution of inflammation and wound healing. The impact of these types of macrophages in TB pathogenesis is being increasingly realized [11-16,69].
3.3. AMs
AMs reside beneath the surfactant monolayer on the luminal side of the alveolar epithelial cells and are a first line of immune cell defense in the alveolus (Fig. 1). It is estimated that there are 8-12 AMs per alveolus [70-72] , which through irradiation studies, were determined to originate from blood monocyte precursors [73]. Following differentiation in the lung, resident AMs are relatively long lived, with a turnover rate of around 40% of the population per year [74]. However, there is also evidence in mice for local production of macrophages from stem cells [75].
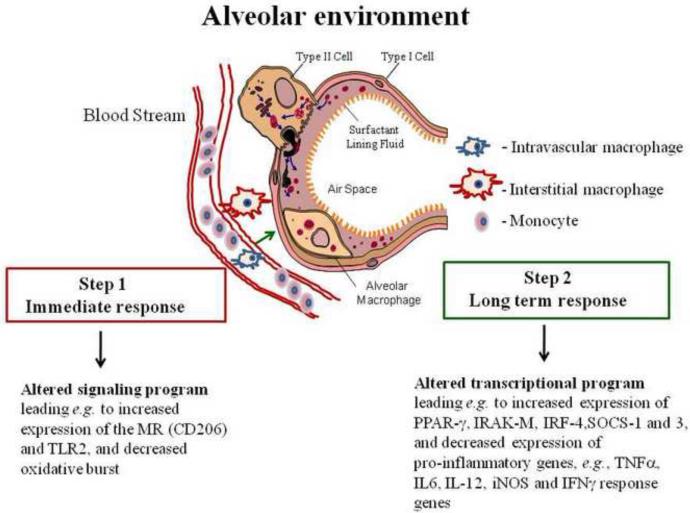
Figure 1. Depiction of the generation of AMs within the alveolar tissue environment.
Blood monocytes traverse the pulmonary alveolar capillary bed and differentiate into intravascular macrophages in situ or transit to the interstitium to become interstitial macrophages (IMs), or to the alveolar space to become AMs (there is also evidence for local production of macrophages in mice). On route, the macrophages are “shaped” by locally produced determinants such as cytokines like GM-CSF, etc. Upon entering the alveolar space, AMs encounter surfactant components which play a role in differentiating these cells to their unique immunoregulatory phenotype, enabling them to acquire their primary function of enhancing clearance of particulates while limiting excessive pro-inflammatory “collateral” damage. Step 1 AM changes are immediate as a result of surfactant components, and other ligands, engaging their cognate receptors to generate a signaling cascade which alters the phenotype and function. Step 2 is long term resulting from an alteration in transcriptional programming. The unique AM phenotype requires constant input from the alveolar environment.
AMs are uniquely adapted to functioning in the alveolar environment, where they act as sentinels against invading organisms but also serve to limit inflammation and minimize lung injury to preserve alveolar function. AM activation is tightly regulated and involves a complex balancing act between activating and repressing signals. On the one hand, PRRs like Toll-like Receptors (TLRs) recognize pathogen associated molecular patterns (PAMPs) and initiate inflammatory responses, while receptors for inflammatory cytokines such as TNFα, IL-1β and IFN-γ perpetuate inflammation [7]. On the other hand, signaling through IL-10 and TGFβ, as well as non-TLR PRRs such as the mannose receptor (MR, CD206) limit the progression of inflammation [7]. Negative regulation of inflammation is also achieved by cell-to-cell interactions, via epithelial cell ligation of AM receptors such as CD200 Receptor (CD200R) [76], triggering receptor expressed by myeloid cells 2 (TREM2) [77,78] and signal-regulatory protein-α (SIRP-α) [79]. AMs control inflammation by suppressing the induction of innate and adaptive immunity through decreased antigen presentation, limited oxidant production, and enhanced release of anti-inflammatory cytokines [7,80]. AMs from healthy human donors make low levels of O2 metabolites when compared to neutrophils or PBMCs following stimulation [80-82] and there is no detectable oxidative burst following M.tb infection of MDMs [23]. The lack of robust oxidative responses in human AMs and MDMs are an important contrast to the substantial evidence for a protective role of ROIs and NO for elimination of M.tb in the murine model of infection [83-88]. AMs are ineffective APCs [80], which delays and limits the initiation of T cell inflammatory responses [7]. In concert, CD28 co-stimulation is defective in AMs [89] and the APC ability of DCs in vitro and in vivo and can be inhibited by AMs [90].
Due to the unique, intermediate phenotype exhibited by AMs (Fig. 2), the conventional categorization of CAM versus AAM does not hold [7]. In accordance with their distinct role in maintaining lung homeostasis, our group has designated AMs as “immunoregulatory macrophages”. Additional efforts in characterizing AMs are required, particularly in regard to human AMs.
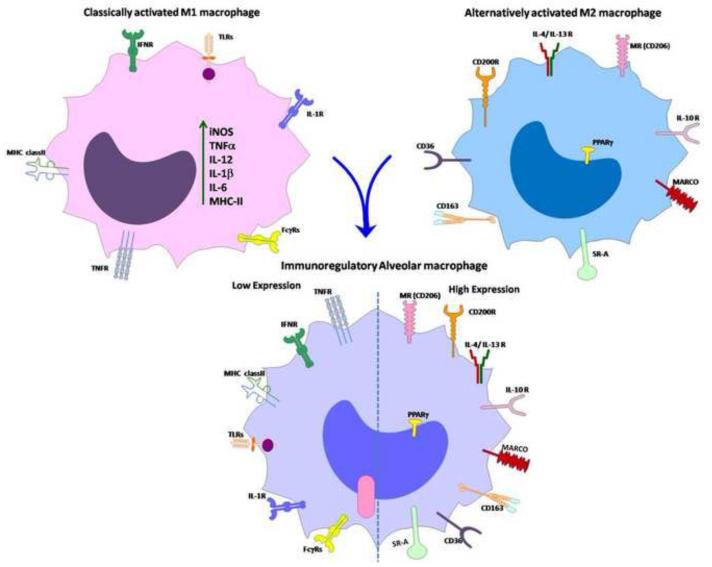
Figure 2. Schematic of macrophage activation by different stimuli that drive macrophage differentiation to different phenotypes.
(a) Classically activated M1 macrophages are differentiated by IFN-γ, LPS and TNFα which lead to increased expression of pro-inflammatory mediators such as cytokines (TNFα, IL-1β, IL-12 and IL-6), iNOS, MHC-II molecule, and TLRs, as well as increased expression of FcγRs. (b) Alternatively activated M2 macrophages are differentiated by IL-4 and IL-13 which lead to an anti-inflammatory signature with increased expression of the MR (CD206), PPARγ, IL-10, CD200R, CD163, CD36, MARCO and SR-A. (c) AMs are unique immunoregulatory cells which express both M1 and M2 markers. Their activation is tightly regulated by molecules such as PPARγ, IRAK-M, IL-10 and SOCS proteins.
3.4. Intravascular and interstitial macrophages (IMs)
Intravascular and interstitial macrophages (IMs) differ in location and function from AMs. IMs are believed to function in regulating tissue fibrosis, inflammation, and antigen presentation [91] whereas intravascular macrophages function in the cross talk between APCs in the lung interstitium for recruiting neutrophils or myeloid cells [92]. The existence of lung macrophage subsets with different functional properties requires additional exploration to better understand their contributions to M.tb pathogenesis, although challenges exist in isolating and modeling them. There is recent evidence that IMs from rhesus macaque lungs exhibit short lived and higher turnover rates compared to AMs. In contrast to AMs, ex-vivo stimulation of IMs with IFNγ and LPS significantly increased TNFα expression providing support for their role in regulating mucosal macrophage functions [93].
4. Macrophage PRRs
AMs express PRRs, which initiate signaling cascades in response to ligand recognition, and/or the engulfment and destruction of fluid and particulate matter via endocytosis. Involvement of specific receptors in the phagocytic process determines the cytoskeletal elements and signals involved in phagosome formation and maturation, resulting inflammatory response and, ultimately, intracellular fate of pathogen [94]. Several macrophage membrane and cytosolic PRRs are involved in recognition and response to M.tb that differ significantly depending on the macrophage source and experimental conditions.
4.1. Toll-like receptors
TLRs are generally considered to be pro-inflammatory, since their ligation leads to phosphorylation and deactivation of the NFκB -inhibitor, IκBα, resulting in NFκB activation and an inflammatory response. However, various TLRs also activate the signaling molecules TRIF and IRF, which result in increased IFN-β gene expression and an anti-inflammatory response [95]. Furthermore, recent evidence has reinforced the idea that TLRs can form complexes with other surface receptors to modulate the nature of the inflammatory response (reviewed in [96,97]). Relative to monocytes, human AMs express lower levels of TLR2, comparable levels of TLR4 and increased levels of TLR9 [98], and contrastingly, murine AMs do not express TLR9 [99] . The mycobacterial 19 kDa lipoprotein (LpqH) interacts with TLR2 to induce TNFα, IL-12 and NO in both murine and human macrophages [100]. Other mycobacterial molecules, e.g., LprA, LprG, PhoS1, LAM, LM and PIMs act as ligands for TLR2 leading to the production of IL-10, IL-4 and TGFβ [101] which can inhibit IFN-γ signaling in macrophages, allowing M.tb to evade host innate and adaptive immune responses. Other mycobacterial antigens, e.g. the 38kDa glycolipoprotein and PIM6 are sensed by TLR4/TLR2 and generate Th1-polarized cytokine responses in M.tb-infected mouse lungs [102,103]. Mycobacterial DNA generates a robust immune response through TLR9/TLR2 in murine macrophages [104]. Much work remains to be done in clarifying the role of TLRs in mycobacterial infection, particularly in regards to TLR cross-talk with non-TLR PRRs and phagocytic receptors in human cells.
4.2. Mannose receptor
The MR (CD206), a member of the C-type lectin family, is highly expressed on AMs [105,106], monocyte derived macrophages (MDMs) and DCs [107], but not on monocytes [108,109]. It interacts with endogenous mannose N-linked glycoproteins via its eight CRDs to maintain homeostasis [110] and also associates with mannose-containing surface PAMPs found on pathogenic microbes [reviewed in [111], representing a form of molecular mimicry for intracellular pathogens like M.tb [112]. It is the dominant C-type lectin on human AMs and monocyte-derived macrophages (MDMs) and, along with complement receptors, mediates the phagocytosis of M.tb [113]. This interaction shows specificity in that the MR can differentiate between M.tb strains [114] and binds to the mannose caps of mannosylated LAM (ManLAM) [115] and higher order PIMs (more mannosylated) found in greater amounts on pathogenic mycobacteria [25]. This receptor-specific interaction affects the fate of M.tb since entry through the MR leads to a decrease in phagosome lysosome (P-L) fusion [24,25] and an increase in the activity of Peroxisome Proliferator-Associated Receptor-γ (PPARγ, see below), leading to survival of intracellular bacteria [12]. The importance of the MR in mycobacterial infection is corroborated by evidence that MR polymorphisms are associated with enhanced susceptibility to M. leprae infection [116-120].
The MR is also a prototypic PRR linking innate and adaptive immunity [65,107,121-127]. These properties have been exploited to deliver DNA vaccines to APCs [126,128-130]. The MR may also contribute to the chronic stages of M.tb infection by mediating homotypic cellular adhesion and giant cell formation [131], which are characteristic in granulomas, and is a marker of AAMs [61,132]. The MR is being used in several ways to modulate the immune system for both therapeutic and vaccine purposes [130].
4.3. Scavenger receptors
Scavenger receptors comprise a large family (divided into 8 classes) that generally contribute to homeostatic functions of macrophages, being best characterized for their role in binding to oxidized low density lipoproteins (oxLDL) in the context of atherosclerosis [reviewed in [133]. However, scavenger receptor ligation leads to several downstream events, with outcomes ranging from the removal of unwanted self-molecules and apoptotic cells to the recognition of PAMPS in conjunction with TLR signaling [reviewed in [134]. Scavenger receptors play a role in M.tb binding to macrophages [135]. Three class A scavenger receptors are present on AMs: Scavenger Receptor A isoforms I and II (SRA-I/II), and macrophage receptor with collagenous structure (MARCO). SR-A binds to most polyanionic molecules [136,137], and MARCO binds to and removes unopsonized environmental particles in the lung [138]. MARCO can tether mycobacterial trehalose-6,6-dimycolate (TDM) to the host cell surface, enabling pro-inflammatory signaling through TLR2 [139]. Knockout mice for SRA-I/II and MARCO are more susceptible to pulmonary pathogens and have a greater inflammatory response to inhaled particulate matter [140-142] indicating a host protective role for these receptors. Expression of the class B scavenger receptor CD36 is induced through PPARγ in human AMs [143] and contributes to the removal of apoptotic cells and oxLDL (reviewed in [144]). CD36 involvement in lipid removal contributes to foam cell generation during TB [145]. CD36 knockout mice fared better during early stages of M.tb infection [146] although the redundancy in scavenger receptor expression patterns complicates the interpretation of these results [147]. Much remains to be elucidated, but scavenger receptors’ role in maintaining lung homeostasis places them in an important position during TB pathogenesis.
4.4. Mincle
Mincle (Clec4e), a C-type lectin family member, is highly expressed on mouse macrophages upon stimulation with LPS, TNFα, IL-6 and IFNγ [148]. Since Mincle does not contain an activating signal motif in the cytoplasmic region, it associates with the immunoreceptor tyrosine-based activation motif containing Fc receptor γ chain (FCRγ) to function as an activating receptor for damaged cells and fungi [149,150]. Mincle serves as a receptor for M.tb TDM to enhance inflammatory cytokine production leading to granuloma formation in mice [151,152].
4.5. Dectin I and II
Dectin I, a non-classical C-type lectin, is highly expressed in DCs, macrophages, monocytes and neutrophils [153]. There are 2 isoforms of Dectin I in mice and eight in humans, although only 2 have a CRD [154,155]. The Dectin I cytoplasmic region contains an ITAM motif for initiation of signaling cascades [156] by its ligands β-1,3 and β-1,6 linked glucans [157]. Dectin I activation by non-pathogenic mycobacteria M. smegmatis and M. phlei, and attenuated BCG and M.tb H37Ra strains enhances TNFα, IL-6 and RANTES in macrophages but fails to induce an inflammatory response to virulent M.tb H37Rv [158]. However, Dectin I and TLR4 mediate an IL-17A response by M.tb [159]. Consistent with earlier findings, we found that Dectin I activation by β-glucans inhibits the intracellular growth of M. bovis BCG but not virulent M.tb in human macrophages [160]. Inducible expression of Dectin I on non-phagocytic airway type II epithelial cells mediates antimicrobial effects against M.tb [161].
Expression of Dectin II, another C-type lectin [162] , is restricted to macrophages and DC subsets [163] and it associates with FcRγ for generating activation signals [164]. Dectin II can function as a receptor for M.tb ManLAM, inducing both a pro-and anti-inflammatory cytokine response and promoting T cell-mediated adaptive immunity in mice [165]
4.6. NOD proteins
NOD (nucleotide binding oligomerization domain-containing protein) like receptors (NLRs) are intracellular PRRs that recognize pathogens or PAMPs [166,167] and are expressed on antigen presenting cells and epithelial cells. Both NOD1 and NOD2 detect bacterial peptidoglycan fragments such as d-glutamyl-meso-diaminopimelic acid (DAP) and muramyl dipeptide (MDP), respectively, to regulate innate immune responses [168]. M.tb produces a glycolated form of MDP (GMDP) that activates NOD2-dependent host immune responses in human and murine macrophages and mice [169,170]. M.tb-mediated immune responses are significantly reduced upon NOD2 knockdown in human macrophages and M.tb growth increased [171]. Consistent with this, NOD2 expressed in AMs recognizes MDP and induces IL-1β, IL-6 and TNF-α. Pretreatment of AMs with MDP significantly increased the intracellular killing of virulent M.tb by increasing expression of LL37 and autophagy proteins IRGM and LC3 [172].
5. Macrophage negative regulators of inflammation
Various regulatory molecules such as IRAK-M, the SOCS proteins and CD200R can suppress macrophage activation and thus are important in protecting the alveolar space from inflammatory damage[49,76,173]. There is some evidence for their role in TB pathogenesis [49,76,174-178].
5.1. IRAK-M
Interleukin-1 receptor associated M (IRAK-M) is an important negative regulator of innate immune responses [179-181]. Its expression is enhanced by TLR ligands indicating a negative feedback loop to dampen inflammation [180]. We have shown that basal expression of IRAK-M is higher in human AMs relative to MDMs and that MDM treatment with SP-A or Survanta (commercially available bovine surfactant) enhances IRAK-M expression, subsequently decreasing LPS-induced pro-inflammatory cytokine responses [49]. These data support the idea that AMs are immunologically shaped by the proteins and lipids in the lung alveolus. LAM-mediated up-regulation of IRAK-M dampens TLR4-mediated IL-12 p40 expression in murine macrophages [182] and M.tb infection delays the Th1 immune response by up-regulating the expression of IRAK-M expression in lung APCs in mice [183].
5.2. SOCS proteins
Expression of the 8 suppressor of cytokine signaling (SOCS) family of proteins is induced in macrophages following cytokine stimulation, which blocks further inflammatory signaling in a classic feedback loop [184,185]. Several microorganisms, including M.tb, have developed sophisticated strategies to hijack SOCS protein pathways in order to block immune defense signaling pathways [174]. M. avium infection enhances SOCS1 and SOCS3, which correlates with a lack of IFNγ-mediated signaling in human macrophages [175]. Similarly, M.tb infection enhances SOCS expression in murine and human macrophages and the subsequently impaired IFNγ secretion results in enhanced bacterial load [176,186]. SOCS1 knockdown in murine macrophages significantly enhances the ability of infected macrophages to clear M.tb through an IFNγ–dependent mechanism [176,178]. Finally, SOCS1 mRNA transcripts are increased in the peripheral blood mononuclear cells (PBMCs) of advanced pulmonary TB patients when compared to moderately infected patients [187,188].
5.3. CD200R
CD200R is an immunoglobulin superfamily member expressed on most leukocytes, particularly cells of the myeloid lineage [7,189-191] and Th2 polarized T cells [189]. Engagement of CD200R by its only known ligand, CD200, inhibits the activation of both myeloid and T cells. CD200R is expressed on murine AMs and plays a critical role in mediating homeostasis in the lung environment [76]. CD200 is highly expressed on type II alveolar epithelial cells and apoptotic leukocytes in the inflamed airway [76,173]. CD200 knockdown results in an increase in AM number and spontaneous up-regulation of activity [76]. CD200 knockout mice succumb to a sub-lethal dose of influenza virus due to uncontrolled inflammation [76]. These data illustrate the critical role of CD200R in the chronic suppression of AM activation, a topic of current interest in our laboratory.
6. Macrophage immunoregulatory cytokines
Cytokines fall in 3 general classes: innate, adaptive, and stimulators of hematopoiesis. Studies of the host cytokine responses TB infection are abundant and vary markedly depending on the model and mammalian species used. It has become clear that M.tb generates cytokine responses in all 3 categories and that the “optimal” protective response to infection will represent a balance of the various cytokines produced. In addition, most of these cytokines have pleiotropic effects depending on the context of their activation and local tissue environment. Thus, it is difficult to correlate host protection with any particular cytokine. Here we will focus on two important immunoregulatory macrophage cytokines for M.tb: IL-10 and type I IFNs.
6.1. Interleukin 10
IL-10 is produced by many cell types and was initially shown to inhibit synthesis of IFNγ in Th1 cells [192-194]. Human IL-10 shares 80% homology with murine IL-10 [195]. Binding of IL-10 to its receptors, IL-10 R1 and IL10R2, results in activation of Jak1 and Tyk2, which subsequently activate STAT-1, 3 and 5 [195]. IL-10 can suppress Ag presentation as well as production of pro-inflammatory cytokines, chemokines, adhesion molecules and co stimulatory molecules in macrophages and other cell types [196]. IL-10 also inhibits nuclear translocation of NFκB and blocks DNA binding activity of translocated NFκB [197]. IFNγ-inducible genes are suppressed by IL-10 through inhibition of STAT1- and 3-mediated induction of SOCS3 [196,198-200]. Pulmonary TB patients have increased levels of IL-10 and TGFβ in their bronchoalveolar lavage (BAL) fluid [201,202] and serum [203]. Both IL-10 and TGFβ can inhibit CD4 T cell proliferation and IFNγ production in PBMCs from healthy PPD+ patients, possibly through the inhibition of APC function [204,204,205]. Additionally, neutralization of IL-10 produced by PBMCs from active TB patients results in increased T cell proliferation and enhanced IFNγ production [204,206]; the same trend was observed in patients with anergic TB [207]. Increased sputum IL-10 levels of active TB patients correlate with increased levels of M.tb antigen CFP32 [208]. Also IL-10 production following M.tb infection of human MDMs or AMs blocks phagosome maturation [209]. Finally, IL-10 polymorphisms are associated with susceptibility to TB [210-212]. Collectively, these human studies provide strong evidence that IL-10 acts a limiting factor for optimal host immune responses to M.tb.
Results on IL-10 in M.tb pathogenesis in mice are variable, partly dependent on the mouse strain used. Early studies in the IL 10 -/- mouse (C57Bl/6 background) showed that despite increased IFNγ levels during every stage of infection, IL-10 deficiency did not result in a significant difference in lung bacterial load compared to wild type mice following aerosol infection [213-215]. In contrast, others have reported that IL-10 deficient mice (C57BL/6, CBA/J and BALB/c) show increased resistance to aerosol M.tb infection, with reduced lung bacterial load and increased IFNγ [216-218]. IL-10 over-expression in mice did not increase the susceptibility of mice during early stages of M.tb infection, but there was increased reactivation and higher lung bacterial burdens during the chronic phase of infection [219]. Another study demonstrated that M.tb infection of IL10 -/- mice treated with anti-IL-10R monoclonal antibody have reduced lung and spleen bacterial growth when compared to control mice [217]. Macrophages infected with M.tb or BCG have reduced surface expression of IFNγ-mediated MHC class II molecules due to IL-10-dependent inhibition [220]. Finally, blockade of IL-10 signaling during BCG vaccination increases the efficacy of the vaccine against M.tb [221]. On balance, mouse studies are generally congruent with human studies with regard to the immunoregulatory effects of IL-10.
6.2. Type I interferons
Type 1 Interferons (IFNs) are produced in response to many pathogens and can subvert anti-TB host defenses by inhibiting production of iNOS, IL-12 p40, IL1-α and IL-1β, while inducing mediators of immune suppression such as IL-10 and IL1R antagonist [222,223]. Although type I and type II IFNs share similar STAT1-dependent signaling pathways, type I IFNs appear to promote bacterial growth. M.tb and activation of TLR2 significantly reduce the expression of TLR9-mediated type I IFNα/β and DC Ag presentation [224]. Type I IFNs are increased during M.tb infection and M.tb-infected type I IFN receptor deficient mice display lower bacterial burden when compared with wild type mice [225,226]. Intranasal delivery of a type I IFN inducer to M.tb-infected mice results in exacerbated lung pathology and increased bacterial load in the lungs [227]. The M.tb ESX-1-mediated secretion system can mediate the Type I IFN response during infection [228,229]. The relevance of these findings to human TB is supported by evidence that type I and II IFN-induced genes dominate the whole blood transcriptional profile of TB patients and this gene signature correlates highly with disease severity [230]. In human leprosy the expression of host protective IFN-γ and host susceptible IFN-β is inversely correlated [231]. The IFN-γ induced vitamin D-dependent macrophage antimicrobial peptide response was significantly inhibited by IFN-β and downstream IL-10 during M. leprae infection [231]. Recent studies demonstrate that IL-1 confers host resistance through the induction of eicosanoids that limit excessive type I IFN production and foster bacterial containment [232]. Further, the study showed that reduced IL-1 response and excessive type I IFN induction in infected mice and humans are linked to an eicosanoid imbalance associated with disease exacerbation [232].
7. Macrophage immunoregulatory transcription factors
7.1. Peroxisome Proliferator-Associated Receptor-γ
The nuclear receptor-associated transcription factor PPARγ acts as a negative regulator of macrophage activation by trans-repression of the transcription factors NF-κB, AP-1 and STAT [233-235] and attenuating the respiratory burst [234,236,237]. PPARγ expression is high in AMs and its deletion in AMs increases Th1-associated gene expression such as iNOS, IFN-γ, IL-12 p40, MIP1α and IP-10 [238]. These attributes have important implications for controlling M.tb infection. Mycobacterial infection causes increased PPARγ expression in human and murine macrophages through PRRs such as the MR and TLRs [12,239], while PPARγ knockdown in infected human macrophages increases anti-mycobactericidal activity and TNFα production [12,145]. M.tb-mediated induction of PPARγ also regulates host cell metabolism, leading to increased lipid body formation and down-regulation of the host immune response [239].
7.2. Liver X receptor
The liver X receptors (LXRs) are a second class of nuclear receptors activated by oxidized lipids. LXRs are mainly considered cholesterol sensors that regulate the expression of genes in lipid metabolism in response to specific oxysterol ligands [240,241]. In macrophages, these ligands may be derived from internalized oxLDL or generated intracellularly through cholesterol modification. Macrophage lipid import and export mechanisms are tightly regulated, since unbalanced lipid homeostasis affects the inflammatory status of the organism. Recently, LXRα and LXRβ have emerged as master regulators of macrophage transcriptional programs involved in cholesterol, fatty acid and glucose homeostasis [242-244] [241,245], and also negatively regulate macrophage inflammatory gene expression such as iNOS, COX2 and IL-6 [241]. Persisting M.tb in lung granulomas use lipids as the sole carbon source for growth [246] and mycobacterial persistence is critically linked to its ability to acquire and catabolize cholesterol from the host [247]. In addition, cholesterol is essential for M.tb phagocytosis by macrophages and for inhibition of phagosome maturation [248]. Intratracheal infection of mice with M.tb enhances LXR and LXR-dependent gene expression [249]. Further, mice deficient in both LXR isoforms were susceptible to M.tb infection, developing higher bacterial burdens and increased granulomatous lesions [249]. LXR knockout mice exhibit dysregulation of both pro- and anti-inflammatory functions [249]. An LXR gene polymorphism is associated with M.tb susceptibility [250] further emphasizing the importance of LXRs in the protective immune response against M.tb.
7.3. TR4
Testicular orphan receptor 4 (TR4) (aka TAK1 and Nrc2c2) does not have an identified ligand but is expressed in numerous tissues, including in macrophages [251,252].Upon activation, TR4 can repress genes targeted by PPARγ, LXR, vitamin D3 receptor and thyroid hormone receptor [252]. TR4 deficiency decreases CD36 expression and reduces foam cell formation in mice [253]. TR4-mediated CD36 transactivation is enhanced by polyunsaturated fatty acids and their metabolites, as well as the synthetic PPAR agonists [253]. TR4 knockdown in human macrophages enhances their ability to control M.tb growth by lowering CD36 expression and decreasing foam cell formation [145]. The same group reported that M.tb oxygenated keto-mycolic acids function as ligands for TR4 and increase its transcriptional activity, resulting in increased abundance of foam cells and granuloma formation [254]. Thus, PPARγ and TR4 appear to synergistically contribute to M.tb-induced lipid biogenesis through increased CD36 expression and modulate foam cell formation by driving macrophages towards an immunosuppressive phenotype.
8. Macrophage lipid metabolism
Host-derived lipid mediators play an important role during M.tb infection and glycolipids from the mycobacterial cell wall can also function as potent antigens and immunomodulators [255,256]. Mycobacterial metabolism of host lipids serves as a source of nutrients [247] and protection from oxidative damage during infection [257], while dysregulation of host lipid metabolism contributes to foamy macrophage generation and granuloma caseation, leading to bacterial persistence [246]. This section will focus on macrophage prostaglandins, lipoxins and leukotrienes during M.tb infection. These polyunsaturated eicosanoids (20-carbon fatty acids) are hydroxylated during enzymatic modification of essential fatty acids and contribute to both homeostasis of the immune system and disease processes (reviewed in [258]).
8.1. Prostaglandins
Prostaglandins (PGs) are derived from the membrane fatty acid arachidonate and are produced by virtually all cells [259,260]. PGs can induce chemokines to enhance trafficking of myeloid cells to inflamed tissues [261] and contribute to the amplification of pro-inflammatory cytokine production [262]. The type of PG produced is tissue specific; however, the bulk of these molecules are deactivated in the lungs after entering the circulatory system [258]. PGJ2, in opposition to the majority of PGs, has predominantly anti-inflammatory effects and acts as an endogenous ligand for PPARγ [263]. As mentioned earlier, PPARγ activity can increase intracellular survival of virulent M.tb in macrophages with a decrease in TNFα production [12]. Through PPARγ activation, PGJ2 production may thus be detrimental to the host during M.tb infection.
PGE2 expression in mice gradually increases during M.tb infection [264]. Low levels of PGE2 during early infection were necessary to control the infection, due to PGE2-dependent iNOS production [264] and stabilization of the T cell response [265]. However, during later chronic infection, lipid-laden infected macrophages express high PGE2 levels, which can inhibit the inflammatory response and contribute to progressive pneumonia [264]. Thus, the concentration and kinetics of PG expression are important in considering their role during TB pathogenesis.
8.2. Lipoxins
Lipoxins (LXs) are produced by leukocytes [266], pulmonary endothelial cells and macrophages during M.tb infection [267]. LXs are known as pro-resolving lipid mediators, a class which also includes resolvins and protectins [266], contributing to the cessation of the inflammatory response [268]. They have autocrine and paracrine effects [268]. To mediate resolution of inflammation, LXs function to inhibit neutrophil migration and degranulation [269], and prevent pre-mature macrophage apoptosis. The 5- and 15-lipoxygenases (LOs) use arachidonic acid as a substrate for the production of 15-epilipoxin-A4 and leukotriene B4 (see below). 15-epi-LXA4 can inhibit the production of IL-6, TNF-α and IL-8 [270,271]. Mice lacking 5LO, the enzyme responsible for LXA4, are more resistant to M.tb infection than wild type controls, attributed to increased production of IL-12, IFNγ and iNOS. Knockout mice given an LXA4 analog reverted to the wild type infection response [267]. Infection of human and murine monocytes with M.tb increases LXA4 production, which blocks PGE2-dependent repair of the cell membrane, causing necrosis and dissemination of bacteria [272]. As with PGJ2 and PPARγ, the anti-inflammatory functions of LXA4 appear to work against the host in this disease.
8.3. Leukotrienes
Lipoxygenase enzymes are responsible for the production of leukotrienes (LTs) through the conversion of arachidonate to hydroxyeicosatetraenoic acid (HETE). 5-HETE is further converted to the unstable LTA4, the precursor to all other LTs [273]. The main sources of LTs are macrophages, neutrophils, DCs and mast cells. LTs are pro-inflammatory molecules that induce smooth muscle contraction and vasoconstriction, contributing to allergies [274,275], asthma [276] and autoimmune diseases [277].
LTs are host protective in the mouse. M.tb-infected BALB/c mice given the drug celecoxib (LTB4 stimulator/PGE2 inhibitor) exhibited a slight increase in survival. Conversely, the LTB4 synthesis inhibitor MK886 reduced 60 day survival rates by half [278]. LTB4 is also important for human neutrophil killing during BCG infection [279]. However, BALB/c mice treated with MK866 are still protected from TB when pre-treated with a prime-boost heterologous vaccine [280], indicating that the effects of LTs can be overridden. LTB4 is induced in both human TB and the zebrafish model of M.tb infection [281,282], and its levels regulate host protection [283].
9. Macrophage microRNAs
9.1. miRNA function and biogenesis
MicroRNAs (miRNAs) are endogenous, non-coding, small RNAs that function as gene regulators by primarily mediating translational repression or degradation of target mRNAs [284]. They are implicated in a variety of biological processes [285] and are transcribed from intergenic or intragenic regions of the genome in pri-miRNA form, which may contain one or multiple miRNAs. [286]. Although the role of miRNAs in regulation of the immune system and response to infection is becoming clearer, we do not yet have a clear picture of the global impact of miRNAs on the immune response to M.tb. Recent evidence indicates that there is one and that macrophage immune pathways play a role.
9.2. miRNAs in M.tb infection
Several studies have compared miRNA profiles in TB patients (active or latent) verses healthy controls, with little agreement between the reports. These profiles were obtained from serum and sputum, which mostly rely on detection of extracellular sources of miRNAs; thus, miRNA profiles obtained in this fashion may not be truly indicative of M.tb-induced processes [287-291].
There are recent studies of miRNAs in M.tb infection with validated targets. However, some were carried out exclusively in murine models or murine cell lines and although miRNAs are conserved between species, regulation of their expression may differ, and so miRNA expression patterns and their targets must be validated in human model systems [292-294]. It was reported early that there is increased expression of miR-144* in the PBMCs of pulmonary TB patients. This increase was later localized to the T cell population and observed to inhibit IFN-γ and TNF-α secretion[295]. Soon after, we reported increased miR-125b expression with virulent M.tb infection (but not with avirulent M. smegmatis) which resulted in destabilization of TNF-α mRNA and decreased TNF-α secretion in human primary macrophages [255]. These studies indicate that modulation of cytokine production by miRNAs may be an effective mechanism for M.tb to escape immune control.
M.tb may also inhibit apoptosis through miRNAs. miR-29a targets the anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2), myeloid cell leukemia -1 (Mcl-1) p85a and GTP-binding protein CDC42 [296] and M.tb infection can suppress miR29a [297], thereby potentially inhibiting apoptosis. Additionally, miR-29a targets the 3’UTR region of IFN-γ mRNA, suppressing IFN-γ production. miRNA-29a expression was down-regulated after BCG infection and miR-29a knockdown induced IFN-γ expression in NK cells and T cells, and promoted murine survival after M.tb infection [297].
Mycobacteria can modulate TLR signaling pathways through up-regulation of various miRNAs. In murine AMs and cell lines, high levels of miR-124 were detected in response to BCG infection. miR-124 inhibits TLR signaling by down-regulating the expression of MyD88, TRAF6 and TLR6 [298]. Patients with active TB have increased levels of miR-147 [299], which acts as a negative regulator of TLR/NFκB-mediated pro-inflammatory cytokines such as TNF-α and IL-6 [300]. However, TNFα and IL-6 levels in sputum did not differ significantly between active TB and control groups. The anti-inflammatory miRNA, miR-99b, was highly up-regulated in M.tb-infected DCs and macrophages and blockade of miR-99b expression significantly reduced bacterial growth in DCs and increased TNFα, IL-6, IL-12 and IL-1β [294]. Autophagy pathways are also influenced by miRNAs. miR-155 expression was enhanced in murine macrophages upon M.tb infection, leading to increased autophagy-mediated killing [301]. miR-155 targets the 3’-UTR region of Ras homologue enriched in brain (Rheb), a negative regulator of autophagy. Finally, a host-protective increase in miR-223 expression was observed during miRNA expression profiling of TB patient blood and lung tissue, followed by functional studies in a miR-223 knockout mouse [302]. These studies indicated that miR-223 directly targets CXCL2 and CCL3, and IL-6 to regulate neutrophil chemotaxis and inflammatory function.
miRNAs potentially serve as a biomarker for TB disease diagnosis and prognosis. However, importantly, individual miRNAs or miRNA families can regulate hundreds of genes and, conversely, gene targets can be regulated by different miRNAs. Thus, a clear picture of miRNAs and other non-coding RNAs in TB pathogenesis will require further study.
10. Differences in macrophage biology between man and mouse
Although murine models have been used extensively to study TB pathogenesis, or identify and test drug and vaccine candidates for subsequent human trials, there is more evidence and attention to the substantial differences between the two species in the nature of their inflammatory pathways, in ways that impact our understanding of TB pathogenesis [303]. For example, in terms of fundamental aspects of host defense, macrophages from humans and mice differ in expression/activity of PRRs, including TLRs and C-type lectins [304-306], signaling pathways [307,308], autophagy pathways [305], and host defense molecules such as antimicrobial peptides [309] and NO [310,311] to name a few. Mouse KO studies targeting host defense pathways have often yielded negative findings leading to interpretations contrary to in vitro findings despite these known differences between species as well as the known redundancy in the innate immune system, a cardinal feature of this evolutionarily conserved arm of immunity. Thus, future studies must take these differences into account and continue to foster new animal models that better reflect human in vitro and in vivo systems to advance the field.
11. Conclusions
Recent focus on the plasticity and functional diversity of macrophages has raised new and important questions for the TB community in terms of assessing: 1) organ-specific effects, particularly in regards to the lung; 2) macrophage activation states; 3) differences in macrophage function and protective responses during the continuum of TB (primary infection, latency and re-activation); 4) in vitro cultivation conditions [e.g. recent studies on TB and diabetes highlight the importance of using autologous serum with macrophages to maintain undefined soluble immune factors [312], and 5) similarities and differences among mammalian macrophage sources. As we gain more knowledge about macrophage responses in the context of organ-specific microenvironments, our understanding of the molecular details underlying the pivotal M.tb-macrophage interactions occurring during TB will become more defined.
Highlights.
We review current concepts in macrophage responses to M. tuberculosis
We emphasize macrophage heterogeneity and the unique immunoregulatory alveolar macrophage
We describe major macrophage immunoregulatory pathways that influence the host inflammatory response to M. tuberculosis
We compare similarities and differences in macrophage responses among different sources
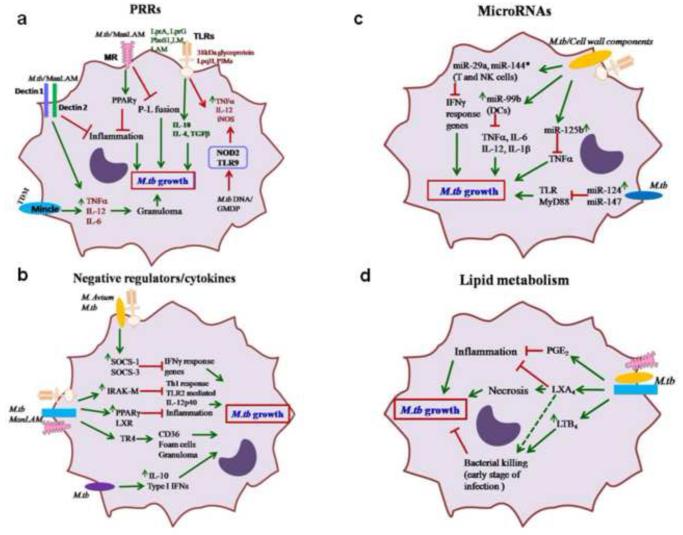
Figure 3. Schematic of macrophage regulatory factors that alter M.tb growth.
(a) Activation of PRRs by M.tb and/or M.tb cell wall components, and downstream responses. TLR activation by M.tb cell wall components 38 kDa glycoprotein, LpqH, or PIMs promote inflammation; in contrast, LprA, LprG, PhoS1, LM and LAM increase the expression of anti-inflammatory mediators such as IL-10, IL-4 and TGF-β which enhance M.tb growth. MR-mediated phagocytosis of M.tb or MR stimulation by ManLAM delays P-L fusion and enhances PPARγ expression, leading to increased M.tb growth. TDM binding to Mincle or ManLAM to Dectin II enhance TNF1, IL-12 and IL-6 leading to granuloma formation and M.tb control. (b) Negative regulators of macrophage responses to M.tb and/or cell wall components. M.tb or ManLAM stimulation enhances the expression of various negative regulators, e.g. IRAK-M, SOCS-1 and SOCS-3, PPARγ, and TR4 as well as IL-10 and type I-IFNs which suppress host protective inflammatory mediators and promote M.tb growth. (c) Regulation of macrophage microRNAs and their functions in response to M.tb and/or cell wall components. miRNAs are important immune regulators during M.tb infection. Increased miR-125b targets TNFα for degradation, and miR-124 and miR-147 target TLR pathway genes such as MyD88 and TRAF6, are highly induced by M.tb infection and promote M.tb growth. (d) Impact of macrophage lipid mediators in response to M.tb and/or cell wall components. Lipid metabolites such as PGE2, LXA4 and LTB4 also regulate the macrophage immune response during M.tb infection. PGE2 and LXA4 are anti-inflammatory and promote M.tb growth. In contrast, LTB4 enhances bacterial killing during the early stage of infection and later promotes M.tb growth.
Abbreviations
- IM
Interstitial Macrophage
- DPPC
Dipalmitoylphosphatidylcholine
- CRD
Carbohydrate recognition domain
- CAM
Classically activated macrophage
- AAM
Alternatively activated macrophage
- SP
surfactant protein
- ManLAM
mannosylated lipoarabinomannan
- PIM
phosphatidyl inositol mannoside
- LM
lipomannan
- P-L fusion
phagosome-lysosome fusion
- AM
alveolar macrophage
- DPPC
dipalmitoyl phosphatidylcholine
- BAL
bronchoalveolar lavage
- M.tb
Mycobacterium tuberculosis
- PG
prostaglandin
- LX
lipoxin
- LT
leukotriene
- miRNA
microRNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol. 2013;35:563–583. doi: 10.1007/s00281-013-0388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van CR. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014;141:506–513. doi: 10.1111/imm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington WR, Hawn TR. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev. 2007;219:167–186. doi: 10.1111/j.1600-065X.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt K, Salgame P. Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol. 2007;27:347–362. doi: 10.1007/s10875-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 6.Parasa VR, Rahman MJ, Ngyuen Hoang AT, Svensson M, Brighenti S, Lerm M. Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue. Dis Model Mech. 2014;7:281–288. doi: 10.1242/dmm.013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day J, Friedman A, Schlesinger LS. Modeling the immune rheostat of macrophages in the lung in response to infection. Proc Natl Acad Sci U S A. 2009;106:11246–11251. doi: 10.1073/pnas.0904846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verreck F, de Boer T, Langenberg DM, Hoeve M, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc.Natl.Acad.Sci USA. 2004 Mar 30;101(13):4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leemans JC, Juffermans NP, Florquin S, Van Rooijen N, Vervoordeldonk MJ, Verbon A, et al. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol. 2001;166:4604–4611. doi: 10.4049/jimmunol.166.7.4604. [DOI] [PubMed] [Google Scholar]
- 15.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur.J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson S, da Gloria M, Bonecini-Almeida MDG, Silva JRLE, Nathan C, Xie QW, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001;345:189–200. doi: 10.1056/NEJM200107193450307. [DOI] [PubMed] [Google Scholar]
- 18.Creuwels LA, van Golde LM, haagsman HP. The pulmonary surfactant system: biochemical and clinical aspects. Lung. 1997;175:1–39. doi: 10.1007/PL00007554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 20.Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol. 2011;187:372–381. doi: 10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson TK, Brooks M, Meyer D, Henning L, Murugesan V, et al. Pulmonary innnate immunity: Soluble and cellular host defenses of the lung. In: Marsh C, Tridandapani S, Piper M, editors. Regulation of Innate Immune Function. Transworld Research Network; Kerala: 2010. pp. 165–211. [Google Scholar]
- 22.1999;163:312–321. [Google Scholar]
- 23.Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, et al. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–7009. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 26.Weikert LF, Lopez JP, Abdolrasulnia R, Chroneos ZC, Shephert VL. Surfactant protein A enhances mycobacterial killing by rat macrophages through a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L216–L223. doi: 10.1152/ajplung.2000.279.2.L216. [DOI] [PubMed] [Google Scholar]
- 27.Borron P, McCormack FX, Elhalwagi BM. Surfactant protein A inhibits T cell proliferation via its collagen-like tail and a 210-kDa receptor. Am J Physiol. 1998;275:L679–L686. doi: 10.1152/ajplung.1998.275.4.L679. [DOI] [PubMed] [Google Scholar]
- 28.Wright JR, Borron P, Brinker KG, Folz RJ. Surfactant protein A. Regulation of innate and adaptive immune responses in lung inflammation. Am J Respir Cell Mol Biol. 2001;24:513–517. doi: 10.1165/ajrcmb.24.5.f208. [DOI] [PubMed] [Google Scholar]
- 29.Weikert LF, Edwards K, Chroneos ZC, Hager C, Hoffman L, Shepherd VL. SP-A enhances uptake of bacillus Calmette-Guerin by macrophages through a specific SP-A receptor. Am J Physiol Lung Cell Mol Physiol. 1997;272:L989–L995. doi: 10.1152/ajplung.1997.272.5.L989. [DOI] [PubMed] [Google Scholar]
- 30.Hansen S, Lo B, Evans K, Neophytou P, Holmskov U, Wright JR. Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens. Am J Respir Cell Mol Biol. 2007;36:94–102. doi: 10.1165/rcmb.2006-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senft AP, Korfhagen TR, Whitsett JA, Shapiro SD, LeVine AM. Surfactant protein-D regulates soluble CD14 through matrix metalloproteinase-12. J Immunol. 2005;174:4953–4959. doi: 10.4049/jimmunol.174.8.4953. [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guilliams M, Lambrecht BN, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013;6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 34.Hasenberg M, Stegemann-Koniszewski S, Gunzer M. Cellular immune reactions in the lung. Immunol Rev. 2013;251:189–214. doi: 10.1111/imr.12020. [DOI] [PubMed] [Google Scholar]
- 35.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 36.Mason RJ, Voelker DR. Regulatory mechanisms of surfactant secretion. Biochim Biophys Acta. 1998;1408:226–240. doi: 10.1016/s0925-4439(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 37.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 38.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 39.Van Iwaarden JF, Van Strijp JAG, Visser H, haagsman HP, Verhoef J, van Golde LMG. Binding of surfactant protein A (SP-A) to herpes simplex virus type 1-infected cells is mediated by the carbohydrate moiety of SP-A. J Biol Chem. 1992;267:25039–25043. [PubMed] [Google Scholar]
- 40.Hickling TP, Malhotra R, Bright H, McDowell W, Blair ED, Sim RB. Lung surfactant protein A provides a route of entry for respiratory syncytial virus into host cells. Viral Immunol. 2000;13:125–135. doi: 10.1089/vim.2000.13.125. [DOI] [PubMed] [Google Scholar]
- 41.van Iwaarden F, Welmers B, Verhoef J, haagsman HP, van Golde LMG. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 42.haagsman HP. Interactions of surfactant protein A with pathogens. Biochim Biophys Acta. 1998;1408:264–277. doi: 10.1016/s0925-4439(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 43.Downing JF, Pasula R, Wright JR, Twigg HL, III, Martin WJ., II Surfactant protein A promotes attachment of Mycobacterium tuberculosis to alveolar macrophages during infection with human immunodeficiency virus. Proc Natl Acad Sci USA. 1995;92:4848–4852. doi: 10.1073/pnas.92.11.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- 45.Pasula R, Downing JF, Wright JR, Kachel DL, Davis TE, Jr., Martin WJ., II Surfactant protein A (SP-A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17:209–217. doi: 10.1165/ajrcmb.17.2.2469. [DOI] [PubMed] [Google Scholar]
- 46.Sidobre S, Nigou J, Puzo G, Riviere M. Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria. J Biol Chem. 2000;275:2415–2422. doi: 10.1074/jbc.275.4.2415. [DOI] [PubMed] [Google Scholar]
- 47.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 48.Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, et al. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen HA, Rajaram MV, Meyer DA, Schlesinger LS. Pulmonary surfactant protein A and surfactant lipids upregulate IRAK-M, a negative regulator of TLR-mediated inflammation in human macrophages. Am J Physiol Lung Cell Mol Physiol. 2012;303:L608–L616. doi: 10.1152/ajplung.00067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson JS, Voelker DR, Ufnar JA, Schlesinger LS. Surfactant protein D inhibition of human macrophage uptake of Mycobacterium tuberculosis is indepenndent of bacterial agglutination. J Immunol. 2002;168:1309–1314. doi: 10.4049/jimmunol.168.3.1309. [DOI] [PubMed] [Google Scholar]
- 51.Hook GE. Extracellular hydrolases of the lung. Biochemistry. 1978;17:520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- 52.Hook GE, Gilmore LB. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982;257:9211–9220. [PubMed] [Google Scholar]
- 53.Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–1988. [PubMed] [Google Scholar]
- 54.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, reuddre-Bosquet N, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 57.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 58.Ezekowitz RAB, Austyn J, Stahl PD, Gordon S. Surface properties of bacillus Calmette-Guerin-activated mouse macrophages: reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981;154:60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 61.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon S, Fraser I, Nath D, Hughes D, Clarke S. Macrophages in tissues and in vitro. Curr Opin Immunol. 1992;4:25–32. doi: 10.1016/0952-7915(92)90119-y. [DOI] [PubMed] [Google Scholar]
- 64.Linehan SA, Martinez-Pomares L, Gordon S. Macrophage lectins in host defence. Microbes Infect. 2000;2:279–288. doi: 10.1016/s1286-4579(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Pomares L, Mahoney JA, Kaposzta R, Linehan SA, Stahl PD, Gordon S. A functional soluble form of the murine mannose receptor is produced by macrophages in vitro and is present in mouse serum. J Biol Chem. 1998;273:23376–23380. doi: 10.1074/jbc.273.36.23376. [DOI] [PubMed] [Google Scholar]
- 66.Fang FC, Vazquez-Torres A. Nitric oxide production by human macrophages: there's NO doubt about it. Am J Physiol Lung Cell Mol Physiol. 2002;282:L941–L943. doi: 10.1152/ajplung.00017.2002. [DOI] [PubMed] [Google Scholar]
- 67.Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geiser M. Update on macrophage clearance of inhaled micro- and nanoparticles. J Aerosol Med Pulm Drug Deliv. 2010;23:207–217. doi: 10.1089/jamp.2009.0797. [DOI] [PubMed] [Google Scholar]
- 71.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 72.Stone KC, Mercer RR, Freeman BA, Chang LY, Crapo JD. Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Am Rev Respir Dis. 1992;146:454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- 73.Blusse van out Alblas A, Van Furth R. Origin, kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979;149:1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol. 2013;174:193–202. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 76.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 77.Gao X, Dong Y, Liu Z, Niu B. Silencing of triggering receptor expressed on myeloid cells-2 enhances the inflammatory responses of alveolar macrophages to lipopolysaccharide. Mol Med Rep. 2013;7:921–926. doi: 10.3892/mmr.2013.1268. [DOI] [PubMed] [Google Scholar]
- 78.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 79.Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, et al. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med. 2008;178:158–167. doi: 10.1164/rccm.200711-1661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fels A, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 81.Arias M, Zabaleta J, Rodriguez JI, Rojas M, Paris SC, Garcia LF. Failure to induce nitric oxide production by human monocyte-derived macrophages. Manipulation of biochemical pathways. Allergol Immunopathol (Madr ) 1997;25:280–288. [PubMed] [Google Scholar]
- 82.Greening AP, Lowrie DB. Extracellular release of hydrogen peroxide by human alveolar macrophages: the relationship to cigarette smoking and lower respiratory tract infections. Clin Sci (Lond) 1983;65:661–664. doi: 10.1042/cs0650661. [DOI] [PubMed] [Google Scholar]
- 83.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MacMicking JD. Recognizing macrophage activation and host defense. Cell Host Microbe. 2009;5:405–407. doi: 10.1016/j.chom.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–2404. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reece ST, Loddenkemper C, Askew DJ, Zedler U, Schommer-Leitner S, Stein M, et al. Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J Clin Invest. 2010;120:3365–3376. doi: 10.1172/JCI42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011;6:e19105. doi: 10.1371/journal.pone.0019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chelen CJ, Fang Y, Freeman GJ, Secrist H, Marshall JD, Hwang PT, et al. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Invest. 1995;95:1415–1421. doi: 10.1172/JCI117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johansson A, Lundborg M, Sköld CM, Lundahl J, Tornling G, Eklund A, et al. Functional, morphological, and phenotypical differences between rat alveolar and interstitial macrophages. Am J Respir Cell Mol Biol. 1997;16:582–588. doi: 10.1165/ajrcmb.16.5.9160840. [DOI] [PubMed] [Google Scholar]
- 92.Schneberger D, Aharonson-Raz K, Singh B. Pulmonary intravascular macrophages and lung health: what are we missing? Am J Physiol Lung Cell Mol Physiol. 2012;302:L498–L503. doi: 10.1152/ajplung.00322.2011. [DOI] [PubMed] [Google Scholar]
- 93.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol. 2014;192:2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861–868. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Cambi A, Koopman M, Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 97.Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev. 2003;16:637–646. doi: 10.1128/CMR.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Juarez E, Nunez C, Sada E, Ellner JJ, Schwander SK, Torres M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir Res. 2010;11:2. doi: 10.1186/1465-9921-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzuki K, Suda T, Naito T, Ide K, Chida K, Nakamura H. Impaired toll-like receptor 9 expression in alveolar macrophages with no sensitivity to CpG DNA. Am J Respir Crit Care Med. 2005;171:707–713. doi: 10.1164/rccm.200408-1078OC. [DOI] [PubMed] [Google Scholar]
- 100.Brightbill HD, Libraty DH, Krutzik SR, Yang R-B, Belisle JT, Bleharski JR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 101.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, et al. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, et al. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169:3155–3162. doi: 10.4049/jimmunol.169.6.3155. [DOI] [PubMed] [Google Scholar]
- 103.Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74:2686–2696. doi: 10.1128/IAI.74.5.2686-2696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci USA. 1986;83:2501–2505. doi: 10.1073/pnas.83.8.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 108.Stahl PD. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990;2:317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- 109.Speert DP, Silverstein SC. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: Maturation and inhibition by mannan. J Leukocyte Biol. 1985;38:655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- 110.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 111.Medzhihtov R, Janeway C., Jr. Innate Immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 112.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb ) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fenton MJ, Riley LW, Schlesinger LS. Receptor-Mediated Recognition of Mycobacterium tuberculosis by Host Cells. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr, editors. Tuberculosis and the Tubercle Bacillus. ASM Press; New York: 2005. pp. 405–426. [Google Scholar]
- 114.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marciando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- 115.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 116.Siddiqui MR, Meisner S, Tosh K, Balakrishnan K, Ghei S, Fisher SE, et al. A major susceptibility locus for leprosy in India maps to chromosome 10p13. Nature Genetics. 2001;27:439–441. doi: 10.1038/86958. [DOI] [PubMed] [Google Scholar]
- 117.Mira MT, Alcais A, Van TN, Thai VH, Huong NT, Ba NN, et al. Chromosome 6q25 is linked to susceptibility to leprosy in a Vietnamese population. Nat Genet. 2003;33:412–415. doi: 10.1038/ng1096. [DOI] [PubMed] [Google Scholar]
- 118.Tosh K, Meisner S, Siddiqui MR, Balakrishnan K, Ghei S, Golding M, et al. A region of chromosome 20 is linked to leprosy susceptibility in a South Indian population. J Infect Dis. 2002;186:1190–1193. doi: 10.1086/343806. [DOI] [PubMed] [Google Scholar]
- 119.Zhang X, Jiang F, Wei L, Li F, Liu J, Wang C, et al. Polymorphic Allele of Human MRC1 Confer Protection against Tuberculosis in a Chinese Population. Int J Biol Sci. 2012;8:375–382. doi: 10.7150/ijbs.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alter A, de LL, Van TN, Thai VH, Huong NT, Ba NN, et al. Genetic and functional analysis of common MRC1 exon 7 polymorphisms in leprosy susceptibility. Hum Genet. 2010;127:337–348. doi: 10.1007/s00439-009-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martínez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S, Bonnefoy JY, et al. Fe chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Linehan SA, Martiniz-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Engering AJ, Cella M, Fluitsma DM, Hoefsmit EC, Lanzavecchia A, Pieters J. Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv Exp Med Biol. 1997;417:183–187. doi: 10.1007/978-1-4757-9966-8_31. [DOI] [PubMed] [Google Scholar]
- 124.Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, et al. Mannose receptor mediated uptake of antigens strongly enhances HLA-class II restricted antigen presentation by cultured dendritic cells. Adv Exp Med Biol. 1997;417:171–174. doi: 10.1007/978-1-4757-9966-8_28. [DOI] [PubMed] [Google Scholar]
- 125.Berney C, Herren S, Power CA, Gordon S, Martinez-Pomares L, Kosco-Vilbois MH. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190:851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 127.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 128.Hattori Y, Kawakami S, Lu Y, Nakamura K, Yamashita F, Hashida M. Enhanced DNA vaccine potency by mannosylated lipoplex after intraperitoneal administration. J Gene Med. 2006;8:824–834. doi: 10.1002/jgm.910. [DOI] [PubMed] [Google Scholar]
- 129.Lu Y, Kawakami S, Yamashita F, Hashida M. Development of an antigen-presenting cell-targeted DNA vaccine against melanoma by mannosylated liposomes. Biomaterials. 2007;28:3255–3262. doi: 10.1016/j.biomaterials.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 130.Azad AK, Rajaram MV, Schlesinger LS. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J Cytol Mol Biol. 2014:1. doi: 10.13188/2325-4653.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McNally AK, DeFife KM, Anderson JM. Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol. 1996;149:975–985. [PMC free article] [PubMed] [Google Scholar]
- 132.Gordon S, Keshav S, Stein M. BCG-induced granuloma formation in murine tissues. Immunobiology. 1994;191:369–377. doi: 10.1016/S0171-2985(11)80442-0. [DOI] [PubMed] [Google Scholar]
- 133.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 134.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 135.Zimmerli S, Edwards S, Ernst JD. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–770. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]
- 136.Doi T, Higashino K, Kurihara Y, Wada Y, Miyazaki T, Nakamura H, et al. Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J Biol Chem. 1993;268:2126–2133. [PubMed] [Google Scholar]
- 137.Yamamoto K, Nishimura N, Doi T, Imanishi T, Kodama T, Suzuki K, et al. The lysine cluster in the collagen-like domain of the scavenger receptor provides for its ligand binding and ligand specificity. FEBS Lett. 1997;414:182–186. doi: 10.1016/s0014-5793(97)01006-5. [DOI] [PubMed] [Google Scholar]
- 138.Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol. 2005;175:6058–6064. doi: 10.4049/jimmunol.175.9.6058. [DOI] [PubMed] [Google Scholar]
- 139.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, et al. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol. 2006;35:474–478. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hollifield M, Bou GE, de Villiers WJ, Garvy BA. Scavenger receptor A dampens induction of inflammation in response to the fungal pathogen Pneumocystis carinii. Infect Immun. 2007;75:3999–4005. doi: 10.1128/IAI.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Asada K, Sasaki S, Suda T, Chida K, Nakamura H. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am J Respir Crit Care Med. 2004;169:195–200. doi: 10.1164/rccm.200207-740OC. [DOI] [PubMed] [Google Scholar]
- 144.Reddy RC. Immunomodulatory role of PPAR-gamma in alveolar macrophages. J Investig Med. 2008;56:522–527. doi: 10.2310/JIM.0b013e3181659972. [DOI] [PubMed] [Google Scholar]
- 145.Mahajan S, Dkhar HK, Chandra V, Dave S, Nanduri R, Janmeja AK, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol. 2012;188:5593–5603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 146.Hawkes M, Li X, Crockett M, Diassiti A, Finney C, Min-Oo G, et al. CD36 deficiency attenuates experimental mycobacterial infection. BMC Infect Dis. 2010;10:299. doi: 10.1186/1471-2334-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Court N, Vasseur V, Vacher R, Fremond C, Shebzukhov Y, Yeremeev VV, et al. Partial redundancy of the pattern recognition receptors, scavenger receptors, and C-type lectins for the long-term control of Mycobacterium tuberculosis infection. J Immunol. 2010;184:7057–7070. doi: 10.4049/jimmunol.1000164. [DOI] [PubMed] [Google Scholar]
- 148.Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. [PubMed] [Google Scholar]
- 149.Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 151.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 154.Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY, et al. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171:4569–4573. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- 155.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, et al. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 156.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 157.Tsoni SV, Brown GD. beta-Glucans and dectin-1. Ann N Y Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 158.Yadav M, Schorey JS. The {beta}-glucan receptor Dectin-1 fucntions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van CR, Van der Meer JW, et al. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–232. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- 160.Betz BE, Azad AK, Morris JD, Rajaram MV, Schlesinger LS. beta-Glucans inhibit intracellular growth of Mycobacterium bovis BCG but not virulent Mycobacterium tuberculosis in human macrophages. Microb Pathog. 2011;51:233–242. doi: 10.1016/j.micpath.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee HM, Yuk JM, Shin DM, Jo EK. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol. 2009;29:795–805. doi: 10.1007/s10875-009-9319-3. [DOI] [PubMed] [Google Scholar]
- 162.Ariizumi K, Shen GL, Shikano S, Ritter R, III, Zukas P, Edelbaum D, et al. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J Biol Chem. 2000;275:11957–11963. doi: 10.1074/jbc.275.16.11957. [DOI] [PubMed] [Google Scholar]
- 163.Taylor PR, Reid DM, Heinsbroek SE, Brown GD, Gordon S, Wong SY. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur J Immunol. 2005;35:2163–2174. doi: 10.1002/eji.200425785. [DOI] [PubMed] [Google Scholar]
- 164.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 165.Yonekawa A, Saijo S, Hoshino Y, Miyake Y, Ishikawa E, Suzukawa M, et al. Dectin-2 Is a Direct Receptor for Mannose-Capped Lipoarabinomannan of Mycobacteria. Immunity. 2014;41:1–12. doi: 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 166.Ting JP, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 169.Hansen JM, Golchin SA, Veyrier FJ, Domenech P, Boneca IG, Azad AK, et al. N-glycolylated peptidoglycan contributes to the immunogenicity but not pathogenicity of Mycobacterium tuberculosis. J Infect Dis. 2014;209:1045–1054. doi: 10.1093/infdis/jit622. [DOI] [PubMed] [Google Scholar]
- 170.Coulombe F, Divangahi M, Veyrier F, de LL, Gleason JL, Yang Y, et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009;206:1709–1716. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brooks MN, Rajaram MV, Azad AK, Amer AO, Valdivia-Arenas MA, Park JH, et al. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol. 2011;13:402–418. doi: 10.1111/j.1462-5822.2010.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Juarez E, Carranza C, Hernandez-Sanchez F, Leon-Contreras JC, Hernandez-Pando R, Escobedo D, et al. NOD2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur J Immunol. 2012;42:880–889. doi: 10.1002/eji.201142105. [DOI] [PubMed] [Google Scholar]
- 173.Jiang-Shieh YF, Chien HF, Chang CY, Wei TS, Chiu MM, Chen HM, et al. Distribution and expression of CD200 in the rat respiratory system under normal and endotoxin-induced pathological conditions. J Anat. 2010;216:407–416. doi: 10.1111/j.1469-7580.2009.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vazquez N, Greenwell-Wild T, Rekka S, Orenstein JM, Wahl SM. Mycobacterium avium-induced SOCS contributes to resistance to IFN-gamma-mediated mycobactericidal activity in human macrophages. J Leukoc Biol. 2006;80:1136–1144. doi: 10.1189/jlb.0306206. [DOI] [PubMed] [Google Scholar]
- 176.Carow B, Ye X, Gavier-Widen D, Bhuju S, Oehlmann W, Singh M, et al. Silencing suppressor of cytokine signaling-1 (SOCS1) in macrophages improves Mycobacterium tuberculosis control in an interferon-gamma (IFN-gamma)-dependent manner. J Biol Chem. 2011;286:26873–26887. doi: 10.1074/jbc.M111.238287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Srivastava V, Vashishta M, Gupta S, Singla R, Singla N, Behera D, et al. Suppressors of cytokine signaling inhibit effector T cell responses during Mycobacterium tuberculosis infection. Immunol Cell Biol. 2011;89:786–791. doi: 10.1038/icb.2011.1. [DOI] [PubMed] [Google Scholar]
- 178.Srivastava V, Manchanda M, Gupta S, Singla R, Behera D, Das G, et al. Toll-like receptor 2 and DC-SIGNR1 differentially regulate suppressors of cytokine signaling 1 in dendritic cells during Mycobacterium tuberculosis infection. J Biol Chem. 2009;284:25532–25541. doi: 10.1074/jbc.M109.006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 181.Nakayama K, Okugawa S, Yanagimoto S, Kitazawa T, Tsukada K, Kawada M, et al. Involvement of IRAK-M in peptidoglycan-induced tolerance in macrophages. J Biol Chem. 2004;279:6629–6634. doi: 10.1074/jbc.M308620200. [DOI] [PubMed] [Google Scholar]
- 182.Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12 p40 production in macrophages. J Biol Chem. 2005;280:42794–42800. doi: 10.1074/jbc.M506471200. [DOI] [PubMed] [Google Scholar]
- 183.Jeyanathan M, McCormick S, Lai R, Afkhami S, Shaler CR, Horvath CN, et al. Pulmonary M. tuberculosis infection delays Th1 immunity via immunoadaptor DAP12-regulated IRAK-M and IL-10 expression in antigen-presenting cells. Mucosal Immunol. 2014;7:670–683. doi: 10.1038/mi.2013.86. [DOI] [PubMed] [Google Scholar]
- 184.Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Delgado-Ortega M, Marc D, Dupont J, Trapp S, Berri M, Meurens F. SOCS proteins in infectious diseases of mammals. Vet Immunol Immunopathol. 2013;151:1–19. doi: 10.1016/j.vetimm.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Carow B, Reuschl AK, Gavier-Widen D, Jenkins BJ, Ernst M, Yoshimura A, et al. Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis. PLoS Pathog. 2013;9:e1003442. doi: 10.1371/journal.ppat.1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Masood KI, Rottenberg ME, Salahuddin N, Irfan M, Rao N, Carow B, et al. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC Infect Dis. 2013;13:13. doi: 10.1186/1471-2334-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Masood KI, Rottenberg ME, Carow B, Rao N, Ashraf M, Hussain R, et al. SOCS1 gene expression is increased in severe pulmonary tuberculosis. Scand J Immunol. 2012;76:398–404. doi: 10.1111/j.1365-3083.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- 189.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 190.Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, et al. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- 191.Shiratori I, Yamaguchi M, Suzukawa M, Yamamoto K, Lanier LL, Saito T, et al. Down-regulation of basophil function by human CD200 and human herpesvirus-8 CD200. J Immunol. 2005;175:4441–4449. doi: 10.4049/jimmunol.175.7.4441. [DOI] [PubMed] [Google Scholar]
- 192.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 193.Li L, Elliott JF, Mosmann TR. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–3978. [PubMed] [Google Scholar]
- 194.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 195.Asadullah K, Sterry W, VolK HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 196.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 197.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 198.Yamaoka K, Otsuka T, Niiro H, Nakashima H, Tanaka Y, Nagano S, et al. Selective DNA-binding activity of interleukin-10-stimulated STAT molecules in human monocytes. J Interferon Cytokine Res. 1999;19:679–685. doi: 10.1089/107999099313839. [DOI] [PubMed] [Google Scholar]
- 199.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–1463. [PubMed] [Google Scholar]
- 200.Cassatella MA, Gasperini S, Bovolenta C, Calzetti F, Vollebregt M, Scapini P, et al. Interleukin-10 (IL-10) selectively enhances CIS3/SOCS3 mRNA expression in human neutrophils: evidence for an IL-10-induced pathway that is independent of STAT protein activation. Blood. 1999;94:2880–2889. [PubMed] [Google Scholar]
- 201.Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LC, Santos AR, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol. 2009;183:718–731. doi: 10.4049/jimmunol.0801212. [DOI] [PubMed] [Google Scholar]
- 202.Bonecini-Almeida MG, Ho JL, Boechat N, Huard RC, Chitale S, Doo H, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect Immun. 2004;72:2628–2634. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Verbon A, Juffermans N, van Deventer SJ, Speelman P, van DH, Van der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–113. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin YG, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang M, Lin Y, Iyer DV, Gong J, Abrams JS, Barnes PF. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huard RC, Chitale S, Leung M, Lazzarini LC, Zhu H, Shashkina E, et al. The Mycobacterium tuberculosis complex-restricted gene cfp32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect Immun. 2003;71:6871–6883. doi: 10.1128/IAI.71.12.6871-6883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.O'Leary S, O'Sullivan MP, Keane J. IL-10 Blocks Phagosome Maturation in Mycobacterium tuberculosis-infected Human Macrophages. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0319OC. [DOI] [PubMed] [Google Scholar]
- 210.Pacheco AG, Cardoso CC, Moraes MO. IFNG +874T/A, IL10 -1082G/A and TNF - 308G/A polymorphisms in association with tuberculosis susceptibility: a meta-analysis study. Hum Genet. 2008;123:477–484. doi: 10.1007/s00439-008-0497-5. [DOI] [PubMed] [Google Scholar]
- 211.Ates O, Musellim B, Ongen G, Topal-Sarikaya A. Interleukin-10 and tumor necrosis factor-alpha gene polymorphisms in tuberculosis. J Clin Immunol. 2008;28:232–236. doi: 10.1007/s10875-007-9155-2. [DOI] [PubMed] [Google Scholar]
- 212.Oral HB, Budak F, Uzaslan EK, Basturk B, Bekar A, Akalin H, et al. Interleukin-10 (IL-10) gene polymorphism as a potential host susceptibility factor in tuberculosis. Cytokine. 2006;35:143–147. doi: 10.1016/j.cyto.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 213.North RJ. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–58. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jung YJ, Ryan L, LaCourse R, North RJ. Increased interleukin-10 expression is not responsible for failure of T helper 1 immunity to resolve airborne Mycobacterium tuberculosis infection in mice. Immunology. 2003;109:295–299. doi: 10.1046/j.1365-2567.2003.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Higgins DM, Sanchez-Campillo J, Rosas-Taraco AG, Lee EJ, Orme IM, Gonzalez-Juarrero M. Lack of IL-10 alters inflammatory and immune responses during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb ) 2009;89:149–157. doi: 10.1016/j.tube.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 216.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40:2200–2210. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Roach DR, Martin E, Bean AG, Rennick DM, Briscoe H, Britton WJ. Endogenous inhibition of antimycobacterial immunity by IL-10 varies between mycobacterial species. Scand J Immunol. 2001;54:163–170. doi: 10.1046/j.1365-3083.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- 218.Beamer GL, Flaherty DK, Assogba BD, Stromberg P, Gonzalez-Juarrero M, de Waal MR, et al. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181:5545–5550. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 220.Sendide K, Deghmane AE, Pechkovsky D, Av-Gay Y, Talal A, Hmama Z. Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S. J Immunol. 2005;175:5324–5332. doi: 10.4049/jimmunol.175.8.5324. [DOI] [PubMed] [Google Scholar]
- 221.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–4087. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, et al. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 224.Simmons DP, Canaday DH, Liu Y, Li Q, Huang A, Boom WH, et al. Mycobacterium tuberculosis and TLR2 agonists inhibit induction of type I IFN and class I MHC antigen cross processing by TLR9. J Immunol. 2010;185:2405–2415. doi: 10.4049/jimmunol.0904005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/B. Proc Natl Acad Sci USA. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 227.Antonelli LR, Gigliotti RA, Goncalves R, Roffe E, Cheever AW, Bafica A, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 230.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 234.Ricote M, Huang JT, Welch JS, Glass CK. The peroxisome proliferator-activated receptor(PPARgamma) as a regulator of monocyte/macrophage function. J Leukoc Biol. 1999;66:733–739. doi: 10.1002/jlb.66.5.733. [DOI] [PubMed] [Google Scholar]
- 235.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 236.von Knethen A, Brune B. Activation of peroxisome proliferator-activated receptor gamma by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J Immunol. 2002;169:2619–2626. doi: 10.4049/jimmunol.169.5.2619. [DOI] [PubMed] [Google Scholar]
- 237.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Malur A, Mccoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, et al. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182:5816–5822. doi: 10.4049/jimmunol.0803504. [DOI] [PubMed] [Google Scholar]
- 239.Almeida PE, Silva AR, Maya-Monteiro CM, Torocsik D, D'Avila H, Dezso B, et al. Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol. 2009;183:1337–1345. doi: 10.4049/jimmunol.0900365. [DOI] [PubMed] [Google Scholar]
- 240.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 241.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 242.Ricote M, Valledor AF, Glass CK. Decoding transcriptional programs regulated by PPARs and LXRs in the macrophage: effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:230–239. doi: 10.1161/01.ATV.0000103951.67680.B1. [DOI] [PubMed] [Google Scholar]
- 243.Valledor AF. The innate immune response under the control of the LXR pathway. Immunobiology. 2005;210:127–132. doi: 10.1016/j.imbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 244.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 245.Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 246.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011;7:e1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–1650. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 249.Korf H, Vander BS, Romano M, Steffensen KR, Stijlemans B, Gustafsson JA, et al. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J Clin Invest. 2009;119:1626–1637. doi: 10.1172/JCI35288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Han M, Liang L, Liu LR, Yue J, Zhao YL, Xiao HP. Liver X receptor gene polymorphisms in tuberculosis: effect on susceptibility. PLoS One. 2014;9:e95954. doi: 10.1371/journal.pone.0095954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chang C, Da Silva SL, Ideta R, Lee Y, Yeh S, Burbach JP. Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc Natl Acad Sci U S A. 1994;91:6040–6044. doi: 10.1073/pnas.91.13.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee YF, Lee HJ, Chang C. Recent advances in the TR2 and TR4 orphan receptors of the nuclear receptor superfamily. J Steroid Biochem Mol Biol. 2002;81:291–308. doi: 10.1016/s0960-0760(02)00118-8. [DOI] [PubMed] [Google Scholar]
- 253.Xie S, Lee YF, Kim E, Chen LM, Ni J, Fang LY, et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci U S A. 2009;106:13353–13358. doi: 10.1073/pnas.0905724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dkhar HK, Nanduri R, Mahajan S, Dave S, Saini A, Somavarapu AK, et al. Mycobacterium tuberculosis keto-mycolic acid and macrophage nuclear receptor TR4 modulate foamy biogenesis in granulomas: a case of a heterologous and noncanonical ligand-receptor pair. J Immunol. 2014;193:295–305. doi: 10.4049/jimmunol.1400092. [DOI] [PubMed] [Google Scholar]
- 255.Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, et al. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci U S A. 2011;108:17408–17413. doi: 10.1073/pnas.1112660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Torrelles JB, Sieling PA, Arcos J, Knaup R, Bartling C, Rajaram MV, et al. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem. 2011;286:35438–35446. doi: 10.1074/jbc.M111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lee W, VanderVen BC, Fahey RJ, Russell DG. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem. 2013 doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 259.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ueno N, Murakami M, Tanioka T, Fujimori K, Tanabe T, Urade Y, et al. Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2. J Biol Chem. 2001;276:34918–34927. doi: 10.1074/jbc.M100429200. [DOI] [PubMed] [Google Scholar]
- 261.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 262.Coceani F, Bishai I, Lees J, Sirko S. Prostaglandin E2 and fever: a continuing debate. Yale J Biol Med. 1986;59:169–174. [PMC free article] [PubMed] [Google Scholar]
- 263.Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 264.Rangel MJ, Estrada G, De La Luz Garcia Hernandez, Aguilar LD, Marquez R, Hernandez PR. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology. 2002;106:257–266. doi: 10.1046/j.1365-2567.2002.01403.x. I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90:579–586. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Russell CD, Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141:166–173. doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Widgerow AD. Cellular resolution of inflammation--catabasis. Wound Repair Regen. 2012;20:2–7. doi: 10.1111/j.1524-475X.2011.00754.x. [DOI] [PubMed] [Google Scholar]
- 269.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 270.Ye Y, Lin Y, Perez-Polo JR, Uretsky BF, Ye Z, Tieu BC, et al. Phosphorylation of 5-lipoxygenase at ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epilipoxin a4 production. J Immunol. 2008;181:3515–3523. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- 271.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, et al. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 272.Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kowal-Bielecka O, Kowal K, Distler O, Gay S. Mechanisms of Disease: leukotrienes and lipoxins in scleroderma lung disease--insights and potential therapeutic implications. Nat Clin Pract Rheumatol. 2007;3:43–51. doi: 10.1038/ncprheum0375. [DOI] [PubMed] [Google Scholar]
- 274.Ford-Hutchinson A, Letts G. Biological actions of leukotrienes. State of the art lecture. Hypertension. 1986;8:II44–II49. doi: 10.1161/01.hyp.8.6_pt_2.ii44. [DOI] [PubMed] [Google Scholar]
- 275.Peters-Golden M, Henderson WR., Jr. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 276.Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 277.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Peres-Buzalaf C, de PL, Frantz FG, Soares EM, Medeiros AI, Peters-Golden M, et al. Control of experimental pulmonary tuberculosis depends more on immunostimulatory leukotrienes than on the absence of immunosuppressive prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 2011;85:75–81. doi: 10.1016/j.plefa.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Coffey MJ, Phare SM, Peters-Golden M. Role of leukotrienes in killing of Mycobacterium bovis by neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2004;71:185–190. doi: 10.1016/j.plefa.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 280.Franco LH, Paula MO, Wowk PF, Fonseca DM, Sergio CA, Fedatto PF, et al. Leukotrienes are not essential for the efficacy of a heterologous vaccine against Mycobacterium tuberculosis infection. Braz J Med Biol Res. 2010;43:645–650. doi: 10.1590/s0100-879x2010007500053. [DOI] [PubMed] [Google Scholar]
- 281.El-Ahmady O, Mansour M, Zoeir H, Mansour O. Elevated concentrations of interleukins and leukotriene in response to Mycobacterium tuberculosis infection. Ann Clin Biochem. 1997;34:160–164. doi: 10.1177/000456329703400205. Pt 2. [DOI] [PubMed] [Google Scholar]
- 282.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tobin DM, Roca FJ, Ray JP, Ko DC, Ramakrishnan L. An enzyme that inactivates the inflammatory mediator leukotriene b4 restricts mycobacterial infection. PLoS One. 2013;8:e67828. doi: 10.1371/journal.pone.0067828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 286.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 287.Yi Z, Fu Y, Ji R, Li R, Guan Z. Altered microRNA Signatures in Sputum of Patients with Active Pulmonary Tuberculosis. PLoS ONE. 2012;7:e43184. doi: 10.1371/journal.pone.0043184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Miotto P, Mwangoka G, Valente IC, Norbis L, Sotgiu G, Bosu R, et al. miRNA signatures in Sera of patients with active pulmonary tuberculosis. PLoS ONE. 2013;8:e80149. doi: 10.1371/journal.pone.0080149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang X, Guo J, Fan S, Li Y, Wei L, Yang X, et al. 2013;8:e81076. doi: 10.1371/journal.pone.0081076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Qi Y, Cui L, Ge Y, Shi Z, Zhao K, Guo X, et al. Altered serum microRNAs as biomarkers for the early diagnosis of pulmonary tuberculosis infection. BMC Infect Dis. 2012;12:384. doi: 10.1186/1471-2334-12-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fu Y, Yi Z, Wu X, Li J, Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J Clin Microbiol. 2011;49:4246–4251. doi: 10.1128/JCM.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang J, Yang K, Zhou L, Minhaowu Wu Y, Zhu M, et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9:e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kumar R, Halder P, Sahu SK, Kumar M, Kumari M, Jana K, et al. Identification of a novel role of ESAT-6-dependent miR-155 induction during infection of macrophages with Mycobacterium tuberculosis. Cell Microbiol. 2012;14:1620–1631. doi: 10.1111/j.1462-5822.2012.01827.x. [DOI] [PubMed] [Google Scholar]
- 294.Singh Y, Kaul V, Mehra A, Chatterjee S, Tousif S, Dwivedi VP, et al. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J Biol Chem. 2013;288:5056–5061. doi: 10.1074/jbc.C112.439778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Liu Y, Wang X, Jiang J, Cao Z, Yang B, Cheng X. Modulation of T cell cytokine production by miR-144* with elevated expression in patients with pulmonary tuberculosis. Mol Immunol. 2011;48:1084–1090. doi: 10.1016/j.molimm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 296.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 297.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 298.Ma C, Li Y, Li M, Deng G, Wu X, Zeng J, et al. microRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol Immunol. 2014;62:150–158. doi: 10.1016/j.molimm.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 299.Yi Z, Fu Y, Ji R, Li R, Guan Z. Altered microRNA signatures in sputum of patients with active pulmonary tuberculosis. PLoS One. 2012;7:e43184. doi: 10.1371/journal.pone.0043184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang J, Yang K, Zhou L, Minhaowu Wu Y, Zhu M, et al. MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb. PLoS Pathog. 2013;9:e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Dorhoi A, Iannaccone M, Farinacci M, Fae KC, Schreiber J, Moura-Alves P, et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest. 2013 doi: 10.1172/JCI67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Heuts F, Gavier-Widen D, Carow B, Juarez J, Wigzell H, Rottenberg ME. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci U S A. 2013;110:6482–6487. doi: 10.1073/pnas.1219985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mestas J, Hughes CC. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 305.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 306.He LZ, Crocker A, Lee J, Mendoza-Ramirez J, Wang XT, Vitale LA, et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol. 2007;178:6259–6267. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 307.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Risso A. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukoc Biol. 2000;68:785–792. [PubMed] [Google Scholar]
- 310.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 311.Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fc-gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS One. 2014;9:e92977. doi: 10.1371/journal.pone.0092977. [DOI] [PMC free article] [PubMed] [Google Scholar]